Abstract
Lymphangioleiomyomatosis (LAM) is a rare neoplastic metastatic disease affecting women of childbearing age. LAM is caused by hyperactivation of the mechanistic target of rapamycin complex 1 (mTORC1) as a consequence of tuberous sclerosis complex (TSC) 1/2 inactivation. Clinically, LAM results in cystic lung destruction. mTORC1 inhibition using rapamycin analogs (rapalogs) is partially effective in reducing disease progression and improving lung function. However, cessation of treatment results in continued progression of the disease. In the present study, we investigated the effectiveness of the combination of rapamycin treatment with resveratrol, an autophagy inhibitor, in the TSC2-null xenograft tumor model. We determined that this combination inhibits phosphatidylinositol-4,5-bisphosphate 3-kinase PI3K/Akt/mTORC1 signaling and activates apoptosis. Therefore, the combination of rapamycin and resveratrol may be an effective clinical strategy for treatment of LAM and other diseases with mTORC1 hyperactivation.
Keywords: rapamycin, resveratrol, LAM, TSC2, mTORC1
Clinical Relevance
Rapamycin is an effective treatment for lymphangioleiomyomatosis (LAM), but the disease progresses in some patients and in patients who discontinue treatment for various reasons. The combination of resveratrol and rapamycin may achieve the goal of eliminating TSC2-deficient cells and allow the patients to stop rapamycin without further disease progression. Going forward, it would be interesting to proceed to a clinical trial of the use of resveratrol in combination with rapamycin compared with rapamycin monotherapy in patients with LAM. Because resveratrol has a low toxicity profile, it may be an attractive option.
Lymphangioleiomyomatosis (LAM) is a rare, slowly progressing neoplastic disorder primarily affecting women of childbearing age (1, 2). It is characterized by the presence of metastatic, smooth-muscle–like cells in the lungs that destroy pulmonary connective tissue and form multiple cysts (3, 4). LAM is caused by inactivating mutations in the tumor suppressor proteins tuberous sclerosis complex (TSC) (5, 6). The TSC1/TSC2 heterodimer is a negative regulator of the mechanistic target of rapamycin complex 1 (mTORC1). TSC1/2 acts as a GTPase-activating protein for the small GTPase Rheb (7, 8), which directly binds to mTOR and activates it in a GTP-dependent manner (9). Therefore, the phosphorylation and negative regulation of TSC2 leads to the activation of mTORC1. mTOR, a serine/threonine kinase that belongs to the phosphatidylinositol 3-kinase–related kinase family, was discovered as a target of a naturally occurring molecule called rapamycin (10). mTOR is a master sensor of nutrient availability in the cell that regulates proliferation, cellular metabolism, protein and lipid synthesis, and autophagy in response to extracellular signals such as nutrient availability and growth factors (11). mTOR is a key component of two distinct complexes: mTORC1 and mTORC2. These complexes differ in their protein composition, downstream targets, and sensitivity to rapamycin. mTORC1 is acutely rapamycin sensitive, and mTORC2 is rapamycin insensitive (11). The growth factor input to mTORC1 via the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt signaling cascade is mediated through the inhibition of TSC2 protein (12–14), and several growth factor–stimulated signaling pathways converge on TSC2. Akt, acting downstream of activated PI3K, phosphorylates TSC2 at S939 and T1462 (15), whereas Ras-activated extracellular signal-regulated protein kinase (ERK)1/2 phosphorylates TSC2 at residue S664, also leading to inactivation of TSC2 (16). Finally, ribosomal S6 kinase, a downstream effector of ERK, has also been shown to directly phosphorylate TSC2 on S1798 (17).
In LAM, loss of TSC1/2 leads to uncontrolled activation of mTORC1 and unrestricted cell growth. LAM can occur sporadically or is associated with TSC syndrome, an inherited, autosomal-dominant tumor suppressor disorder that is characterized by benign tumors of the brain, heart, and kidneys as well as seizures, cognitive impairment, and skin lesions (18). Whereas LAM in women with TSC is caused by mutations in either the TSC1 or TSC2 genes, sporadic LAM is almost exclusively caused by mutations in TSC2. Because of the hyperactivation of mTORC1 in LAM, mTORC1 inhibitors are an effective therapeutic tool for blocking LAM cell proliferation. Clinical trials with rapamycin and its analog (rapalog) everolimus reduced disease-related symptoms and stabilized pulmonary function in patients; however, treatment was not able to reverse the preexisting damage, and pulmonary function decline resumed after cessation of treatment (19, 20). Several factors likely contribute to the partial effectiveness of rapalogs. First, inhibition of mTORC1 relieves the negative feedback loop to PI3K/Akt, promoting Akt activation, an oncogene that suppresses apoptosis and promotes cell survival. Thus, rapamycin is cytostatic rather than cytotoxic. Second, increased mTORC1 activity in LAM cells negatively regulates autophagy (21), rendering LAM-derived cells acutely dependent on autophagy for survival. Thus, rapamycin causes reactivation of autophagy, which paradoxically allows LAM cells to survive. Therefore, the use of autophagy inhibitors in addition to rapamycin treatment could cause LAM cells to undergo apoptosis (22, 23).
