Abstract
Background: In the United States, African American infants experience the highest mortality, and their mothers report the lowest breastfeeding rates. Science reports decreased infant mortality among breastfed infants and suggests that milk immune component (MIC) levels are associated with maternal stressors. Little is known about these relationships among African Americans; therefore the aim was to explore the relationships of African American mothers' stressors and MICs 1–14 days postdelivery.
Materials and Methods: Mothers meeting eligibility requirements were approached for consent 48–72 hours postdelivery of a healthy term infant and given instructions to collect milk (Days 3, 9, and 14) and saliva (Day 9), as well as complete three Perceived Stress Scale questionnaires (Days 3, 9, and 14) and a survey of pregnancy stressors experiences. Pearson correlations and linear regressions were performed to assess the relationships of maternal stressors with MICs.
Results: There was at least one statistically significant correlation of a maternal stressor with nine of the 10 MICs (effect sizes ranging from r = 0.22 to 0.38) on Days 3 and 9. Of all MICs, epidermal growth factor had the most associations with maternal stress indicators. No mediational relationship of cortisol with MICs was observed.
Conclusions: Many of the MIC changes observed could potentially impact the health of term and preterm infants. Further research is warranted.
Introduction
Scientific evidence supports that the immunogenic properties of human milk improves infant/maternal health outcomes worldwide. In a meta-analysis of breastfeeding outcomes in developed countries, 43 primary studies and 28 systematic reviews identified the associations between breastfeeding and improved infant outcomes.1 Infants who were breastfed for at least 3 months had significantly decreased odds of ear and respiratory infections, diarrhea, and sudden infant death syndrome during infancy, as well as decreased odds of diabetes, obesity, and leukemia through adolescence.1 Maternal benefits included the decreased incidence of obesity and diabetes mellitus as well as improved cardiovascular health that extended past menopause.2,3 The scientific evidence of the breastfeeding benefits is so strong that health leaders such as the World Health Organization recommend that breastfeeding is the optimal source of nutrition for infants even during emergent crises (war or natural disasters) or maternal human immunodeficiency virus infection.4 Little is known about the occurrence of specific immunological changes in breastmilk as a result of maternal stress. Exploring these relationships could lead to supportive interventions that improve maternal and infant health for vulnerable populations such as African American mothers and infants.
Historically, African American women have been at risk for health disparities due to limited access to health care; however, recent evidence suggests that chronic stress may also play a role in health disparities such as hypertension, diabetes, and adverse birth outcomes.5,6 In a systematic review, Beckie7 reported that race/ethnicity and socioeconomic status were associated with increased stress and health disparities. Chronic stress is associated with elevated serum cortisol levels, which can influence the immune response.8 Originally, the stress-immune theory proposed that elevated serum cortisol levels inhibited the production and function of immune cells.9 More current evidence suggests that cortisol selectively influences immune cell response, creating a shift in cytokine production and distribution.9 Cytokines help regulate the pro-inflammatory and anti-inflammatory immune responses.8 A shift in the cytokine response could increase susceptibility to infection and chronic immune-mediated diseases such as diabetes, obesity, and hypertension in pregnant and lactating women.10 Here in the United States, African American women have greater rates of obesity, diabetes, and hypertension than non-Hispanic white and Hispanic women.11,12
African American infants have the highest incidence of mortality during the first year of life, and African American mothers have the lowest breastfeeding initiation rates regardless of socioeconomic or educational background.13–16 Maternal stressors (physical, psychological, and environmental) may play a role in health disparities among African American mothers and infants.7
Physical and environmental stressors (advanced maternal age, increased parity, mode of delivery, smoking, diabetes, hypertension, infection, lower socioeconomic status, relationship status, and increased number of children under care) have been associated with negative birth outcomes and decreased breastfeeding duration/exclusivity.17–37 Self-perceived stress is the cognitive appraisal of physical and environmental stressors and has been associated with increased levels of prenatal and postnatal serum cortisol.38–41 Prolonged elevation of maternal serum cortisol levels can influence the infant immune system response and potentially the milk immune components (MICs).42 Groer et al.42,43 reported higher self-perceived stress was associated with higher serum cortisol (r = 0.25, p = 0.040) as well as greater levels of milk secretory immunoglobulin A (SIgA). Furthermore, greater levels of maternal serum cortisol were associated with significantly greater levels of milk SIgA (r = 0.26, p = 0.035) at 4–6 weeks postdelivery (n = 50). This suggests a potential mediation model of cortisol on the direct associations of self-perceived stress and milk SIgA. It is important to explore whether maternal stress (physical, psychological, and environmental) is related to changes in levels of MICs over time postdelivery to determine the full potential of the protective properties of breastmilk for infant health.
The purpose of this study was to describe (1) the associations of maternal stress with MICs and (2) the potential mediation of cortisol on the direct associations of maternal stress and MICs among African American mothers of healthy term infants. The study aims included the following:
1. Assess the associations of maternal stress with MICs from African American mothers of term infants during the first 14 days postdelivery.
