Abstract
ATP-binding cassette transporter subfamily G member 2 (Abcg2)-expressing cardiac-side population cells have been identified in the developing and adult heart, although the role they play in mammalian heart growth and regeneration remains unclear. In this study, we use genetic lineage tracing to follow the cell fate of Abcg2-expressing cells in the embryonic and adult heart. During cardiac embryogenesis, the Abcg2 lineage gives rise to multiple cardiovascular cell types, including cardiomyocytes, endothelial cells, and vascular smooth muscle cells. This capacity for Abcg2-expressing cells to contribute to cardiomyocytes decreases rapidly during the postnatal period. We further tested the role of the Abcg2 lineage following myocardial injury. One month following ischemia reperfusion injury, Abcg2-expressing cells contributed significantly to the endothelial cell lineage, however, there was no contribution to regenerated cardiomyocytes. Furthermore, consistent with previous results showing that Abcg2 plays an important cytoprotective role during oxidative stress, we show an increase in Abcg2 labeling of the vasculature, a decrease in the scar area, and a moderate improvement in cardiac function following myocardial injury. We have uncovered a difference in the capacity of Abcg2-expressing cells to generate the cardiovascular lineages during embryogenesis, postnatal growth, and cardiac regeneration.
Introduction
ATP-binding cassette transporter subfamily G member 2 (Abcg2), a member of the ABC (ATP-binding cassette) transporter family, is expressed by stem cells isolated from a wide range of tissues, including the heart. Abcg2 is an energy-dependent transporter located in the plasma membrane and is capable of effluxing a variety of substances, including cytotoxic drugs [1]. Side population (SP) cells, defined by their ability to efflux the DNA-binding dye Hoechst 33342, rely principally on Abcg2 for the efflux of Hoechst 33342 dye [2]. SP cells have been shown to possess stem cell activity in numerous tissues, including bone marrow and the heart [2,3]. When isolated by flow cytometry and cultured with the proper stimulation, cardiac SP cells have the ability to differentiate into all cardiac cell lineages, including cardiomyocytes, smooth muscle cells, and endothelial cells [3–7]. When delivered to animals following cardiac injury, isolated cardiac SP cells can migrate to the area of injury and differentiate into the cardiac lineages [6,7].
In the adult heart, Abcg2 expression is predominantly localized to the interstitial spaces located between muscle fibers. The majority of Abcg2-positive cells express microvasculature markers, including coexpression with endothelial cell markers [8–10]. Following myocardial injury, cardiac expression of Abcg2 increases [11]. In addition to its well-described expression in adult tissue-specific stem cell populations, Abcg2 is expressed throughout embryonic development [12]. At embryonic day (e)8.5 in mouse development, Abcg2 is expressed in the yolk sac and the heart tube. Expression in the heart decreases significantly between e11.5 and e13.5 [3]. Although Abcg2 is widely expressed in the developing embryo, its role during embryonic development has not been previously investigated.
Recent genetic lineage-tracing studies have expanded the stem cell characterization of Abcg2 by allowing for cell fate analysis of Abcg2-expressing cells and their progeny [13]. Using this lineage-tracing technique, Fatima et al. demonstrated that Abcg2 labels tissue-specific progenitor cell populations in multiple tissues, including marking of hematopoietic stem cells, intestinal stem cells, and interstitial cells in cardiac and skeletal muscle [13]. Labeled cells in cardiac and skeletal muscle are predominantly vascular and coexpress the endothelial gene CD31 [13–15]. Upon introduction of systemic oxidative stress, the contribution of the Abcg2 lineage to vascular cells increased [15]. Following either chemical injury in skeletal muscle or response to cardiac oxidative stress, Abcg2-labeled cells contribute to microvascular cells but contribute only minimally to regenerated skeletal muscle fibers and do not contribute to cardiomyocytes [14,15]. Furthermore, when cardiac injury is performed on Abcg2-null mice, they exhibit decreased cardiac function resulting in increased mortality contributing to impaired angiogenesis and antioxidant responses [9,10]. Despite the importance of Abcg2 in cardiac regeneration, the fate of Abcg2-expressing cells following cardiac injury has not been assessed.
We have undertaken studies using an inducible lineage-tracing technique to further investigate the cell fate of cardiac Abcg2-expressing cells in two settings. First, we examined the cell fate of Abcg2-labeled cells during cardiovascular development. We show significant Abcg2 labeling of cardiomyocytes throughout the developing heart, as well as labeling of endothelial cells and smooth muscle cells. The ability of Abcg2-labeled cells to contribute to cardiomyocytes decreases in the early postnatal period. Second, we analyzed the Abcg2 lineage contribution in the adult heart following ischemia reperfusion (IR) injury. In combination with preconditioning using the oxidative stress agent paraquat dichloride (PQ), we show that the Abcg2 lineage contributes to endothelial cells but not to newly formed cardiomyocytes following IR injury.
For the first time, we have analyzed the cardiac role of Abcg2 during embryonic development and we observed a striking difference in the cell lineages that Abcg2 contributes to in the embryonic and adult heart. Abcg2-expressing cells contribute to multiple cardiovascular lineages during embryonic heart development and also primarily to vascular cells in the adult heart.
Materials and Methods
Experimental animals
All experiments were performed in accordance with the Guide for the Care and Use Laboratory Animals, and the experimental protocol was submitted and approved by the University of Minnesota Institutional Animal Care and Use Committee. All studies used adult mice aged 2–6 months. Abcg2:CreERT2 mice were obtained from Dr. B.P.S. (St. Jude Children's Research Hospital Memphis, TN). C57Bl/6J wild-type (000664) and RosaCAGZsGreen (007906) mice were purchased from The Jackson Laboratories.