We have previously demonstrated that the autophagy inhibitor resveratrol synergized with rapamycin in suppressing PI3K/mTORC1 activity, preventing rapamycin-induced autophagy and promoting death of TSC2-deficient cells in vitro (23). In this study we investigated whether the combination of rapamycin and resveratrol is effective in reducing TSC2-deficient tumor burden in vivo, thereby increasing the clinical usefulness of rapamycin therapy.
Materials and Methods
Wound Healing Assay
Cells were seeded in 12-well plates in complete DMEM and grown to confluency in monolayer overnight. Diagonal wound/scratch was created using a 200-μl pipette tip. Cell debris was removed by washing once with PBS, followed by the addition of fresh media containing 20 nM rapamycin and/or 100 μM resveratrol for 17 hours. Vehicle alone served as control. For each condition, three different areas were imaged, and three measurements were taken per image. Images were collected, and migration distance was measured using an EVOS FL Auto microscope and software (Life Technologies, Grand Island, NY) at 20× magnification.
Boyden Chamber Assay
Cells were cultured in media without serum in the presence of rapamycin and/or resveratrol. Nineteen hours later, 1 × 105 cells per well were plated on tissue culture inserts with 8.0-μm pores (BD Biosciences, San Jose, CA). The inserts were incubated with serum-free media containing 20 nM rapamycin and/or 100 μM resveratrol. Complete media were added to the lower chamber and allowed to migrate for 6 hours. After 6 hours, cells remaining on the upper side of the membrane were scraped off, and the cells that had migrated to the lower side of the membrane were fixed in 4% paraformaldehyde. The insert membranes were removed, stained, and mounted on coverslips using DAPI Fluoromount (Southern Biotech, Birmingham, AL). Images were collected at 10× magnification using an EVOS FL Auto miscroscope. Nuclei were counted manually, and data were analyzed using two-tailed Student’s t test.
Animal Studies
All animal work was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee-Children's Hospital Boston (IACUC-CHB). Intact female CB17-SCID mice, 6 to 8 weeks of age, were purchased from Taconic (Hudson, NY). Eker rat uterine leiomyoma–derived TSC2-deficient (ELT3) cells were developed by Howe and colleagues (24, 25). ELT3 cells were transduced with Luciferase tag for bioluminescent imaging. For xenograft tumor establishment, 2.5 × 106 cells were inoculated bilaterally into the posterior back region of mice. Five weeks after cell inoculation, mice bearing subcutaneous tumors were randomized into four groups: vehicle control (n = 5; 10% ethanol in 10% 2-hydroxypropyl-β-cyclodextrin, 200 μl/d, orally), rapamycin (n = 4; 3 mg/kg/d, intraperitoneally), resveratrol (n = 5; 200 mg/kg/d in 10% 2-hydroxypropyl-β-cyclodextrin, orally), and rapamycin plus resveratrol (n = 5; 3 mg/kg/d, intraperitoneally and 200 mg/kg/d, orally). The concentrations were selected based on effective doses as previously determined (23). Animal toxicity was analyzed using body weight as previously described (21). Drug treatment was initiated 5 weeks after cell inoculation. Body weight was measured every week. Tumor volume [(length × width)2/2] was measured weekly using calipers.
Bioluminescent Reporter Imaging
Ten minutes before imaging, animals were injected with luciferin (Xenogen, Alameda, CA) (120 mg/kg, intraperitoneally). Bioluminescent signals were recorded using the Xenogen IVIS System. Total photon flux of tumors was analyzed (26).
Statistical Analysis
For animal experiments, all data are presented as means ± SEM. Measurements at single time points were analyzed by ANOVA and, if demonstrated significance, were further analyzed by a two-tailed Student’s t test. Time courses were analyzed by a repeated measures (mixed model) ANOVA with Bonferroni post t tests. Statistical tests and graphs were performed with GraphPad Prism 6.0 (GraphPad Software, San Diego, CA). P < 0.05 indicates statistical significance.
For in vitro experiments, statistical analysis was performed using a two-tailed Student’s t test and graphed using Excel (Microsoft, Redmond, WA).
Further details are provided in the online supplement.