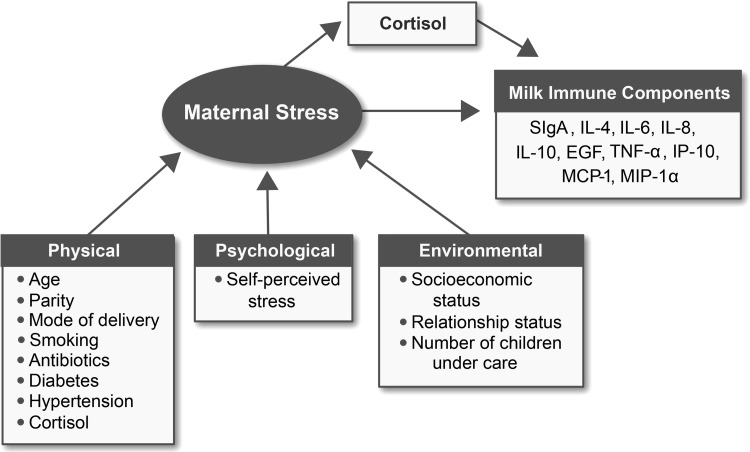
2. Assess the mediation (indirect) effect of maternal cortisol on the direct association of maternal stress and MIC from African American mothers of term infants. Figure 1 gives the proposed mechanism of cortisol mediation.
FIG. 1.
Proposed cortisol mediation pathway on the direct associations of maternal stress indicators with milk immune components. EGF, epidermal growth factor; IL, interleukin; IP-10, interferon-gamma-induced protein-10; MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1alpha; SIgA, secretory immunoglobulin A; TNF-α, tumor necrosis factor-alpha.
Materials and Methods
A descriptive longitudinal design was used to assess MIC levels in relation to maternal stress and salivary cortisol levels. The setting was a large urban southern hospital with 2,500 deliveries/year. The sample consisted of 139 African American women who delivered healthy term infants. A healthy term infant was defined as gestational age of ≥37 weeks, birth weight of >1,800 g, and a 5-minute Apgar score of ≥7. Sample size was calculated as a minimum of 75 participants to achieve statistical significance (80% statistical power, alpha = 0.050) for a 0.30 correlation (small), as well as associations with shared variability of at least 10%.44 Approval was obtained from the organizational Institutional Review Board.
Mothers received a study packet containing the survey instruments and all study collection materials/instructions for breastmilk and saliva collections. The specimen containers were prelabeled with the day/time of collection and study ID. The study staff instructed the mothers to collect approximately 1 teaspoon of hindmilk of the first feeding on Days 3, 9, and 14 postdelivery using hand expression or a breastmilk pump. Hindmilk collections were chosen to reduce the variability of MIC values related to changes in milk constituents from beginning to end of feeding.45–47
As a reminder, the study staff called each mother the evening before collection days to review collection/storage instructions and clarify any questions. The milk collection days were chosen to represent the three phases of milk production: colostrum (Days 0–3), transition milk (Days 4–10), and mature milk (>Day 11).
The study staff instructed the mothers to collect the saliva sample by placing the cotton sponge (Sarstedt®; Sarstedt AG & Co, Nümbrecht, Germany) into their cheek and gently rolling the sponge for approximately 2 minutes before spitting the sponge back into the collection container on the morning of Day 9 (upon awakening, 30 minutes after awaking, and 60 minutes after awaking). Additional instructions included no food or drink 30 minutes prior to collections, as well as no touching the sponge with fingers. As with the milk collections, the study staff called each mother the evening before to review collection/storage instructions and clarify any questions. The time frame for saliva collections was selected to represent the cortisol awakening response.
The study staff instructed the mothers to store all study samples in their home freezers and to transport the samples to the principal investigator using the supplied transport bag with ice-packs. A gift card of $25 was given to mothers who returned all biological samples and surveys. To be eligible for lab analysis, the study specimens had to remain frozen during transport. The Principal Investigator placed the frozen samples in the research setting biobank freezer (–80°C) until analysis.
The mothers completed a self-report survey of physical and environmental stressors, including maternal age, parity, mode of delivery, smoking status, infection (antibiotic usage), diabetes, hypertension, socioeconomic status (health payment status), relationship status, and number of children under the mother's care. The Principal Investigator collected infant demographics (gender, gestation, weight) from the electronic medical record.
The Perceived Stress Scale (PSS) was used to measure maternal self-perceived stress. The mothers completed the 14-item instrument with Likert-type response options ranging from 0 to 4 (where 0 = never and 4 = very often).48 Internal reliability of the 14-item PSS among pregnant women has an alpha range of 0.84–0.86, as well as construct validity in women postdelivery with the Tennessee Post-Partum Stress Scale (r = 0.62, p < 0.001), serum cortisol (r = 0.25, p = 0.040), and milk SIgA (r = 0.25, p = 0.006).42,43,49 A summed score was created by reversing the scores on the seven positive items (items 4–7, 9, 10, and 13) (e.g., 0 = 4, 1 = 3, 2 = 2, etc.) and then summing across all 14 items.48 The Cronbach's alpha assessments of internal reliability for the scores in this study were 0.88 (Day 3) and 0.89 (Days 9 and 14).
The salivary cortisol and milk SIgA levels were measured using enzyme-linked immunosorbent assays carried out according to the kit's instructions (ALPCO, Salem, NH). The previously frozen saliva samples were defrosted and centrifuged at 2,652 g for 10 minutes; the clear supernatant was used in the analysis. Results outside the quality control values were reported as not valid. Cortisol results were reported in ng/mL.
Milk samples were thawed and then centrifuged at 20,000 g for 10 minutes at 4°C, and then the fat layer was removed by scooping it off with a Corning (Corning, NY) small spoon and discarding it. The whey supernatant was removed and syringe-filtered using a Millipore low protein-binding Durapor® (polyvinylidene) filter (pore size, 0.45 μm; Merck Millipore, Cork, Ireland). The filtered whey was then placed in a labeled Eppendorf tube (Eppendorf AG, Hamburg, Germany) and frozen at −80°C until analysis. Milk SIgA was reported in g/L.