Tamoxifen administration
Tamoxifen was administered by intraperitoneal injection. For the determination of embryonic ages, noon on the day of the postcoital plug was considered e0.5. For the cell fate analysis of the embryonic heart, timed pregnant females (RosaCAGZsGreen heterozygous mated to Abcg2:CreERT2 heterozygous males) received one to three doses of 60 mg/kg body weight tamoxifen (Sigma) dissolved in corn oil beginning at embryonic day 7.5. To reduce the abortion rate associated with tamoxifen, 30 mg/kg body weight progesterone (Sigma) was included for intraperitoneal injections as previously described [16]. For cell fate analysis in the postnatal heart, postnatal day 1 to day 21 pups received a single dose of 1 mg tamoxifen subcutaneously. Animals were sacrificed at 8 weeks of age to determine the Abcg2-labeled cells in the heart. For adult heart injury studies, each mouse received 60 mg/kg body weight of tamoxifen (Sigma) dissolved in corn oil or vehicle control (corn oil) for 12 consecutive days, 24 h apart.
Immunofluorescence staining
Adult hearts and embryos (various ages) were harvested, fixed with 4% paraformaldehyde, cryoembedded, and processed for tissue staining. Ten-microgram sections were stained overnight at 4°C with the following primary antibodies: desmin (1:500; Novus Biologicals), CD31 (1:500; R&D Systems), c-kit (1:200; R&D Systems), dystrophin (1:500; Abcam), endomucin (1:500; Abcam), Sca-1 (Ly6a, 1:200; BD Biosciences), Sox10 (1:500; Santa Cruz), α-smooth muscle actin (α-SMA: 1:500; Sigma, clone 1A4), Wt1 (1:200; Abcam), and OSF2 (periostin, 1:500; BioVendor). Secondary staining was performed for 1 h at room temperature using the appropriate AF555- or AF594-conjugated secondary antibody (Life Technologies). Images were taken using a Zeiss LSM 510 Meta Confocal Microscope.
Cell expression quantification
All immunohistochemical images were quantified using the Image Pro Plus 6.0 or the FIJI Software analysis. For all analyses following IR injury, five random fields of view were chosen per mouse from at least three mice per group. For postnatal Abcg2 lineage analysis, three whole heart sections were analyzed from at least three mice per group. Dystrophin membrane staining was used to identify and count the total number of cardiomyocytes, and then the number of ZsGreen-positive cardiomyocytes was counted. The total number of Abcg2 lineage-positive cells was determined by counting ZsGreen-positive cells and normalized to section area.
Myocardial injury
Following tamoxifen treatment, Abcg2:CreERT2, RosaCAGZsGreen double heterozygous mice then received two doses of 20 mg/kg PQ [Sigma; dissolved in sterile 1× phosphate-buffered saline (PBS)] or vehicle control (sterile 1× PBS) 1 week apart beginning 2 weeks before cardiac injury. Sex-matched mice aged 3–4 months underwent temporary ligation of the left anterior coronary artery. Briefly, mice were anesthetized with 1.5% isofluorane and intubated to the level of the carina, placed on a heating pad, and put on a ventilator to aid respiration. Thoracotomy was performed, the heart exposed, and the proximal left anterior coronary artery was ligated below the atrial appendage using a 9-0 silk suture for 30 min. Ligature was then released, and blood flow was confirmed by visualization. Thoracic wall was approximated using 3-0 silk sutures and extubation was achieved. Mice were kept on a heating pad and observed until fully recovered.
Echocardiography
Echocardiography was obtained under 2% isoflurane anesthesia using a Vevo 770 (VisualSonics) high-frequency ultrasound biomicroscope using a 30-Mhz probe operating at a range of 100–120 frames per second. Echocardiography was performed at baseline, 1 week, and 1 month following myocardial injury. Ejection fraction (EF%) and shortening fraction (FS%) values were calculated from M- and B-mode images of the left ventricle in standard long- and short-axis views, respectively. EF and FS were calculated using the following formulas:
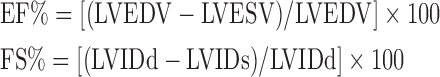 |
Scar size assessment
Hearts were perfused with cold PBS and cut transversely into three 3-mm rings (base, middle, and apex). Ten-microgram sections at the landmark level of the papillary muscles were stained with Sirius Red–Fast Green. Mosaic images were taken on a Zeiss AxioObserver Z1 Inverted Microscope with the Zeiss AxioVision software and scar size, indicated by red-stained collagen, was calculated as a percent of the entire left ventricle using the ImageJ software.
Statistical analysis
All results are mean ± SEM. Significance was established by two-tailed Student's t-test, P < 0.05.
Results
Cell fate of Abcg2-positive progenitor cells in the embryonic heart
Abcg2 is expressed by stem cells in the adult heart and it is expressed during heart development. To explore the role of Abcg2 during cardiac development, we used the previously described Abcg2:CreERT2 mouse [13] crossed to RosaCAGZsGreen reporter mouse. In double transgenic animals, the reporter allele is expressed following inducible activation of Cre recombinase resulting in reporter expression in Abcg2-positive cells and their progeny allowing us to track the cell fate of Abcg2-expressing cells throughout heart development. We initially delivered a single dose of tamoxifen to timed pregnant females at e8.5 and analyzed the reporter expression 2 days later at e10.5. A small number of Abcg2-labeled cells were present in the heart and they coexpress the muscle interfilament protein desmin (Supplementary Fig. S1A; Supplementary Data are available online at www.liebertpub.com/scd).