Results
The Combination of Rapamycin and Resveratrol Suppresses PI3K/mTORC1 Signaling, Promotes Apoptosis, and Reduces Motility of TSC2-Null LAM Cells
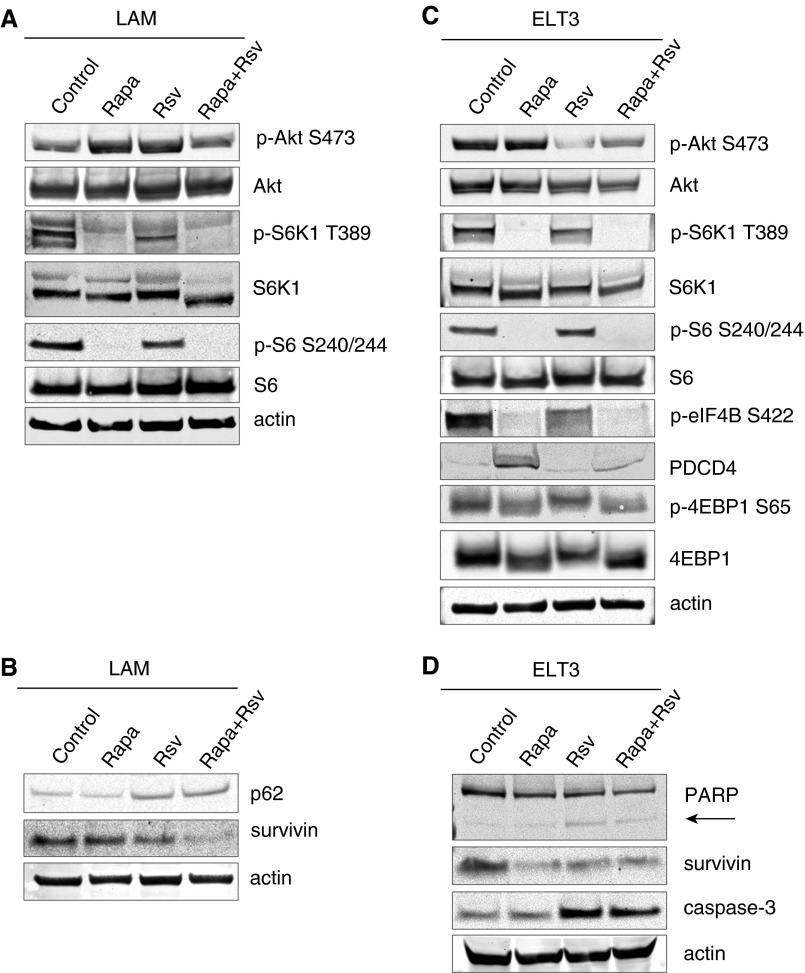
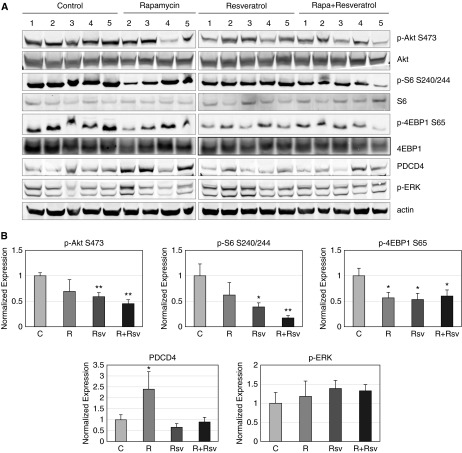
We first confirmed the ability of the rapamycin and resveratrol combination to inhibit PI3K/mTORC1 activation in human LAM-derived cells, which were obtained from biopsies of lung lesions from patients with LAM (5). Due to TSC2 deficiency and hyperactivation of mTORC1, these cells have high basal levels of p-S6K1 and p-S6 (Figure 1A). Combination treatment with rapamycin and resveratrol was able to inhibit S6K1 signaling, as evidenced by the reduction of S6K1 phosphorylation at T389 and S6 at S240/244. We also observed that rapamycin-induced phosphorylation of Akt on S473 was suppressed by the addition of resveratrol, and these results are in agreement with our previous findings (23). Combination therapy also induced stabilization of the autophagy protein p62/SQSTM1, corresponding to suppression of autophagy induction, and we observed reduced levels of survivin (Figure 1B). Similar results were seen with the use of combination therapy in ELT3 uterine leiomyoma cells (ELT3) derived from Eker rat (24). As shown in Figure 1C, combination or rapamycin and resveratrol inhibited TORC1/S6K1 signaling as indicated by the suppression of S6K1 phosphorylation at T389, eIF4B at S422, and S6 at S240/244, as well as stabilization of PDCD4 protein levels. mTORC1 signaling to 4EBP1 was also suppressed, as evidenced by dephosphorylation of 4EBP1 at S65. Additionally, the rapamycin and resveratrol combination was able to inhibit rapamycin-induced activation of Akt by decreasing p-Akt levels compared with that of vehicle-treated controls. Combination therapy with rapamycin and resveratrol also dramatically down-regulated survivin levels and induced the apoptotic markers cleaved poly(ADP-ribose) polymerase-1 (PARP) and caspase-3 (Figure 1D).
Figure 1.
Combination of rapamycin (Rapa) and resveratrol (Rsv) inhibits phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt and mechanistic target of rapamycin (mTOR) signaling pathways in lymphangioleiomyomatosis (LAM) and Eker rat uterine leiomyoma-derived TSC2-deficient (ELT3) cells. (A) LAM cells were treated with 20 nM rapamycin and/or 100 μM resveratrol for 24 hours. Cells were lysed, and indicated proteins were detected by immunoblot. (B) LAM cells were treated as described in (A). (C and D) ELT3 cells were treated as described in (A). Each experiment was performed at least three times to ensure reproducibility. PARP, poly(ADP-ribose) polymerase-1.