The milk cytokines, chemokines, and growth factors (CCGFs) (interleukin [IL]-4, IL-6, IL-8, IL-10, epidermal growth factor [EGF], tumor necrosis factor-alpha [TNF-alpha], interferon-gamma-induced protein-10 [IP-10], monocyte chemotactic protein-1 [MCP-1], and macrophage inflammatory protein-1-alpha [MIP-1alpha])] were measured in a Luminex (Austin, TX) MAGPIX® instrument with a Milliplex® MAP kit purchased from EMD Millipore Corp. (Billerica, MA). The kit contains fluorescent-coated magnetic beads (microspheres) with antibody capture that bind with the individual CCGF. The prepared plates were placed in the MAGPIX, where the microspheres were passed through a laser that excited a specific fluorescent dye on each microsphere. A high-speed digital processor identified each microsphere and quantified each CCGF assay. Control samples were analyzed for each run, and those values outside the controls were reported as not valid. Values were reported as pg/mL. When the value was below the limit of detection (LOD), half the value between 0 and the LOD was used. When the value was above the LOD, the high LOD value was used.
The demographic and stress indicator data from those enrolled who completed the study were compared with those who did not complete the study. Chi-squared tests of independence were performed to test for differences in the nominal data; Mann–Whitney tests were used for the non-normally distributed continuous data.
Descriptive statistics were used to summarize and assess the distributional characteristics of all study biological variables. The MICs except EGF and salivary cortisol values were positively skewed. Square root transformations resulted in suitable distributions for use in parametric statistical analyses. The validity of the observed changes in the levels of milk components over time was assessed using mixed-level general linear models. Pearson correlations were used to assess the associations of maternal stress indicators with MICs on each day of collection as well as with salivary cortisol on Day 9. Multiple linear regressions were used to assess the mediational effect of maternal cortisol on the association of maternal self-reports of maternal stress with MICs. Although an alpha level of 0.05 was used for statistical significance, associations were considered to be clinically meaningful if they indicated at least 10% variance shared (i.e., r [unadjusted] or beta [adjusted] = 0.33).
Results
Enrollment
There were 865 African American deliveries of term infants; 328 (32%) met eligibility requirements, and of those 139 mothers consented. Eighty-nine women (64%) returned study samples and survey materials. The attrition rate (36%) was attributed largely to cessation of breastfeeding by Day 9 or Day 14. Four women returned incomplete surveys, and the number of milk and saliva samples sufficient for lab analysis varied. Due to the nonrandom nature of the incomplete data, no values were imputed; therefore the data from 85 (61%) women were used for data analyses.
Descriptive summaries
The average age of the mother was 27.8 years. More than half (52%) of the infants were male. The average infant gestation was 39.3 weeks, and median infant birth weight was 3,245 grams (7.2 pounds). The majority of the mothers (65%) experienced vaginal delivery, 34% had no prior pregnancies, 34% received antibiotics, 7% reported hypertension and diabetes, and 3.5% reported smoking. Approximately 46% of the subjects were married, 53% cared for more than one child, and 64% reported Medicaid enrollment. The self-report of the number of times breastfed within the last 24 hours of each milk collection remained consistent over the study period (9 ± 3.8), indicating there was no active weaning process during study procedures. The PSS summed score median and interquartile range varied over time [Day 3, 16 (10, 21); Day 9, 19 (12, 25); Day 14, 18 (11, 26)], but these differences were not statistically significant. Maternal cortisol values represented the cortisol awakening response, with the peak value being 30 minutes after awakening (Table 1). With the exception of IL-4, IP-10, MCP-1, and EGF, statistically significant changes in MIC levels were observed from Day 3 to Day 14 (p < 0.05). All of those changes were decreasing levels except for EGF levels, which increased (Table 2).
Table 1.
Summary of Cortisol Values
| Inadequate data (n = 9) | Study sample (n = 73) | p value | |
|---|---|---|---|
| Salivary cortisol (ng/mL) | |||
| Awake | 11 (7.7, 38.2) | 19 (11.4, 25.0) | 0.598 |
| 30 minutes | 16 (5.3, 35.0) | 21 (12.3, 30.0) | 0.372 |
| 60 minutes | 18 (8.4, 30.0) | 18 (10.0, 27.0) | 0.694 |
| AUC | 30 (13.7, 67.8) | 40 (24.3, 53.1) | 0.501 |
Data are median values (interquartile range). Mann–Whitney tests were performed using a significance of 0.050.
AUC, area under the curve.
Table 2.