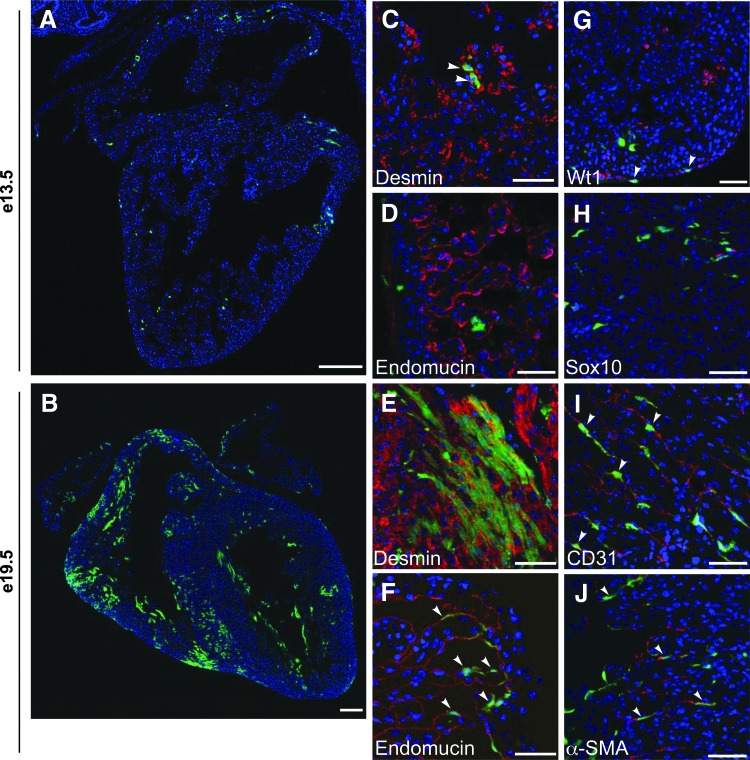
To maximize the number of labeled cells, we administered multiple doses of tamoxifen 24 h apart to timed pregnant females beginning at e7.5 for analysis at later embryonic and fetal time points. We found that for embryos to survive to fetal time points, we could deliver a maximum of two tamoxifen doses to pregnant females, even with the addition of progesterone. In double transgenic embryos at e13.5, we saw scattered endogenous ZsGreen-positive cells throughout the myocardium and epicardium in both the atria and ventricles (Fig. 1A). At e19.5, there was extensive ZsGreen expression throughout the ventricular myocardium and in the atrium (Fig. 1B). We continued to see ZsGreen-positive cells in the epicardium and we now also saw ZsGreen-positive cells in the endocardium. No reporter expression was observed with the delivery of tamoxifen in embryos lacking either the Abcg2:CreERT2 or RosaCAGZsGreen allele, and it was also not observed with the vehicle control delivered heterozygous to embryos, indicating low recombination leakiness (data not shown).
FIG. 1.
The Abcg2 lineage contributes to multiple cardiovascular cell types during embryonic heart development. Embryos were analyzed at e13.5 (A, C, D, G, H) or e19.5 (B, E, F, I, J) following induction with tamoxifen at e7.5–e9.5 or e7.5–e8.5, respectively. Abcg2 lineage-labeled cells (ZsGreen-positive) are green, lineage markers are red, and nuclei are labeled with DAPI (blue) in all images. ZsGreen is coexpressed in desmin-positive cardiomyocytes (C, E) and in Wt1 (G), endomucin (F), CD31 (I), α-actin, smooth muscle isoform (α-SMA) (J) positive cells. ZsGreen is not coexpressed with endomucin (D) or Sox10 (H) at e13.5. Arrowheads indicate cells that coexpress ZsGreen with the appropriate lineage marker. Scale bars = 200 μm (A, B) and 50 μm (C–J). Abcg2, ATP-binding cassette transporter subfamily G member 2.
At both e13.5 and e19.5, some ZsGreen-positive cells resemble adjacent cardiomyocytes in size and shape and coexpress the muscle protein desmin (Fig. 1C, E, Supplementary Figs. S2A and S3A). ZsGreen-positive cells did not coexpress endomucin at e13.5 (Fig. 1D and Supplementary Fig. S2B), however, by e19.5 there was coexpression of ZsGreen and endomucin in the endocardial cell layer (Fig. 1F and Supplementary Fig. S3B). Together these observations indicate that the Abcg2-positive progenitor cells contribute to cardiomyocytes early in heart development and subsequently contribute to endocardial cells as well. To confirm the presence of reporter-positive cells in the epicardium, we assessed the coexpression of ZsGreen with the epicardial cell marker Wt1 (Wilms' Tumor 1) [17] and observed coexpression of Wt1 in some ZsGreen-positive cells (Fig. 1G and Supplementary Fig. S2C).
Additionally, because a subset of cardiac SP cells express neural precursor markers and cardiac SP cells may derive from neural crest cells in the heart [18], we evaluated the coexpression of ZsGreen with the neural crest marker Sox10. Although we observed Sox10-positive and ZsGreen-positive cells in the outflow tract (OFT) region, we did not see any cells that coexpressed ZsGreen and Sox10 (Fig. 1H and Supplementary Fig. S2D), suggesting that it is unlikely Abcg2-labeled cells in the heart are derived from neural crest cells.
