Abstract
Significance: Chronic wounds remain a significant public health problem. Alterations in normal physiological processes caused by aging or diabetes lead to impaired tissue repair and the development of chronic and nonhealing wounds. Understanding the unique features of the wound environment will be required to develop new therapeutics that impact these disabling conditions. New drug-delivery systems (DDSs) may enhance current and future therapies for this challenging clinical problem.
Recent Advances: Historically, physical barriers and biological degradation limited the efficacy of DDSs in wound healing. In aiming at improving and optimizing drug delivery, recent data suggest that combinations of delivery mechanisms, such as hydrogels, small molecules, RNA interference (RNAi), as well as growth factor and stem cell-based therapies (biologics), could offer exciting new opportunities for improving tissue repair.
Critical Issues: The lack of effective therapeutic approaches to combat the significant disability associated with chronic wounds has become an area of increasing clinical concern. However, the unique challenges of the wound environment have limited the development of effective therapeutic options for clinical use.
Future Directions: New platforms presented in this review may provide clinicians and scientists with an improved understanding of the alternatives for drug delivery in wound care, which may facilitate the development of new therapeutic approaches for patients.

Geoffrey C. Gurtner, MD, FACS
Scope and Significance
Chronic wounds, often manifesting in elderly and diabetic patients, are thought to result from impairments in the cellular and molecular mechanisms of wound repair.1 These wounds can result in significant disability, amputation, and increased mortality.2 Understanding the normal mechanisms that promote regeneration and restore organ function is crucial for the development of effective therapeutic approaches to impaired healing.3 In addition, the complex challenges of the wound environment, including hypoxia, ischemia, oxidative stress, bacterial infection, as well as the critical role played by inflammatory cells, should be considered (Table 1). There is a need for therapeutics that maintain efficacy despite the obstacles presented by the wound environment.
Table 1.
Challenges of the wound environment
| Challenge | Characteristics | Possible solutions |
|---|---|---|
| Mechanical | Difficulty in retaining locally delivered therapeutics during normal movements or change of dressing. | Protected delivery (i.e., patch delivery or bandage based). |
| Oxidative stress | Free radicals generated by uncontrolled oxidative stress can cause cellular damage and death. | Deferoxamine and other free radical scavengers have been shown to reduce oxidative stress in wounds. |
| Enzymatic | Proteases present during normal and pathological wound healing degrade growth factors. | Controlled and sustained release using drug-delivery systems. |
| Hypoxia/ischemia | Arterial or venous insufficiency impairing blood supply to injured tissue; edema impairing oxygen diffusion to injured tissue. | Hyperbaric oxygen therapy, negative pressure wound therapy, and pro-angiogenic therapeutics. |
| Infected/necrotic tissue | Infected tissue is chronically inflamed and unable to progress through phases of wound healing; necrotic tissue lacks the ability to heal and is prone to infection. | Debridement, systemic antibiotics, and antimicrobial dressings. |
Translational Relevance
After injury, the human body initiates a sequence of events that aims at restoring tissue integrity and homeostasis. Wound healing relies on the coordination of intracellular, intercellular, and extracellular pathways, and offers both challenges and opportunities in clinical medicine.5 Newer therapies based on growth factors and/or stem cells (biologics), small-molecule drugs, or RNA interference (RNAi) attempt to replicate and enhance the mechanisms of normal wound healing. For instance, deferoxamine (DFO), which for many years has been clinically used for the treatment of hemochromatosis, has now been shown to accelerate wound healing in vivo by scavenging iron needed for degradation of hypoxia-inducible factor 1-alpha (HIF-1α).6 This novel application of a known drug, based on an understanding of the underlying mechanism of disease, represents an appealing approach to wound therapy with the potential for rapid clinical translation.
Clinical Relevance
More than 6.5 million Americans suffer from chronic, nonhealing wounds, generating annual healthcare costs of more than $25 billion.4 These numbers are expected to increase with the growth of high-risk populations, including diabetics, the obese, and the elderly. In addition to the economic cost, there is a significant deterioration in the quality of life for patients suffering from chronic wounds.4 The immense socioeconomic burden of nonhealing wounds in the United States necessitates the continued development of technologies that aim at modifying wound microenvironments and optimizing biological repair.