Summary of Milk Overall Changes by Day Postdelivery
| Day 3 | Day 9 | Day 14 | ||||||
|---|---|---|---|---|---|---|---|---|
| Milk immune component | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | p value | Change |
| SIgA | 48 | 26.2 (10.5–47.8) | 48 | 9.3 (5.5–15.7) | 48 | 10.5 (6.1–20.6) | <0.001 | Day 3 > 9, 14 |
| EGF | 51 | 9,189 (6,731–12,435) | 51 | 11,697 (6,732–12,437) | 51 | 12,436 (6,974–14,285) | 0.001 | Day 9, 14 > 3 |
| IL-10 | 51 | 4.6 (1.3–13.8) | 51 | 1.4 (0.8–3.3) | 51 | 1.6 (0.7–4.2) | <0.001 | Day 3 > 9, 14 |
| IL-4 | 51 | 1 (0.4–1.6) | 51 | 0.7 (0.4–1.6) | 51 | 1 (0.4–1.6) | 0.560 | |
| IL-6 | 51 | 38.5 (18.8–81.8) | 51 | 16.6 (5–35) | 51 | 12.6 (5–40.2) | <0.001 | Day 3 > 9, 14 |
| IL-8 | 51 | 163.4 (63.1–739) | 51 | 31.7 (6.8–97) | 51 | 34.8 (7.5–97.7) | <0.001 | Day 3 > 9, 14 |
| IP-10 | 51 | 1,392 (763–2,498) | 51 | 1,087 (575–2,317) | 51 | 1,164 (633–2,138) | 0.327 | |
| MIP-1alpha | 51 | 61.4 (29.7–199.8) | 51 | 12.7 (4.9–32.6) | 51 | 12.5 (6.4–49.2) | <0.001 | Day 3 > 9, 14 |
| MCP-1 | 51 | 729.4 (198.5–1,977) | 51 | 475 (108.4–2,549) | 51 | 352 (122–891.5) | 0.168 | |
| TNF-alpha | 51 | 29.5 (15.1–39.5) | 51 | 15.2 (8.6–26.6) | 51 | 16.1 (11.5–28.3) | <0.001 | Day 3 > 9, 14 |
Secretory immunoglobulin A (SIgA) values are reported as mg/mL; all other values are reported as pg/mL.
EGF, epidermal growth factor; IL, interleukin; IP-10, interferon-gamma-induced protein-10; IQR, interquartile range; MCP-1, monocyte chemotactic protein-1; MIP-1alpha, macrophage inflammatory protein-1alpha; SIgA, secretory immunoglobulin A; TNF-alpha, tumor necrosis factor-alpha.
Aim 1
Statistically significant (p < 0.05) associations of maternal stress indicators with the MICs are summarized in Table 3. Parity status, use of antibiotics, self-reported diabetes, self-reported hypertension, self-perceived stress, cortisol levels, Medicaid status, and number of children under care were statistically significantly associated with six of the 10 MICs (effect sizes ranging from r = 0.25 to 0.38) on Day 3. EGF was the single MIC with the most associations with maternal stress indicators (Table 3). The only remaining statistically significant associations at Day 9 were previous pregnancy with MCP-1 and TNF-alpha (r = −0.24 and −0.31, respectively; p < 0.05), use of antibiotics and cortisol levels with EGF (r = −0.24 and –0.32, respectively; p < 0.05), and number of children under care with IL-6 and TNF-alpha (r = −0.26 and −0.31, respectively; p < 0.05).
Table 3.
Statistically Significant Correlations of Maternal Stressors with Milk Immune Components on Day 3
| SIgA | EGF | IL-4 | IL-6 | IL-8 | IL-10 | MCP-1 | MIP-1alpha | IP-10 | TNF-alpha | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||
| Parity | ||||||||||
| Mode of delivery | ||||||||||
| Previous pregnancy | −0.34 | |||||||||
| Smoking | ||||||||||
| Antibiotics | −0.29 | |||||||||
| Hypertension | 0.28 | |||||||||
| Diabetes | −0.30 | 0.27 | ||||||||
| Perceived stress | −0.32 | −0.25 | −0.27 | |||||||
| Cortisol | 0.38 | |||||||||
| Medicaid | 0.32 | |||||||||
| Children (n) under care | −0.26 | |||||||||
Significance was defined by p < 0.05.
EGF, epidermal growth factor; IL, interleukin; IP-10, interferon-gamma-induced protein-10; MCP-1, monocyte chemotactic protein-1; MIP-1alpha, macrophage inflammatory protein-1alpha; SIgA, secretory immunoglobulin A; TNF-alpha, tumor necrosis factor-alpha.
Aim 2
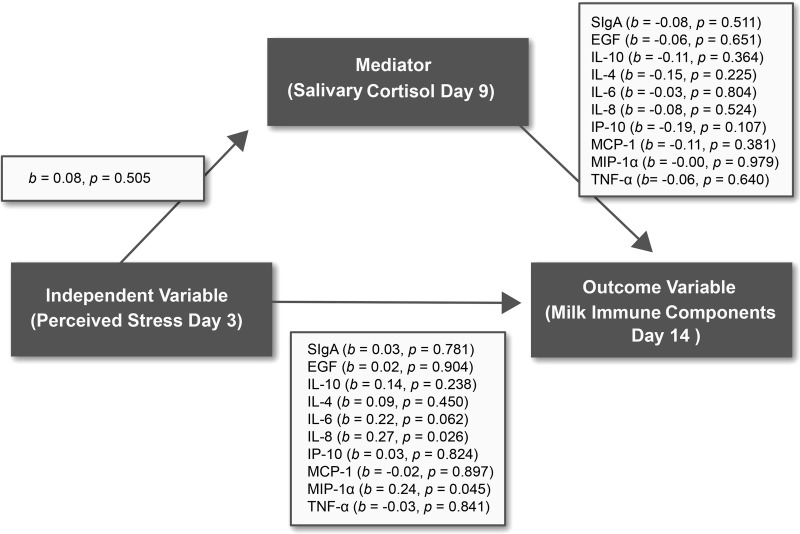
No statistically significant correlations of maternal cortisol level with MICs were observed. A mediation relationship of cortisol on the associations of maternal stressors and milk values also was not found (p > 0.05) (Fig. 2).
FIG. 2.
Cortisol mediation model. b, standardized regression beta coefficient; EGF, epidermal growth factor; IL, interleukin; IP-10, interferon-gamma-induced protein-10; MCP-1, monocyte chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1alpha; SIgA, secretory immunoglobulin A; TNF-α, tumor necrosis factor-alpha.