In addition to labeling cardiomyocytes, we also saw a high expression of ZsGreen in interstitial cells located between the cardiomyocytes, so we looked at coexpression with interstitial cell population markers. At e19.5, we saw considerable coexpression of ZsGreen with the endothelial cell marker CD31 (Fig. 1I and Supplementary Fig. S3C) in both small and large blood vessels and some coexpression with the smooth muscle isoform of α-actin (α-SMA) (Fig. 1J and Supplementary Fig. S3D). We did not observe ZsGreen coexpression with periostin (OSF2)-expressing cardiac fibroblasts [19] (Supplementary Fig. S3E). Therefore, during embryonic heart development, the Abcg2 lineage contributes to multiple cardiovascular lineages, including cardiomyocytes, endothelial cells, and vascular smooth muscle cells (VSMCs).
The ability of Abcg2-labeled cells to contribute to cardiomyocytes decreases rapidly postnatally
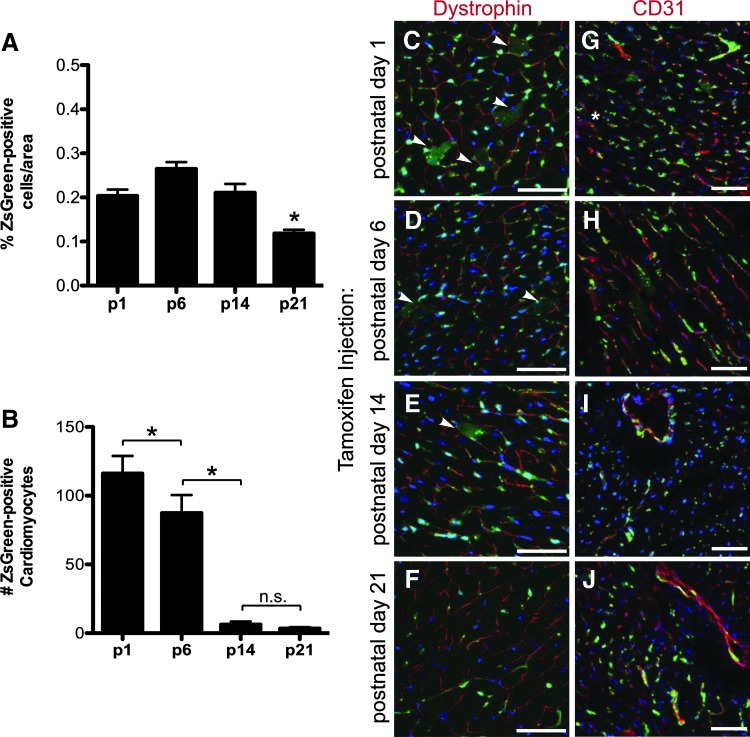
Because we observed Abcg2 lineage contribution to cardiomyocytes during heart development, we evaluated the potential of Abcg2-labeled progenitor cells to contribute to cardiomyocytes postnatally. To do so, we delivered a single dose of tamoxifen to postnatal pups at four time points, postnatal day 1 (p1), day 6 (p6), day 14 (p14), and day 21 (p21) and analyzed the contribution of Abcg2-derived cells in the heart at 8 weeks of age. When tamoxifen is delivered at p1, p6, or p14, a similar number of ZsGreen-positive cells were detected throughout the heart. In animals treated with tamoxifen at p21, we observed approximately a 50% decrease in the total number of ZsGreen-positive cells (Fig. 2A; P < 0.05). We assessed the coexpression of ZsGreen with dystrophin-positive cardiomyocytes. Interestingly, we observed ∼116 ± 33.7 Abcg2-labeled cardiomyocytes in a whole heart cross section when lineage labeling is done at p1 (Fig. 2B, C; n = 3). This represented about 1% of total cardiomyocytes. When tamoxifen was delivered at p6, the number of ZsGreen-positive cardiomyocytes decreased significantly to 87 ± 29.2 (Fig. 2B, D; P < 0.05, n = 3). The ability of Abcg2-labeled cells to contribute to cardiomyocytes continued to decrease significantly and they were on average 6 ± 5.8 and 3 ± 2.5 ZsGreen-positive cardiomyocytes when tamoxifen was delivered at p14 and p21, respectively (Figs. 2B, E, F; P < 0.05, n = 3). The observed decrease in Abcg2-derived cardiomyocytes was greater than the change in the expression of total ZsGreen-positive cells throughout the studied postnatal period, thus supporting the conclusion that the ability of the Abcg2 lineage to contribute to cardiomyocytes decreases with age.
FIG. 2.
The ability of Abcg2-derived cells to generate cardiomyocytes diminishes between postnatal day 6 and 14. The total number of ZsGreen-positive cells in adult heart sections (8 weeks old) were scored and normalized to section area: percent ZsGreen-positive cells/area for p1 is 0.20, p6 is 0.26, p14 is 0.21, and p21 is 0.12, n = 3, for each time point. *P < 0.05 (A). ZsGreen-positive cells were further analyzed for coexpression with dystrophin (cardiomyocytes, C–F) or CD31 (endothelial cells, G–J) following a single dose of tamoxifen at p1 (C, G), p6 (D, H), p14 (E, I), or p21 (F, J). Arrowheads indicate ZsGreen- and dystrophin-double positive cardiomyocytes. The number of dystrophin-positive cardiomyocytes that coexpress ZsGreen when tamoxifen was administered at p1 is 116 ± 33.7, at p6 is 87 ± 29.2, at p14 is 6 ± 5.8, and at p21 is 3 ± 2.5, n = 3 for each time point. *P < 0.05 (B). Extensive coexpression of ZsGreen with the endothelial cell marker CD31 (red) is observed in adult heart tissue (8 weeks old) when tamoxifen is given at all time points (p1 to p21, G–J). Nuclei labeled with DAPI (blue) in all images. Scale bars = 50 μm.