Background
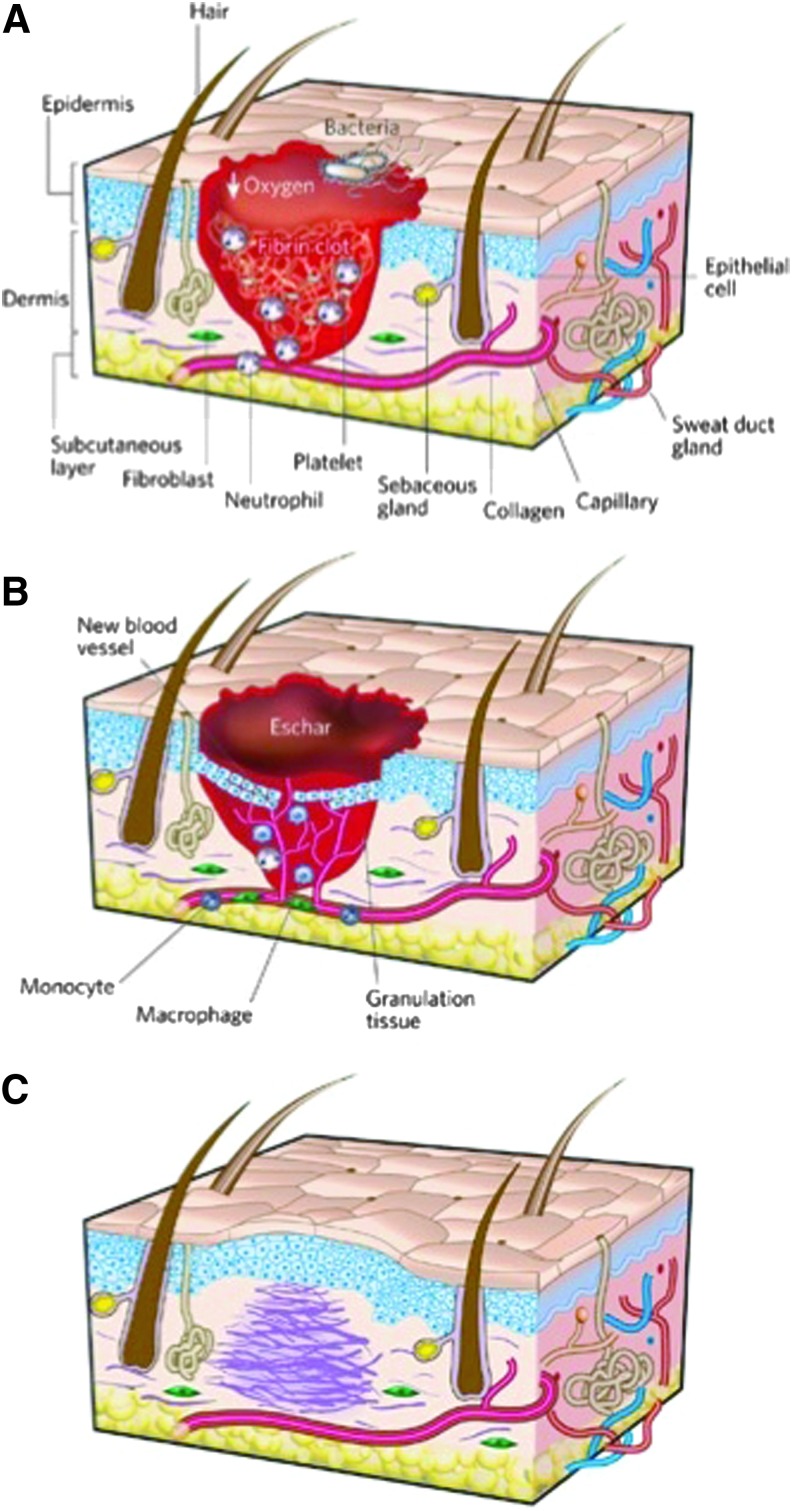
Wound healing is a dynamic process that is characterized by three overlapping but distinct cellular phases: inflammation, new tissue formation, and remodeling5 (Fig. 1). The inflammatory phase is characterized by hemostasis, as well as the recruitment of neutrophils and macrophages in preparation for new tissue formation.7 The formation of a vascularized extracellular matrix (ECM) from endothelial-fibroblast interactions provides structural support and promotes angiogenesis to increase nutrient and oxygen delivery to the wound.7 Re-epithelialization of the dermal bridge resurfaces the wound in parallel with the onset of wound contraction.7 The final stage of wound healing, remodeling, commences as apoptosis of macrophages and myofibroblasts occurs in the wound site. During the months after injury, a predominantly type III reticular collagen matrix is actively remodeled into the more disordered type I collagen ECM characteristic of scar tissue.5
Figure 1.