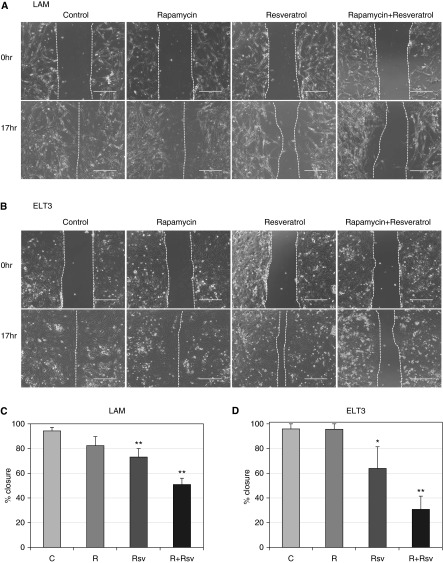
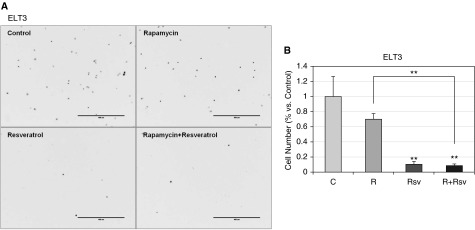
To test whether combination therapy with rapamycin and resveratrol affects cell motility, migration of ELT3 and LAM cells was measured by means of the wound-healing assay (Figure 2). Although rapamycin treatment alone resulted in complete wound closure 17 hours after treatment (Figures 2A and 2C), the newly populated area showed lower cell density than the untreated controls, indicating that rapamycin treatment delays cell migration (27). Additionally, as previously reported, resveratrol treatment alone impaired cell migration by approximately 25% compared with controls (28). Significantly, the combination treatment produced a synergistic effect on wound healing: only approximately 50% of the scratched area was repopulated. Similar effects on wound healing were seen with ELT3 cells (Figures 2B and 2D), where rapamycin treatment alone did not prevent complete would closure, resveratrol treatment alone produced approximately 65% repopulation, and the combination of rapamycin and resveratrol resulted in approximately 30% repopulation. Additionally, transwell cell migration assay confirmed that the combination of rapamycin and resveratrol reduced cell migration of TSC2-null ELT3 cells by over 90% (Figure 3). Thus, our data indicate that the combination of rapamycin and resveratrol is effective in inhibiting PI3K/mTORC1 signaling and reducing cell motility of TSC2-null cells.
Figure 2.
The combination of rapamycin and resveratrol reduces cell migration of LAM and ELT3 cells. (A) LAM cells grown to confluent monolayer were scratched and treated with 20 nM rapamycin and/or 100 μM resveratrol for 17 hours. Representative images at the 0- and 17-hour time points are shown. (B) ELT3 cells were treated as described in (A). (C) Measurement and quantification of LAM cell migration was performed using EVOL FL Auto and Excel software. Statistical analysis was performed using two-tailed Student’s t test. (D) Measurement and quantification of the ELT3 cell migration was performed as described in (C). Scale bars, 400 μm. *P < 0.05; **P < 0.01. C, control; R, rapamycin.
Figure 3.
The combination of rapamycin and resveratrol prevents cell migration. Cell migration/Boyden chamber assay was performed as described in Materials and Methods. (A) Representative images of ELT3 cells stained with 4′,6-diamidino-2-phenylindole after a 6-hour migration assay. (B) Histogram representing the number of cells migrated relative to untreated control. Scale bars, 400 μm. **P < 0.01.
Combined Administration of Rapamycin and Resveratrol Has Low Toxicity in Mice and Causes a Significant Reduction of TSC2-Deficient Xenograft Tumor Size and Growth
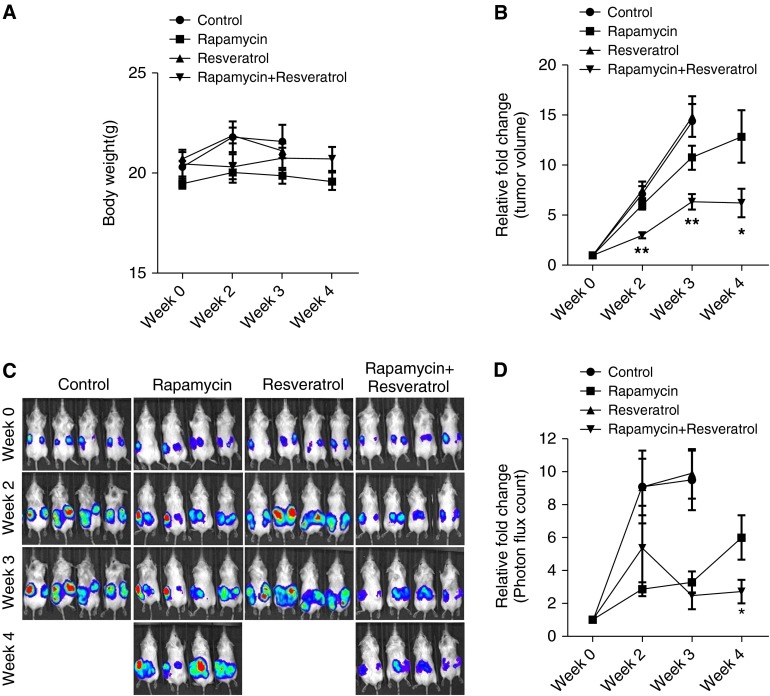
To evaluate the role of rapamycin and resveratrol inhibition in cells with mTORC1 hyperactivation in vivo, we used an ELT3 cell xenograft model (26). Mice bearing ELT3-luciferase–expressing xenograft tumors were treated with rapamycin and resveratrol as single-agent treatments or in combination. Drug toxicity was evaluated by body weight changes, and no significant effect was observed after the drug treatments (Figure 4A). It is important to note that tumors from control and resveratrol groups grew faster than the ones from the rapamycin alone or the rapamycin with resveratrol groups; thus, ulcers developed in the skin of tumor areas. We therefore had to kill these two groups at Week 3. We next assessed the possible benefits of combination treatment on tumor development by analysis of the xenograft tumor size. As previously published (21), rapamycin treatment alone showed a slight but significant reduction in tumor size in just 3 weeks of treatment. Strikingly, the combination of rapamycin and resveratrol caused a statistically significant reduction in tumor size as compared with rapamycin treatment alone by as early as Week 2 of treatment, and this reduction in tumor size was maintained at 4 weeks after treatment (Figure 4B). Subsequently, in comparison with the vehicle control, single-agent treatment of rapamycin or the combination of rapamycin and resveratrol for 3 weeks reduced tumor growth by 0.54-fold as measured by bioluminescence intensity (Figures 4C and 4D). More importantly, unlike single-agent rapamycin treatment, the combination of rapamycin and resveratrol suppressed xenograft tumor progression at 4 weeks of treatment (Figures 4C and 4D). Our results indicate that combination therapy of rapamycin with resveratrol is significantly more effective that rapamycin treatment alone in reducing tumor size and tumor growth while maintaining low toxicity.