We also assessed the ability of the Abcg2 lineage to contribute to endothelial cells postnatally and observed a significant expression of ZsGreen in cells expressing CD31 beginning with postnatal day 1 tamoxifen delivery (Fig. 2G–J). In contrast to our findings with the cardiac lineage, we did not observe a difference in Abcg2 lineage-labeled endothelial cells when tamoxifen was delivered at different time points. This observation is consistent with prior observations that the Abcg2 lineage contributes significantly to endothelial cells [15].
Abcg2 labeled cells express the stem cell markers, Sca1, and c-kit
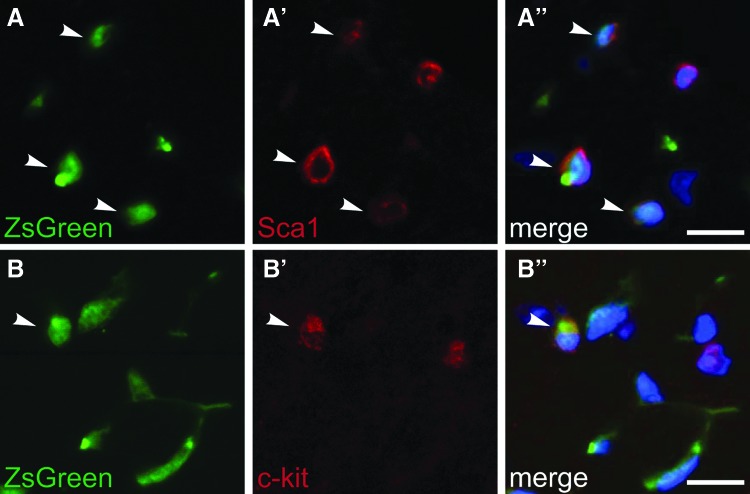
Cardiac SP cells are heterogenous and express the cell surface markers Sca1 and c-kit in addition to expressing Abcg2 [3–5]. We assessed the coexpression of these stem cell markers within the Abcg2 lineage-labeled cells in the adult heart following the induction of Cre recombination at postnatal day 1. ZsGreen-positive cells coexpress Sca1 (Fig. 3A) or c-kit (Fig. 3B) throughout the adult heart, indicating that some of the Abcg2 lineage-marked cells likely retain stem cell characteristics.
FIG. 3.
Abcg2-labeled cells coexpress the stem cell markers Sca1 and c-kit. ZsGreen-positive cells in adult (8 week old) heart sections were analyzed for coexpression of the stem cell markers Sca1 and c-kit following tamoxifen delivery at p1. ZsGreen-positive (A, B) cells coexpressing Sca1 (red, A′) and c-kit (red, B′) are indicated with arrowheads. Merged images shown with DAPI (blue, A″ and B″). Scale bars = 10 μm.
Abcg2-labeled cells contribute to endothelial cells but not to cardiomyocytes following ischemia-reperfusion injury
To evaluate the ability of Abcg2-labeled cells to contribute to the cardiovascular lineages following a significant cardiac injury, we performed an IR injury. We have previously shown that Abcg2 plays a protective role in response to oxidative stress in vivo, and therefore mice were pretreated with PQ, a systemic oxidative stress agent. For injury analysis, Abcg2:CreERT2, RosaCAGZsGreen adult mice were placed in two groups 1 month before cardiac injury. Following 12 consecutive days of tamoxifen administration, one group received two doses of PQ 1 week apart, whereas the control group received two doses of PBS vehicle control. All mice were then subjected to a temporary ligation of the left anterior descending coronary artery for a duration of 30 min and followed for 1-month post-IR injury. There was no difference in survival between treatment and control groups both before and after ligation (data not shown). In our prior study, using the same tamoxifen and PQ delivery strategy, we observed no cardiac damage in the absence of injury [15].