Classic stages of wound repair. There are three classic stages of wound repair: inflammation (A), new tissue formation (B), and remodeling (C). Reprinted with permission from Gurtner et al.5 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Substantial efforts have been made by scientists and clinicians alike to enhance the process of wound healing. Wound care has traditionally relied on dressings, comprising both natural and synthetic materials, to maintain a warm and moist wound environment while minimizing bacterial load.8 Over the last two decades, a number of new dressings and drug delivery models have expanded the armamentarium of wound-care professionals. For example, researchers have recently developed a wound dressing containing nanovesicles that has the ability to release antimicrobials in the presence of pathogenic bacteria and induces a color change in the dressing as an indicator of infection.9 In addition, approaches such as photodynamic therapy and a variety of topical antimicrobial dressings (e.g., silver based) have further facilitated wound decontamination.10 Multiple local delivery methods have been developed for cell- and small-molecule-based therapies, and the descriptions of the most promising approaches are included throughout this review. Despite these promising advances, further research and translation of experimental findings is essential to effectively tackle the healthcare burden of chronic wounds. In this review, we discuss the therapeutic potential of biological, small-molecule, and RNAi-based approaches for wound healing and the challenges associated with their delivery.
Discussion
Drug delivery
As new drugs are developed and old drugs are repurposed to address recently identified targets for wound healing, the delivery of these drugs requires particular attention (Table 2).
Table 2.
Comparison of local versus systemic drug delivery
| Systemic delivery | Local delivery |
|---|---|
| IV injection/oral route | Direct delivery to the wound site |
| Risk of multi-organ toxicity | Enables sustained drug administration |
| Less predictable | Skin irritation |
| Requires higher dosing to achieve therapeutic effect | Avoidance of hepatic first-pass metabolism and the GI tract |
Systemic drug delivery
Systemic delivery, typically oral or intravenous, is the most common approach for administering drugs to patients and continues to be widely used for a variety of indications, including pain relief and antibiotic therapy. Unfortunately, systemic drug delivery relies on adequate perfusion of the target tissue, and many chronic wounds lack this critical blood supply. Moreover, there is significant potential for harmful side-effects in nontarget tissues, as seen in recent in vitro and in vivo preclinical studies assessing the systemic delivery of the small molecules pyrvinium and DFO.11,12 These adverse effects limit the dosing and duration of treatment. Given the risks for systemic toxicity and less predictable drug delivery to the target tissue, there has been a significant shift in clinical focus toward localized delivery of drugs for wound healing.
Local drug delivery
Localized drug delivery permits convenient self-administration for patients while avoiding issues with gastrointestinal tract absorption and hepatic first pass metabolism, thereby improving bioavailability and maintenance of drug concentration within the therapeutic window.13 Furthermore, local delivery enables transmission of the largest fraction of drug molecules to the target area, maximizing therapeutic potential and reducing systemic drug toxicity.14 Despite the advantages of localized delivery, many challenges still remain, including penetration of the stratum corneum in skin at risk for ulceration, maintaining cell survival after delivery, and the development of effective mechanisms for sustained delivery.