Discussion
There were both similarities and differences in the maternal stress indicators among this sample of African American women compared with national data limiting the generalization of the findings. The national incidence of smoking (15.7%) and hypertension (19.2%) in African American women is greater than that self-reported by the study subjects, whereas diabetes (5.1%) among African American women was less.23 The use of antibiotics in this sample was above national data, which reports 27.9% of women (all race/ethnicity) receive antibiotics during pregnancy.23 Medicaid status was higher in this sample than data for viable deliveries in the United States (48%).50,51
The only stress indicator that changed over time was self-perceived stress. Overall, the women in this study reported less perceived stress (PSS scores) across all study days than previously published PSS scores of women 4–6 weeks postdelivery (race/ethnicity not reported) (mean = 21.3–27.7 [95% confidence interval = 19.2, 29.8]).42,43 However, the PSS scores did fall within the reported PSS score ranges of African Americans in two cohorts from 2006 (n = 99; mean = 16.44 [95% confidence interval = 12.8, 20.1]) and 2009 (n = 99; mean = 15.68 [95% confidence interval = 11.3, 20.1]).52 The internal reliability of the PSS item responses across time also fell within published literature values.48,49
The median values of salivary cortisol of African American women in this study were higher than those reported by Hampson et al.53 among women <14 days postpartum (race/ethnicity not reported) (4.61 ± 0.03 ng/mL [95% confidence interval = 4.60, 4.62]). However, the timing of collections in this study used the cortisol awakening response (awake, 30 minutes after waking, 60 minutes after awaking), whereas Hampson et al.53 used noon ±2 hours.
The majority of the MIC values decreased over time postdelivery as expected. However, in this sample, EGF levels steadily increased over time, which has not been previously reported.54 This study also adds to the literature by including the analyses of chemokines (IL-8, IP-10, MCP-1, and MIP-1alpha). Maternal stressors not previously reported in milk immunology include diabetes, hypertension, antibiotics, number of children under care, and relationship status. All of these maternal stressors except relationship status had at least one significant relationship with an MIC. The overall observation of bidirectional relationships of MICs in association with maternal stressors is important in that the majority of these relationships were observed on Day 3 and then disappeared after Day 9 (effect sizes of r = 0.22–0.38).
Day 3 postdelivery is a rapidly changing environment for the mother of a healthy term infant. Physical repair coupled with new maternal role responsibilities and the onset of lactogenesis could contribute to higher levels of maternal stress.35 Preexisting conditions such as hypertension and diabetes could contribute to delayed physical recovery as well as delayed lactogenesis.27,28 In the United States, mothers of healthy term infants are typically discharged by the evening of Day 3; therefore mothers with children at home would anticipate the juggle of existing childcare responsibilities with the newborn's care. In this sample, the majority of the relationships with maternal stressors were observed on Day 3. EGF, essential for infant intestinal wall integrity, demonstrated most of the strongest relationships with maternal stressors on Day 3 (effect size of r = 0.24–0.32), which has the potential to impact infant health.55 It would be important to explore these findings in future research, particularly in mothers of preterm infants for whom an increased risk of intestinal inflammatory conditions such as necrotizing enterocolitis is observed.55
Another important finding is that there were decreased levels of several pro-inflammatory CCGFs (MCP-1, IL-6, and TNF-alpha) observed on both Days 3 and 9 in relation to the number of children under care. Caregiver stress is strongly correlated with the increased incidence of inflammatory processes.56,57 In this study the opposite was observed. One plausible explanation for the observed decrease in milk pro-inflammatory components in this sample could be that immediately postdelivery many women receive an increased level of social support to welcome the newborn. This increased support may have buffered the pro-inflammatory response to caregiver stress temporarily, but more research is warranted to determine if these findings are generalizable.
The proposed mediation of cortisol was not observed in this study. Salivary cortisol is the biomarker of stress that reflects the physical response to all maternal stressors (physical, psychological, and environmental); however, no one particular maternal stressor was significantly associated with salivary cortisol. It could be debated that the measurement of salivary cortisol on Day 9 postdelivery did not provide a sufficient enough washout time period for the elevated levels of maternal serum cortisol associated with delivery.58 Serum cortisol is associated with decreased levels of serum pro-inflammatory chemokines/cytokines, but this also was not observed in the milk.8
There are two important strengths of this study: the longitudinal collections and the population studied. By collecting milk over time, the findings of this study contribute to the knowledge of the natural variations in MICs, and the directions of those variations could have clinical applications to infant health. Regarding the population studied, this is the largest sample of milk collected from African American women reported to date. The levels of the MICs captured in this study fell within the published literature values, which largely represents non-Hispanic white and Hispanic women in the United States, Europe, and Eastern Europe. Thus it could be suggested that race is not likely associated with the known variations in MICs but rather the context of the mother's individual situation (physical, psychological, and environmental characteristics) may be more important than race/ethnicity.
There are several limitations of this study that should be noted. The small sample size could be reflective of the very low breastfeeding initiation rates of African American women.11 Milk volume was assessed using the self-report of the number of times the infant was fed in the last 24 hours. A more objective measurement of milk volume would be to have the mothers weigh the infant before and after each feeding using a valid scale supplied by the investigators, but this was not feasible for this study.59 Lastly, in this study hindmilk was chosen as the method of milk collection to be able to compare results to previous studies where consistency of methods has been reported. Arguably the literature does not support which method of milk collection (foremilk, hindmilk, or pooled sample) is best for milk immune analysis.54
The greater rates of cesarean section delivery and antibiotic usage self-reported in this sample would suggest that there may be medical indicators that could be confounding variables. All of the subjects enrolled were discharged within 3 days postdelivery, and none of those who completed study procedures was rehospitalized within 14 days postdelivery; therefore we can propose the mothers and their infants met the healthy delivery criteria. Also of note is that we did not capture oral contraception usage, which has been reported to influence salivary cortisol levels.60 Currently, progesterone hormonal injection and progesterone-only oral pills are recommended in breastfeeding women <21 days postdelivery.61 Thus it is possible our subjects were receiving progesterone as oral contraception. This would be important to explore in a secondary analysis.