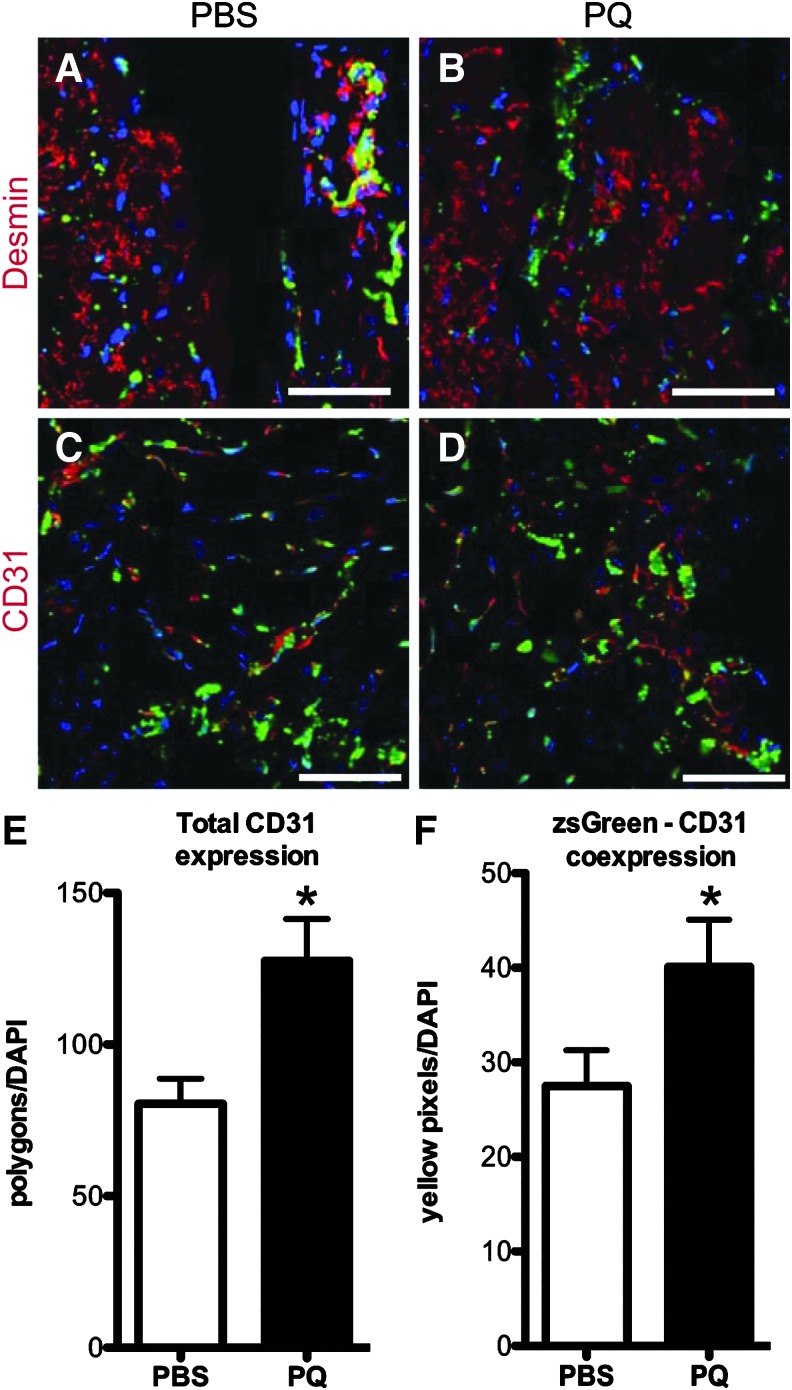
We assessed the expression of ZsGreen-positive cells with cardiac and endothelial cell markers 1 month following IR injury in PBS- and PQ-treated animals. We observed no ZsGreen-positive cells coexpressing desmin in any region of the heart in PBS- or PQ-treated groups, indicating an absence of Abcg2 lineage-positive cardiomyocytes (Fig. 4A, B). We analyzed the Abcg2 lineage contribution to microvasculature following IR injury by staining with CD31 in the peri-scar region. Overall, capillary density was significantly increased in the border zone in PQ-treated mice compared to PBS-treated mice (Fig. 4C–E; total area CD31 normalized to nuclei per field 127.6 ± 13.8 PQ-treated compared to 80.5 ± 8.3p PBS-treated, P < 0.05; n = 6). Furthermore, the coexpression of ZsGreen with CD31 in the peri-scar area was increased in PQ-treated mice following IR injury compared to PBS-treated mice (Fig. 4C, D, F; 40.1 ± 5 merged pixels normalized to nuclei per field PQ vs. 27.5 ± 3.8 PBS, P < 0.05; n = 6), indicating an increase in the number of Abcg2 lineage-positive endothelial cells. This observation is consistent with our previous finding that exposure to the oxidative stress agent PQ leads to an increase in Abcg2 lineage-labeled vascular cells in the heart [15].
FIG. 4.
Oxidative stress pretreatment increases vascular remodeling following ischemia-reperfusion injury. Images show heart sections from PBS-treated (A, C) or PQ-treated (B, D) mice 1 month following IR injury stained for desmin (red, A, B) or CD31 (red, C, D) and imaged with Abcg2 lineage label (green). Desmin-positive cardiomyocytes are negative for ZsGreen (A, B), whereas CD31-positive endothelial cells are positive for ZsGreen (C, D) in both PBS- and PQ-treated mice. Nuclei are labeled with DAPI (blue) for all images. Capillary density (scored by total CD31-positive area normalized to nuclei per field) in the peri-scar region is increased following IR injury in PQ-pretreated mice (127.6 ± 13.8) compared to control (PBS-treated; 80.5 ± 8.3) mice, *P < 0.05 (E). The number of CD31-positive cells coexpressing ZsGreen (Abcg2-labeled) was scored by the number of merged pixels normalized to nuclei per field and is increased in the peri-scar region in PQ-treated mice (40.1 ± 5) versus PBS-treated mice (27.5 ± 3.8), *P < 0.05 (F). n = 6 mice per group for quantitative analysis. Scale bar = 50 μm. IR, ischemia reperfusion; PQ, Paraquat dichloride; PBS, phosphate-buffered saline.
To determine the physiological effects of the increase in the Abcg2-labeled vasculature following pretreatment with PQ and IR injury, we assessed cardiac function 1 month after IR injury. Using echocardiographic analysis of the left ventricle to assess cardiac performance, we observed no significant difference in EF before injury (Supplementary Fig. S4A). One week after IR injury, EF between groups was roughly equivalent and at 1 month EF of the PQ-treated group was greater compared to control but not significantly different (Supplementary Fig. S4A). The EF in the control group continued to decrease significantly between 1 week and 1 month postinjury in contrast to the PQ-treated group, which revealed no significant deterioration at 1 month postinjury. This supports that preconditioning with PQ was protective in preventing continued postinjury functional deterioration and adverse remodeling.