Growth factor and progenitor cell-based therapy research has recently centered on identifying new delivery mechanisms to overcome biological degradation and poor cell survival in the harsh wound environment. Nanoparticles, for example, have successfully been used to increase the half life of therapeutic growth factors delivered to wounds in diabetic rats.15 Recent advances in negative pressure wound therapy have allowed for intermittent fluid instillation, which may enable an alternative delivery method for aqueous wound therapies with the added benefit of providing local debridement during instillation.16 Cell delivery methods such as fibrin sealant sprays or hydrogel scaffolds have been shown to improve cell retention and functional capacity at the application site both in vitro and in vivo.17,18 Experience with these various methods shows that an ideal delivery system is nontoxic, facilitates access of therapies to the wound site, and protects these therapies from premature degradation. It should be noted, however, that development of an ideal delivery vehicle is a dynamic process that greatly depends on the properties of the therapeutic agent being delivered. Further refinement of these delivery vehicles, as well as the delivered therapies themselves, is needed before clinical implementation.
Effective and sustained delivery of small interfering RNA (siRNA) to target cells in vivo continues to limit clinical translation. The use of biological skin equivalents (BSEs), such as biomimetic scaffolds that serve as porous tissue templates, could tremendously enhance the regenerative potential of siRNA. The innovative combination of RNAi with bilayer dermal equivalent (BDE), for example, represents an attractive option for sustained delivery of RNAi.19 The adoption of microfluidic technology in the synthesis of nano-sized drug-delivery systems (DDSs) could also benefit the siRNA platform. For example, flow-focusing microfluidics was recently used to fabricate DNA lipoplexes for nonviral gene therapy and in vitro results demonstrated reduced cytotoxicity, narrower size distribution, and improved efficiency when compared with bulk mixing applications.20
Major strides have been made in the design, fabrication, and testing of DDSs. As knowledge of the dynamics of drug transport in tissues increases, the safety and efficacy of local DDSs should continue to improve. Enhancements in DDSs will facilitate a wider range of clinical application.
Biological therapies
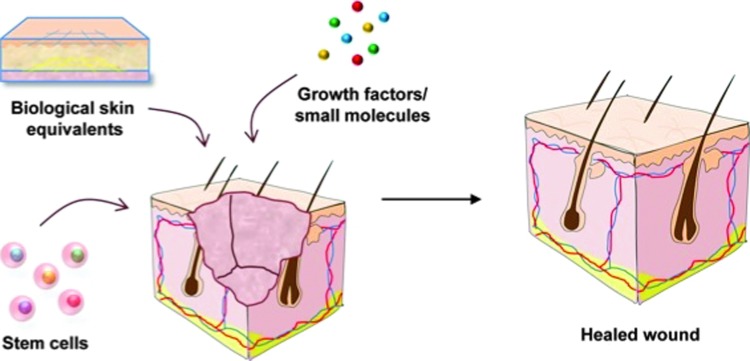
Biological therapies, or biologics, are naturally occurring cells or substances that enhance the body's own reparative capabilities in order to treat complex acute and chronic wounds. Promoting microenvironments that encourage the proliferation of both matrix-forming stromal cells and endothelial cells at the site of injury, biologics facilitate the formation of a vascular network in newly forming tissue. Approaches to biological therapy include growth factor and stem cell-based therapies (Fig. 2).
Figure 2.