The validity of the salivary cortisol and MIC values was dependent on the adherence to collection and storage instructions; however, the reported levels fell within existing literature values, which suggests that field collections might be feasible for future milk research.54 Regarding validity of milk values, free cytokines were measured in the whey fraction. Although there is evidence of soluble receptors for cytokines such as TNF-alpha, IL-1, and IL-6 in colostrum and mature milk that contribute to the anti-inflammatory properties of human milk, very little is known about these receptors in the majority of MICs measured in this study.62 This would be important to explore in the future. Regarding validity of cortisol values, there is evidence that suggests a seasonal variation of cortisol: greater cortisol levels were reported in the winter months.63 This seasonal variation was noted in northern latitudes where there is limited sunshine during winter months. Although this study did not assess seasonal variation, it would be important to explore.
Conclusions
In both animal and human research, lactation has been associated with a decreased stress response; however, this protective effect may be short-lived to the actual act of breastfeeding in humans.41,64 It is not yet known whether there is a threshold of stress in the mother that could influence the MICs, but this is an important question to ask. The origins of MICs are not clear, but three pathways have been proposed: it is suggested that milk cytokines can be derived from the mammary epithelium, lymphocytes, and macrophages within milk, as well as direct transfer from serum across leaky tight junctions of the mammary epithelium.54,65,66 The lymphoid immune system is responsive to stressors, and the mechanisms that influence cytokine production in milk may also be affected by stress. Nonhuman primate work supports the proposal that there are stress effects in milk.67
This study represents a beginning foundation of research on maternal stressors and MICs. It is a springboard for numerous future research questions. To determine if these findings are replicable, future studies should include larger sample sizes of women of diverse race/ethnicities across numerous geographic locations to explore the contextual maternal situation postdelivery. The inclusion of serum immune markers pre-/postdelivery along with MICs would broaden the existing knowledge of the immense variation of MICs and give insight to whether this variation is sensitive to the maternal physical, psychological, or environmental characteristics. Lastly, exploring the relationship of maternal stress and MICs among mothers of preterm infants could lead to supportive interventions to improve the protective properties of milk.
Acknowledgments
This publication described was supported by Vanderbilt Clinical Translational Science Award number UL1TR000445 from the National Center for Advancing Translational Sciences. Additional support was received from the following: REDCap (UL1 TR000445 from the National Center for Advancing Translational Sciences, National Institutes of Health), the Vanderbilt University School of Nursing PhD Student Support Fund, the Sigma Theta Tau International–Epsilon Nu Chapter, Medela, Inc., and Ochsner Health System. Additional thanks to the mothers who participated and the following research team members: Carol Holt, Cynthia Boudreaux, Jennifer Annino, Barbara Conley, Brooke Towne, Alyssa Simon, and Rachel Thompson.
Disclosure Statement
No competing financial interests exist.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ip S, Chung M, Raman G, et al. . Breastfeeding maternal and infant health outcomes in developed countries. Evidence Evid Rep Technol Assess (Full Rep) 2007;(153):1–186 [PMC free article] [PubMed] [Google Scholar]
- 2.Bobrow K, Quigley M, Green J, et al. . Persistent effect of women's parity and breastfeeding patterns on their body mass index: Results from the Million Women Study. Int J Obes (Lond) 2013;37:712–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz E, Brown J, Creasman J, et al. . Lactation and maternal risk of type-2 diabetes: A population based study. Am J Med 2010;123:863e861–e866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Infant and Young Child Feeding. Model Chapter for Textbooks for Medical Students and Allied Health Professionals. Available at www.who.int/nutrition/publications/infantfeeding/9789241597494/en/ (accessed December3, 2015) [PubMed]
- 5.Hicken M, Lee H, Morenoff J, et al. . Racial/ethnic disparities in hypertension prevalence: Reconsidering the role of chronic stress. Am J Public Health 2014;104:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace ME, Harville EW. Allostatic load and birth outcomes among white and black women in New Orleans. Matern Child Health J 2012;17:1025–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckie T. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs 2012;14:311–346 [DOI] [PubMed] [Google Scholar]
- 8.Elenkov I. Glucorticoids and the Th1/Th2 balance. Ann N Y Acad Sci 2004;1024:138–146 [DOI] [PubMed] [Google Scholar]
- 9.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull 2004;130:601–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calgani E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci 2006;1069:62–76 [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Health, United States, 2012. 2012. Available at www.cdc.gov/nchs/data/hus/hus12.pdf#068 (accessed November25, 2013)