We also quantified the extent of scar tissue in heart sections at the level of the papillary muscle using the Sirius Red–Fast Green staining to identify fibrotic tissue. Mice preconditioned with PQ demonstrated a significant decrease in collagen volume fraction compared to control-treated mice (Supplementary Fig. S4B, C). In summary, the data obtained using the Abcg2 lineage-tracing model demonstrate that in the adult environment even following injury, the Abcg2 lineage primarily contributes to the cardiac vascular system.
Discussion
The function of Abcg2 beyond xenobiotic transport has remained largely unknown because its initial characterization as a multidrug resistant protein in cancer cells [20]. Employment of lineage tracing has allowed for insight into the fate commitment of Abcg2-expressing cells in the adult, thus providing a greater understanding of Abcg2 function as well as its veracity as a marker for adult stem cell populations [13]. In this study, we have undertaken cell fate analysis of Abcg2-expressing cells in multiple contexts using a tamoxifen-inducible Cre-lox mouse model (Abcg2:CreERT2): first, during embryonic heart development; second, during the early postnatal period, and last, following cardiac injury in adult mice. Given the low expression of Abcg2 in a rare cell population and the fact that Abcg2 is rapidly downregulated with cell differentiation, this inducible lineage-tracing model is advantageous to investigate the function of Abcg2 in the embryonic and adult heart. We observe a difference in the embryonic versus postnatal differentiation capacity of Abcg2-positive progenitor cells. During embryogenesis, the Abcg2 lineage contributes to cardiomyocytes as well as other cardiovascular cell types, including endothelial cells and VSMCs. In contrast, in adult mice, the Abcg2 lineage contributes to the cardiac vasculature but does not contribute to cardiomyocytes even following cardiac injury.
Although Abcg2 is widely expressed during development and cardiac SP cells have been identified in the developing heart [3], the developmental potential of Abcg2 or cardiac SP cells has not been previously explored. For the first time, we show that endogenous Abcg2-expressing cells have the potential to differentiate into cardiomyocytes. In addition to the generation of cardiomyocytes from Abcg2-expressing progenitor cells, it is clear that in vivo the Abcg2 lineage includes other cardiac cell types, including endothelial cells and VSMCs. The embryonic capacity of Abcg2-derived cells to differentiate into multiple cardiovascular lineages in vivo is consistent with the differentiation potential of cardiac SP cells in vitro [3–6]. The pattern of Abcg2-labeled cells observed during heart development is interesting. Initially, we see the appearance of single Abcg2-labeled cells at e13.5 followed by clusters of neighboring cardiomyocytes late in development (e19.5).
The potential for Abcg2-positive stem cells to differentiate into cardiomyocytes seems to be largely restricted to embryogenesis. Approximately, 1% of adult cardiomyocytes are labeled when Cre recombination is induced at p1. This is considerably less than when Abcg2-expressing cells are labeled during embryogenesis. The capacity of Abcg2-labeled cells to differentiate to cardiomyocytes decreases rapidly in neonatal mice. By p6, it is already ∼25% compared to p1 and is essentially completely extinguished by p21. Interestingly, this seems to correspond to the window of cardiomyocyte proliferation and regenerative capacity [21]. Prior studies have demonstrated that cardiac Abcg2 expression as well as the cardiac SP cell population decreases with age [3,6]. The postnatal decrease in the capacity of Abcg2-expressing cells to contribute to cardiomyocytes may be explained by the decrease in new cardiomyocyte generation during the neonatal period or by the decrease in cardiac SP cells.
The present study is the first time the potential of the endogenous Abcg2 lineage has been evaluated following cardiac injury. Surprisingly in response to IR, the Abcg2 lineage does not contribute to cardiomyocyte renewal. Previously, Oyama et al. demonstrated that isolated neonatal cardiac SP cells could migrate to the site of injury and were able to differentiate into cardiomyocytes, endothelial cells, smooth muscle cells, and fibroblasts in vivo when transplanted following cardiac injury [6]. Similarly, Liang et al. further subfractionated the cardiac SP population based on the expression of Sca1 and CD31 (Sca1+, CD31−) and demonstrated the ability of these cells to migrate to the site of injury and differentiate into cardiomyocytes and endothelial cells following cardiac injury [7]. There are several possibilities that could explain the differences between these results and our results. Both of these results depend on the isolation of cardiac SP cells and transplantation into recipient animals; the manipulation of isolated SP cells may alter their properties to favor cardiomyogenic differentiation. Second, Oyama et al. used cardiac SP cells isolated from neonatal rats. Consistently, our data suggest that the cardiomyocyte differentiation potential of the Abcg2 lineage decreases with age; thus, again highlighting the differing capacity of young and adult cardiac SP cells. The regenerative capacity of Abcg2-derived cells may parallel or even overlap with the ability of other endogenous stem cell populations (c-kit or Sca1 positive) to contribute to cardiomyocytes following injury. However, the regenerative capacity of these cells has been controversial and recent data suggest that it may also be limited [22]. Last, it is possible that the level of CreERT2 expression from the Abcg2 locus is below a sufficient threshold to induce recombination in all adult cardiac SP cells, which may leave a SP cell fraction unstudied in our current model.