Figure 4.
Combination treatment with rapamycin and resveratrol reduces tumor size and inhibits growth. Female CB17-scid mice were inoculated with ELT3-luciferase cells subcutaneously. Mice were treated with vehicle, rapamycin, resveratrol, or a combination of rapamycin and resveratrol for 4 weeks. (A) Body weight was measured every week. (B) Tumor volume was measured weekly using a digital caliper. The y axis indicates the relative fold growth of tumor size versus the baseline measurement before drug treatment. (C and D) Bioluminescent intensity in xenograft tumors was recorded and quantified weekly. The y axis indicates the relative tumor growth versus the baseline quantification before drug treatment. *P < 0.05; **P < 0.01. Vehicle- and resveratrol-treated tumors progressed faster than the tumors in rapamycin or rapamycin–resveratrol groups, and ulcers developed in the skin of tumor areas; thus, those animals had to be killed at Week 3.
The Combination of Rapamycin and Resveratrol Suppresses PI3K/Akt/mTORC1 Activation in the Tumor
We next examined the effect of the rapamycin and resveratrol combination on the activity of the PI3K/Akt/mTORC1 signaling pathways in tumor samples. The combination of rapamycin and resveratrol resulted in severe reduction of S6 phosphorylation and approximately 40% inhibition of 4EBP1 phosphorylation (Figures 5A and 5B). Although rapamycin treatment alone caused a slight inhibition of S6 phosphorylation, it was not statistically significant. This could be due to compensatory S6 phosphorylation by ribosomal S6 kinase (29). Moreover, the combination of rapamycin and resveratrol caused a 45% inhibition of Akt phosphorylation at S473. This is important because inhibition of mTORC1 signaling leads to suppression of the negative feedback loop to Akt, causing reactivation of Akt (30). The combination treatment was also able to stabilize PDCD4 protein levels. Finally, rapamycin and resveratrol, alone or in combination, did not alter p-ERK levels, indicating the specificity of this treatment in inhibiting Akt/mTORC1 signaling, as we have previously observed (31).
Figure 5.
The combination of rapamycin and resveratrol inhibits mechanistic target of rapamycin complex 1/Akt signaling in xenograft tumors. (A) Tumors were lysed as described in Materials and Methods, and indicated proteins were detected by immunoblot. Numbers represent different mice within the treatment group. (B) Quantification of immunoblots was performed using Image Studio 4.0 program and Excel. *P < 0.05; **P < 0.01.