- 12.Office on Women's Health, U.S. Department of Health and Human Services. Minority Women's Health. 2013. Available at http://womenshealth.gov/minority-health/african-americans/pregnancy.html (accessed November25, 2013)
- 13.Centers for Disease Control and Prevention. Breastfeeding Report Card 2014, United States: Outcome Indicators. 2014. Available at www.cdc.gov/breastfeeding/pdf/2014breastfeedingreportcard.pdf (accessed December3, 2015)
- 14.Ludington-Hoe SM, McDonald PE, Satyshur R. Breastfeeding in African-American women. J Natl Black Nurses Assoc 2002;13:56–64 [PubMed] [Google Scholar]
- 15.March of Dimes. Born Too Soon and Too Small in the United States. 2010. Available at www.marchofdimes.com/peristats/pdflib/195/99.pdf (accessed July12, 2010)
- 16.Spencer BS, Grassly JS. African American women and breastfeeding: An integrative literature review. Health Care Women Int 2013;34:607–625 [DOI] [PubMed] [Google Scholar]
- 17.Kenny L, Lavendar T, McNamee R, et al. . Advanced maternal age and adverse pregnancy outcomes: Evidence from a large contemporary cohort. PLoS One 2013;8:e56583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laopaiboon M, Lumbiganon P, Intarut N, et al. . Advanced maternal age and pregnancy outcomes: A multicountry assessment. BJOG 2014;121(Suppl):49–56 [DOI] [PubMed] [Google Scholar]
- 19.Carolan M, Frankowska D. Advanced maternal age and adverse perinatal outcomes: A review of the evidence. Midwifery 2011;27:793–801 [DOI] [PubMed] [Google Scholar]
- 20.Lanier P, Jonson-Reid M. Comparing primiparous and multiparous mothers in a nurse home visiting prevention program. Birth Issues Perinatal Care 2014;41:344–352 [DOI] [PubMed] [Google Scholar]
- 21.Thompson J, Roberts C, Currie M, et al. . Prevalence and persistence of health problems after childbirth: Association with parity and method of birth. Birth 2002;29:83–94 [DOI] [PubMed] [Google Scholar]
- 22.Mund M, Louwen F, Klingelheofer D, et al. . Smoking and pregnancy—A review on the first major environmental risk factor of the unborn. Int J Environ Res Public Health 2013;10:6485–6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbins CL, Zapata LB, Farr SL, et al. . Core state preconception health indicators—Pregnancy Risk Assessment Monitoring System and Behavioral Risk Factor Surveillance System, 2009. MMWR Surveill Summ 2014;63(3):1–62 [PubMed] [Google Scholar]
- 24.Letson G, Rosenberg K, Wu L. Association between smoking during pregnancy and breastfeeding at about 2 weeks of age. J Hum Lact 2002;18:368–372 [DOI] [PubMed] [Google Scholar]
- 25.Sappenfield E, Jamieson DJ, Kourtis AP. Pregnancy and susceptibility to infectious diseases. Infect Dis Obstet Gynecol 2013;2013:752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne MS, Bayatibojakhi S. Exploring preterm birth as a polymicrobial disease: An overview of the uterin microbiome. Front Immunol 2014;5:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bramham K, Parnell B, Nelson-Piercy C, et al. . Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ 2014;348:g2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurst N. Recognizing and treating delayed or failed lactogenesis II. J Midwifery Womens Health 2007;52:588–593 [DOI] [PubMed] [Google Scholar]
- 29.Wendland E, Torloni M, Falavigna M, et al. . Gestational diabetes and pregnancy outcomes—A systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth 2012;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkelstein S, Keely E, Feig D, et al. . Breastfeeding in women with diabetes: Lower rates despite greater rewards. A population-based study. Diabet Med 2013;30:1094–1101 [DOI] [PubMed] [Google Scholar]
- 31.Marinda M, Maxson P, Edwards S. Environmental contribution to disparities in pregnancy outcomes. Epidemiol Rev 2009;31:67–83 [DOI] [PubMed] [Google Scholar]
- 32.Ruiz R, Avant K. Effects of maternal prenatal stress on infant outcomes. Adv Nurs Sci 2005;28:345–355 [DOI] [PubMed] [Google Scholar]
- 33.Bryant Borders A, Grobman W, Amsden L, et al. . Chronic stress and low birth weight neonates in a low-income population of women. Obstet Gynecol 2007;109:331–338 [DOI] [PubMed] [Google Scholar]
- 34.Hung C. The psychosocial consequences for primiparas and multiparas. Kaohsiung J Med Sci 2007;23:352–360 [DOI] [PubMed] [Google Scholar]
- 35.Jevitt C, Groer M, Crist N, et al. . Postpartum stressors: A content analysis. Issues Ment Health Nurs 2012;33:309–318 [DOI] [PubMed] [Google Scholar]
- 36.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2009 period linked birth/infant death data set. Natl Vital Stat Rep 2013;61(8):1–27. Available at www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_08.pdf (accessed May25, 2014) [PubMed]
- 37.Child Trends Data Bank. Births to Unmarried Women: Indicators on Children and Youth. 2014. Available at www.childtrends.org/wp-content/uploads/2015/03/75_Births_to_Unmarried_Women.pdf (accessed December3, 2015)
- 38.Richter J, Bittner A, Petrowski K, et al. . Effects of an early intervention on perceived stress and diurnal cortisol in pregnant women with elevates stress, anxiety, and depressive symptomatology. J Psychosom Obstet Gynecol 2012;33:162–170 [DOI] [PubMed] [Google Scholar]