Although the Abcg2 lineage-tracing studies did not show evidence of Abcg2-derived cardiomyocyte regeneration or proliferation postinjury, this does not eliminate an important physiological role for Abcg2-derived cells in cardiac remodeling. Our studies have previously established a cytoprotective role for Abcg2 in response to oxidative stress [11,15]. Other studies utilizing the Abcg2-null mice have shown decreased cardiac function and increased mortality in two different myocardial infarction models, which has been attributed in part to impaired survival of microvascular endothelial cells under oxidative stress [9,10]. Our current study confirmed that Abcg2 contributes to vascular remodeling following injury and plays an important cytoprotective role in response to oxidative stress.
In summary, utilizing an inducible lineage-tracing technique, we have described the cell fate of Abcg2-expressing cells in cardiac development and adulthood. We have shown that during embryogenesis, the Abcg2 lineage gives rise to multiple cardiovascular cell populations including cardiomyocytes, endothelial cells, and VSMCs. During the postnatal period, the cardiogenic capacity of the Abcg2-expressing cells diminishes, even in response to myocardial injury, however, the endothelial capacity remains. Preconditioning with an oxidative stress agent was confirmed to promote favorable remodeling postmyocardial injury. These data support the idea that the Abcg2-derived lineages play an important role in the cardiovascular development. In adulthood, the vascular contribution remains important in providing protective effects of ventricular remodeling postinjury.
Supplementary Material
Acknowledgments
We would like to thank Danielle Amundsen for assistance with cryosectioning of heart samples. This work was supported by Dr. Thomas Pengo in the University Imaging Centers at the University of Minnesota (http://uic.umn.edu). This work was supported by funding from the National Institutes of Health (K08 HL102157).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Krishnamurthy P. and Schuetz JD. (2006). Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol 46:381–410 [DOI] [PubMed] [Google Scholar]
- 2.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, and Sorrentino BP. (2001). The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7:1028–1034 [DOI] [PubMed] [Google Scholar]
- 3.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD. and Garry DJ. (2004). Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 265:262–275 [DOI] [PubMed] [Google Scholar]
- 4.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA. and Megeney LA. (2002). The post-natal heart contains a myocardial stem cell population. FEBS Lett 530:239–243 [DOI] [PubMed] [Google Scholar]
- 5.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS. and Liao R. (2005). CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97:52–61 [DOI] [PubMed] [Google Scholar]
- 6.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, et al. (2007). Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol 176:329–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang SX, Tan TY, Gaudry L. and Chong B. (2010). Differentiation and migration of Sca1+/CD31- cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol 138:40–49 [DOI] [PubMed] [Google Scholar]
- 8.Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, Fine GC, Mouquet F, Westerman K. and Liao R. (2008). Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res 103:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashikuni Y, Sainz J, Nakamura K, Takaoka M, Enomoto S, Iwata H, Sahara M, Tanaka K, Koibuchi N, et al. (2010). The ATP-binding cassette transporter BCRP1/ABCG2 plays a pivotal role in cardiac repair after myocardial infarction via modulation of microvascular endothelial cell survival and function. Arterioscler Thromb Vasc Biol 30:2128–2135 [DOI] [PubMed] [Google Scholar]
- 10.Higashikuni Y, Sainz J, Nakamura K, Takaoka M, Enomoto S, Iwata H, Tanaka K, Sahara M, Hirata Y, Nagai R. and Sata M. (2012). The ATP-binding cassette transporter ABCG2 protects against pressure overload-induced cardiac hypertrophy and heart failure by promoting angiogenesis and antioxidant response. Arterioscler Thromb Vasc Biol 32:654–661 [DOI] [PubMed] [Google Scholar]
- 11.Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A, Garcia JA, Szweda LI, Garry MG. and Garry DJ. (2008). Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circ Res 102:1075–1081 [DOI] [PubMed] [Google Scholar]
- 12.Sawicki WT, Kujawa M, Jankowska-Steifer E, Mystkowska ET, Hyc A. and Kowalewski C. (2006). Temporal/spatial expression and efflux activity of ABC transporter, P-glycoprotein/Abcb1 isoforms and Bcrp/Abcg2 during early murine development. Gene Expr Patterns 6:738–746 [DOI] [PubMed] [Google Scholar]
- 13.Fatima S, Zhou S. and Sorrentino BP. (2012). Abcg2 expression marks tissue-specific stem cells in multiple organs in a mouse progeny tracking model. Stem Cells 30:210–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle MJ, Zhou S, Tanaka KK, Pisconti A, Farina NH, Sorrentino BP. and Olwin BB. (2011). Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J Cell Biol 195:147–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher TJ, Ren Y, Li Q, Braunlin E, Garry MG, Sorrentino BP. and Martin CM. (2014). ATP-binding cassette transporter Abcg2 lineage contributes to the cardiac vasculature after oxidative stress. Am J Physiol Heart Circ Physiol 306:H1610–H1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura E, Nguyen MT. and Mackem S. (2006). Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn 235:2603–2612 [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR. and Pu WT. (2008). Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomita Y, Matsumura K, Wakamatsu Y, Matsuzaki Y, Shibuya I, Kawaguchi H, Ieda M, Kanakubo S, Shimazaki T, et al. (2005). Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol 170:1135–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snider P, Standley KN, Wang J, Azhar M, Doetschman T. and Conway SJ. (2009). Origin of cardiac fibroblasts and the role of periostin. Circ Res 105:934–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK. and Ross DD. (1998). A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 95:15665–15670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN. and Sadek HA. (2011). Transient regenerative potential of the neonatal mouse heart. Science 331:1078–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Berlo JH. and Molkentin JD. (2014). An emerging consensus on cardiac regeneration. Nat Med 20:1386–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.