Combined Administration of Rapamycin and Resveratrol Reduces Proliferation and Induces Apoptosis within the Tumor
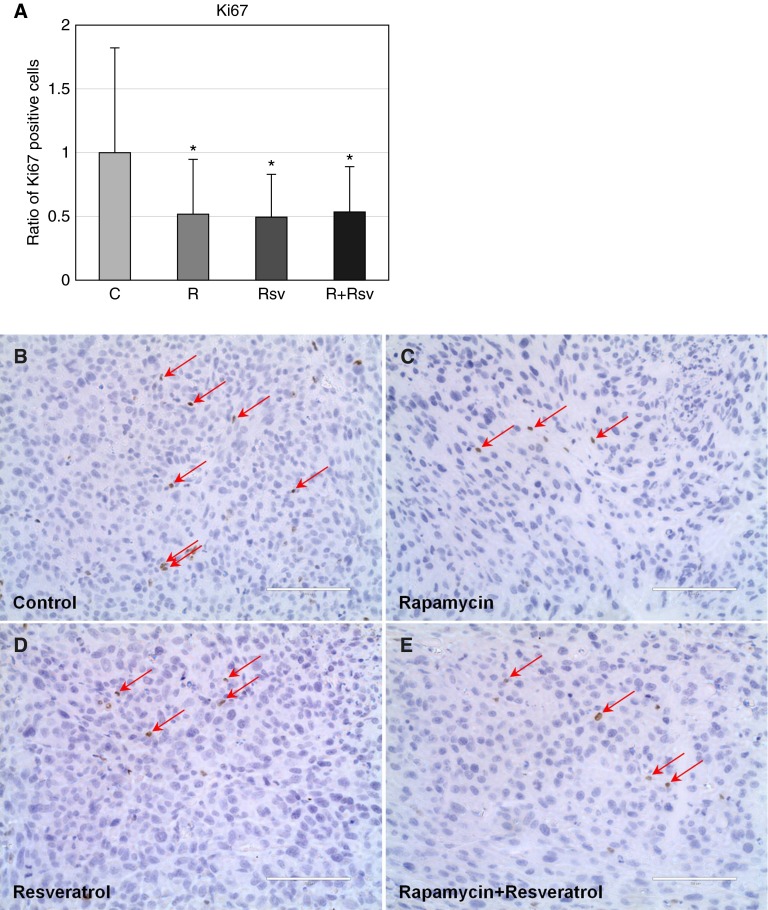
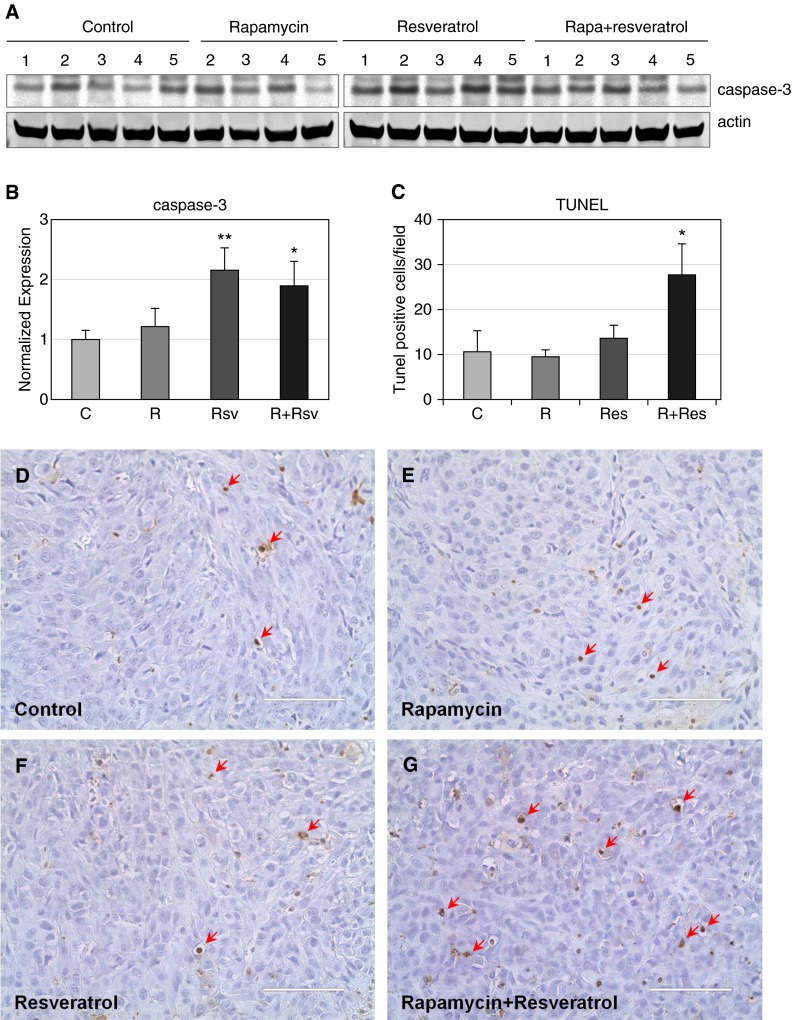
To examine the cause of reduced tumor size as observed within the combination rapamycin and resveratrol treatment group, we first examined tumors for expression of Ki67, a marker of proliferative index. Single-agent or combination treatment reduced the number of proliferating cells by 50% compared with untreated tumors (Figure 6). We subsequently examined the levels of cleaved caspase-3, a marker of apoptosis. Rapamycin and resveratrol combination treatment caused a 2-fold up-regulation of cleaved caspase-3 (Figures 7A and 7B). In addition, analysis of TUNEL staining of tumor sections showed that combination treatment increased the levels of apoptotic TUNEL-positive cells by 3-fold, whereas single-agent treatment with either rapamycin or resveratrol had no effect on apoptosis (Figures 7C–7G). Taken together, these data indicate that a 4-week administration of the combination treatment of rapamycin and resveratrol inhibits mTORC1 signaling, inhibits Akt activation, induces apoptosis, and inhibits cells motility.
Figure 6.
The combination of rapamycin and resveratrol reduces proliferation in xenograft tumors. (A) Quantification of Ki617-positive cells was performed as described in Materials and Methods. *P < 0.05. After immunohistochemistry, representative images for control (B), rapamycin (C), resveratrol (D), or the combination of rapamycin and resveratrol (E) were created using an EVOS Fl Auto microscope (original magnification: ×40). Arrows indicate Ki67-positive cells. Scale bars, 100 μm.
Figure 7.