- 39.Urizar G, Munoz R. Impact of a prenatal cognitive-behavioral stress managment intervention on salivary cortisol levels in low income mothers. Psychoneuroendocrinology 2011;36:1480–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawano A, Emori Y, Miyagawa S. Association between stress-related substances in saliva and immune substances in breast milk in puerperae. Biol Res Nurs 2009;10:350–355 [DOI] [PubMed] [Google Scholar]
- 41.Tu M, Lupien S, Walker C. Diurnal salivary cortisol levels in postpartum mothers as a function of infant feeding choice and parity. Psychoneuroendocrinology 2006;31:812–824 [DOI] [PubMed] [Google Scholar]
- 42.Groer M, Davis M, Steele K. Associations between human milk sIgA and maternal immune, infectious, endocrine, and stress variables. J Hum Lact 2004;20:153–158 [DOI] [PubMed] [Google Scholar]
- 43.Groer M, Davis M, Casey K, et al. . Neuroendocrine and immune relationships in postpartum fatigue. MCN Am J Matern Child Nurs 2005;30:133–138 [DOI] [PubMed] [Google Scholar]
- 44.Malgady R, Krebs D. Understanding correlation coefficients and regression. Phys Ther 1986;66:110–120 [DOI] [PubMed] [Google Scholar]
- 45.Groer M, Beckstead J. Multi-dimensional scaling of multiplex data: Human milk cytokines. Biol Res Nurs 2011;13:289–296 [DOI] [PubMed] [Google Scholar]
- 46.Groer MW, Humenick S, Hill PD. Characterizations and psychoneuroimmunologic implications of secretory immunoglobulin A and cortisol in preterm and term breastmilk. J Perinat Neonatal Nurs 1994;7:42–51 [DOI] [PubMed] [Google Scholar]
- 47.Groer M, Shelton M. Exercise is associated with elevated proinflammatory cytokines in human milk. J Obstet Gynecol Neonatal Nurs 2009;38:35–41 [DOI] [PubMed] [Google Scholar]
- 48.Cohen F, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396 [PubMed] [Google Scholar]
- 49.Nast I, Bolten M, Meinlschmidt G, et al. . How to measure prenatal stress? A systematic review of psychometric instruments to assess psychosocial stress during pregnancy. Paediatr Perinat Epidemiol 2013;27:311–322 [DOI] [PubMed] [Google Scholar]
- 50.Kaiser Family Foundation. Demographics and Economy: State Health Facts. 2010. Available at www.statehealthfacts.org/profilecat.jsp?rgn=20&cat=1 (accessed April15, 2010)
- 51.Medicaid.gov Medicaid & CHIP Program Information—Pregnant Women. 2011. Available at www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Population/Pregnant-Women/Pregnant-Women.html (accessed March9, 2011)
- 52.Cohen S, Janicki-Deverts D. Who's stressed? Distributions of pyschological stress in the Unites States in probablity samples from 1983, 2006, 2009. J Appl Soc Psychol 2012;42:1320–1334 [Google Scholar]
- 53.Hampson E, Phillips SD, Soares CN, et al. . Steroid concentrations in antepartum and postpartum saliva: Normative values in women and correlations with serum. Biol Sex Differ 2013;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agarwal S, Karmaus W, Davis S, et al. . Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J Hum Lact 2011;27:171–186 [DOI] [PubMed] [Google Scholar]
- 55.Chatterton D, Nguyen D, Bering S, et al. . Anti-inflammatory mechanisms of bioactive milk proteins in the intestines of newborns. Int J Biochem Cell Biol 2013;45:1730–1747 [DOI] [PubMed] [Google Scholar]
- 56.Lovell B, Wetherell M. The cost of caregiving: Endocrine and immune implications in elderly and non elderly caregivers. Neurosci Biobehav Rev 2011;35:1342–1352 [DOI] [PubMed] [Google Scholar]
- 57.Miller G, Cohen S, Ritchey K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol 2002;21:531–541 [DOI] [PubMed] [Google Scholar]
- 58.O'Keane V, Lightman S, Patrick K, et al. . Changes in the maternal hypothalamic-pituitary-adrenal axis during the early puerperium may be related to the postpartum ‘blues.’ J Neuroendocrinol 2011;23:1149–1155 [DOI] [PubMed] [Google Scholar]
- 59.Haase B, Barreira J, Murphy PK, et al. . The development of an accurate test weighing technique for preterm and high-risk hospitalized infants. Breastfeed Med 2009;4:151–156 [DOI] [PubMed] [Google Scholar]
- 60.Roche D, King A, Cohoon A, et al. . Hormonal contraceptive use diminishes salivary cortisol response to psychosocial stress and naltrexone in health women. Pharmacol Biochem Behav 2013;109:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2010: Adapted from the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th edition. Available at www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a1.htm?s_cid=rr5904a1_e (accessed December3, 2015)
- 62.Buescher ES, Malinowska I. Soluble receptors and cytokine antagonists in human milk. Pediatr Res 1996;40:839–844 [DOI] [PubMed] [Google Scholar]
- 63.Persson R, Garde AH, Hansen AM, et al. . Seasonal variation in human salivary cortisol concentration. Chronobiol Int 2009;25:923–937 [DOI] [PubMed] [Google Scholar]
- 64.Heinrichs M, Meinschmidt G, Neuman I, et al. . Effects of suckling on hypothalmic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab 2001;86:4798–4804 [DOI] [PubMed] [Google Scholar]
- 65.Garofalo R, Goldman A. Cytokines, chemokines, and colon-stimulating factors in human milk. Biol Neonate 1998;74:134–142 [DOI] [PubMed] [Google Scholar]
- 66.Neville M. Anatomy and physiology of lactation. Pediatr Clin North Am 2001;48:13–34 [DOI] [PubMed] [Google Scholar]
- 67.Hinde K, Skibiel AL, Foster AB, et al. . Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behav Ecol 2015;26:269–281 [DOI] [PMC free article] [PubMed] [Google Scholar]