The combination of rapamycin and resveratrol induces apoptosis in xenograft tumors. (A) Tumors were lysed as described in Materials and Methods, and caspase-3 and actin were detected by immunoblot. (B) Quantification of caspase-3 was performed using Image Studio 4.0 program and Excel. *P < 0.05; **P < 0.01. (C) Quantification of TUNEL-positive cells was performed as described in Materials and Methods. *P < 0.05. After immunohistochemistry, representative images for control (D), rapamycin (E), resveratrol (F), or the combination of rapamycin and resveratrol (G) were created using an EVOS Fl Auto microscope (original magnification: ×40). Arrows indicate TUNEL-positive cells. Scale bars, 100 μm. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Discussion
Because loss of TSC function leads to an enhancement of signaling through mTORC1, rapamycin has always been an attractive therapy for LAM. Clinical trials for sirolimus resulted in significant improvement of symptoms during the treatment period. However after treatment was discontinued, symptoms returned, often to levels comparable with the placebo group (19, 20, 32–34). The cytostatic response of LAM cells to sirolimus may be due to activation of autophagy as a result of the inhibition of mTORC1 activity, causing cell survival (1).
Although it would be expected that a loss of TSC function would lead to an enhancement of cell growth due to increased mTORC1 signaling, this is not observed in LAM cells. mTORC1 activity negatively regulates autophagy, and it has been suggested that the mTORC1-induced inhibition of autophagy maintains the LAM cells in a stagnant metabolic state, preventing cell growth and proliferation that would have otherwise been stimulated by mTORC1 activity (21, 35). In addition, LAM cells seem to be dependent on even the low levels of autophagy because further inhibition of autophagy induces cell death (21).
Our previous studies showed that combination therapy of rapamycin with resveratrol was able to block autophagy and induce apoptosis in TSC2-null cells and in cells with mTORC1 pathway hyperactivation (22, 23). In this study, we observed that, in TSC2-null cells, the combination therapy prevented rapamycin-induced up-regulation of Akt while maintaining inhibition of S6K1 signaling, indicating that this combination therapy can counter hyperactivation of mTORC1 signaling caused by the loss of TSC2. Additionally, resveratrol treatment was able to prevent rapamycin-induced up-regulation of autophagy, as evidenced by stabilization of p62, in accord with our previous findings (23). Interestingly, expression of survivin, an inhibitor of apoptosis in TSC2-null cells, was found to correlate with resistance to chemotherapeutic agents (36). We found that survivin expression is down-regulated by the combination treatment, an important consequence for TSC2-null/LAM cells because rapamycin treatment alone does not suppress survivin expression and could be one of the mechanisms for therapy resistance. In addition, combination treatment significantly induced apoptosis in cell culture, as measured by the increase in cleavage of apoptotic markers caspase-3 and PARP. Rapamycin and resveratrol also synergized in inhibiting cell motility, in accord with reduced lung colonization in vivo we have previously observed (22).
Because LAM is caused by the loss of TSC2, we examined the effectiveness of this combination therapy in the TSC2-null mouse xenograft model. Although resveratrol treatment alone had no effect on the TSC2-deficient xenograft tumor size grown in vivo, the combination of rapamycin and resveratrol had a statistically significant effect on reducing the tumor size by Week 2 of treatment as compared with rapamycin treatment alone, and this effect was maintained throughout the treatment. Additionally, analysis of tumor progression revealed that although both single-agent rapamycin and the combination therapy reduced xenograft tumor growth after 3 weeks of treatment, combination therapy was able to suppress tumor growth, whereas rapamycin delayed tumor growth, as seen by the increase in photon flux from Week 3 to Week 4 of treatment. Signaling data from treated tumor tissue consistently depicted strong suppression of Akt/mTORC1 signaling but had no effect on MAPK signaling. Combination therapy also significantly increased apoptosis in the TSC2-deficient xenograft tumors and inhibited proliferation. Although both single treatments and the combination treatment reduced the proliferative index, only combination therapy induced apoptosis. This further supports the model whereby the synergistic interaction of rapamycin and resveratrol results in reduction of xenograft tumors. Our findings are very exciting because rapamycin and resveratrol combination therapy could benefit patients with LAM not only by arresting tumor growth but also by eliminating lesions, possibly through induction of apoptosis.
Combination therapy with rapamycin and an autophagy inhibitor is an attractive strategy for the treatment of LAM because it may result in better long-term clinical outcomes than rapamycin alone. Based on our previous in vitro work, resveratrol was able to suppress autophagy in TSC2-deficient cells even in the presence of rapamycin. Based on the current in vivo study, rapamycin administered in combination with resveratrol is a potent inhibitor of TSC2-deficient lesion growth partly via induction of apoptosis. Based on these data, resveratrol is a good candidate for a rapamycin cotherapy in patients with LAM.
Acknowledgments
Acknowledgments
The authors thank Dr. Vera Krymskaya (University of Pennsylvania) for generously providing the LAM-derived cells.
Footnotes
This work was supported by National Institutes of Health grants CA151112 (M.K.H.) and HL098216 (J.J.Y.), by the National Cancer Center (A.A.), by the Atol Charitable Trust, by grant 098P0113 from the LAM Foundation, and by grant RSG-13–287–01-TBE from the American Cancer Society.
Author Contributions: A.A. and R.S.S. performed the study and wrote the manuscript. N.S.S. analyzed tumor samples. Y.S., C.L., and J.J.Y. executed xenograft experiments. M.K.H. designed the study and wrote the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0022OC on April 6, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Henske EP, McCormack FX. Lymphangioleiomyomatosis: a wolf in sheep's clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammes SR, Krymskaya VP. Targeted approaches toward understanding and treating pulmonary lymphangioleiomyomatosis (LAM) Horm Cancer. 2013;4:70–77. doi: 10.1007/s12672-012-0128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 4.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13:276–285. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 5.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: a role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM) J Biol Chem. 2002;277:30958–30967. doi: 10.1074/jbc.M202678200. [DOI] [PubMed] [Google Scholar]
- 6.Goncharova EA, Goncharov DA, Spaits M, Noonan DJ, Talovskaya E, Eszterhas A, Krymskaya VP. Abnormal growth of smooth muscle-like cells in lymphangioleiomyomatosis: role for tumor suppressor TSC2. Am J Respir Cell Mol Biol. 2006;34:561–572. doi: 10.1165/rcmb.2005-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 9.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 10.Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic: I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 11.Alayev A, Holz MK. mTOR signaling for biological control and cancer. J Cell Physiol. 2013;228:1658–1664. doi: 10.1002/jcp.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 13.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 14.Tee AR, Anjum R, Blenis J. Inactivation of the tuberous sclerosis complex-1 and -2 gene products occurs by phosphoinositide 3-kinase/Akt-dependent and -independent phosphorylation of tuberin. J Biol Chem. 2003;278:37288–37296. doi: 10.1074/jbc.M303257200. [DOI] [PubMed] [Google Scholar]
- 15.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/Akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 19.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkhitko A, Myachina F, Morrison TA, Hindi KM, Auricchio N, Karbowniczek M, Wu JJ, Finkel T, Kwiatkowski DJ, Yu JJ, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc Natl Acad Sci USA. 2011;108:12455–12460. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alayev A, Berger SM, Kramer MY, Schwartz NS, Holz MK. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J Cell Biochem. 2015;116:450–457. doi: 10.1002/jcb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alayev A, Sun Y, Snyder RB, Berger SM, Yu JJ, Holz MK. Resveratrol prevents rapamycin-induced upregulation of autophagy and selectively induces apoptosis in TSC2-deficient cells. Cell Cycle. 2014;13:371–382. doi: 10.4161/cc.27355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe SR, Gottardis MM, Everitt JI, Goldsworthy TL, Wolf DC, Walker C. Rodent model of reproductive tract leiomyomata: establishment and characterization of tumor-derived cell lines. Am J Pathol. 1995;146:1568–1579. [PMC free article] [PubMed] [Google Scholar]
- 25.Howe SR, Gottardis MM, Everitt JI, Walker C. Estrogen stimulation and tamoxifen inhibition of leiomyoma cell growth in vitro and in vivo. Endocrinology. 1995;136:4996–5003. doi: 10.1210/endo.136.11.7588234. [DOI] [PubMed] [Google Scholar]
- 26.Yu JJ, Robb VA, Morrison TA, Ariazi EA, Karbowniczek M, Astrinidis A, Wang C, Hernandez-Cuebas L, Seeholzer LF, Nicolas E, et al. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci USA. 2009;106:2635–2640. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 1996;98:2277–2283. doi: 10.1172/JCI119038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igura K, Ohta T, Kuroda Y, Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001;171:11–16. doi: 10.1016/s0304-3835(01)00443-8. [DOI] [PubMed] [Google Scholar]
- 29.Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holz MK. The role of S6K1 in ER-positive breast cancer. Cell Cycle. 2012;11:3159–3165. doi: 10.4161/cc.21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alayev A, Doubleday PF, Berger SM, Ballif BA, Holz MK. Phosphoproteomics reveals resveratrol-dependent inhibition of Akt/mTORC1/S6K1 signaling. J Proteome Res. 2014;13:5734–5742. doi: 10.1021/pr500714a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies DM, de Vries PJ, Johnson SR, McCartney DL, Cox JA, Serra AL, Watson PC, Howe CJ, Doyle T, Pointon K, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res. 2011;17:4071–4081. doi: 10.1158/1078-0432.CCR-11-0445. [DOI] [PubMed] [Google Scholar]
- 33.Taveira-DaSilva AM, Hathaway O, Stylianou M, Moss J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann Intern Med. 2011;154:797–805. doi: 10.1059/0003-4819-154-12-201106210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ, Jr, Miles D, Cutler D, Krueger D, Uppot RN, Rabenou R, et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF- D levels decrease. PLoS One. 2011;6:e23379. doi: 10.1371/journal.pone.0023379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Chemaly S, Henske EP. Towards personalised therapy for lymphangioleiomyomatosis: lessons from cancer. Eur Respir Rev. 2014;23:30–35. doi: 10.1183/09059180.00008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carelli S, Lesma E, Paratore S, Grande V, Zadra G, Bosari S, Di Giulio AM, Gorio A. Survivin expression in tuberous sclerosis complex cells. Mol Med. 2007;13:166–177. doi: 10.2119/2006-00091.Carelli. [DOI] [PMC free article] [PubMed] [Google Scholar]