Biological therapies for wound healing. Biologics are designed to stimulate or restore the body's natural wound-healing abilities. The majority of emerging products fall under the following categories: biological skin equivalents, growth factors, and stem cell-based therapies. Reprinted with permission from Rennert et al.29 To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Growth factor-based therapies
The use of growth factors as therapeutics is based on an understanding that specific regulatory pathways govern the host response to wound healing, and can be used to stimulate wound angiogenesis, matrix deposition, and re-epithelialization.5 Growth factors such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and fibroblast growth factor (FGF) have been studied extensively for their role in wound repair. Despite promising preclinical evidence to support growth factor modulation for wound healing, clinical evidence for such a benefit is currently lagging. For example, topical VEGF therapy has been shown to accelerate wound repair in diabetic mice.21 A subsequent phase I clinical trial for topical recombinant human VEGF (rhVEGF) in chronic neuropathic diabetic foot ulcers showed a nonsignificant positive trend toward therapeutic efficacy, but no further clinical trials have established a therapeutic benefit for this drug.22
Recombinant platelet-derived growth factor BB (rPDGF-BB), marketed under the trade name Regranex (Ortho-McNeil, Raritan, NJ), is currently the only FDA-approved growth factor treatment for nonhealing wounds. This drug accelerates the regenerative process by promoting fibroblast migration and wound re-epithelialization. Randomized clinical trials showed that application of rPDGF-BB significantly improved both the probability and time course for complete healing of lower-extremity diabetic neuropathic ulcers.23 Phase II clinical trials showed a potential therapeutic benefit in treating pressure ulcers24; however, this benefit did not persist in subsequent phase III studies.25,26 In addition, a black box warning was placed on the drug after a postmarketing retrospective cohort study that found an increased rate of mortality secondary to malignancy in certain patients.25 Although this drug may be useful in treating diabetic neuropathic wounds, its further clinical utility is limited by its potential for dangerous side effects, as well as by a lack of significant efficacy in alternate wound settings.
Native proteases represent a fundamental challenge for growth factor-based wound therapies. Matrix metalloproteinases, responsible for ECM degradation and cytokine inactivation, are deregulated in the setting of chronic wounds and contribute to impaired ECM remodeling and tissue ulceration.27 The application of protease inhibitors, such as tissue inhibitors of metalloproteinase, could potentially make the wound microenvironment more suitable for growth factor-based therapies, but further studies are needed to justify their clinical use.
Stem cell therapies
Self-renewal and the capacity to differentiate into numerous tissue types via asymmetric replication are the defining characteristics of stem cells. The known trophic activity of these cells has led to the development of progenitor cell-based approaches for the treatment of chronic wounds. Stem cell-derived growth factors act to stimulate cell proliferation and migration, as well as angiogenesis, ECM production, and antimicrobial activity.28
Stem cells from a variety of sources, including bone marrow, adipose tissue, extra-fetal tissue, skin, and blood, have been harvested for use in wound research.29 Local delivery of stem cells to wounds is preferred, as this is minimally invasive and targeted. Unfortunately, several obstacles have impeded the clinical translation of cell transplantation. In vitro studies have shown that cell viability after delivery can be as low as 1–32% due to shear stresses and extensional flow during syringe needle injection.30 The use of a protective matrix, such as a biomimetic hydrogel scaffold, represents an approach that may overcome these challenges.18 Enrichment of therapeutic stem cell populations before delivery may also enhance the regenerative potential of cell-based therapies. The Magellan Autologous Platelet Separator (Arteriocyte, Cleveland, OH), currently being used in a phase I clinical trial for the treatment of burns in wounded soldiers, is one possible solution that can be used to enrich bone marrow aspirates in platelet, hematopoietic stem cell, and mesenchymal stem cell (MSC) fractions before administration.31 While clearly still in early phase development, stem cell therapies, used either independently or in conjunction with BSEs, such as acellular dermal matrices or biomimetic scaffolds, appear to be a promising therapeutic approach.29
Looking to the future, biologics will continue to be an intriguing area for potential clinical extension as spatially controlled drug release systems aim to precisely control the quantity of cells or drug being delivered, reducing adverse effects on surrounding tissues, and reducing overall cost.13 The clinical use of biologics will be contingent on further testing and reduced manufacturing costs. Biologics should be shown to be locally effective with minimal systemic side effects and without risk of malignancy. Comparative studies against current standard-of-care treatments may provide the necessary evidence to support new evidence-based wound-care guidelines including this emerging therapeutic category.
Small-molecule therapies
From a translational perspective, the application of small molecules, rather than cells or proteins, has significant advantages in terms of sterility, shelf life, and regulatory hurdles.32 In addition, their chemical composition may prevent rapid degradation seen in both cell and growth factor-based therapies. Emerging small-molecule therapies for wound healing are based on the modulation of key signaling pathways involved in tissue repair.
Wnt proteins are highly conserved signaling molecules that regulate embryonic development and cell fate, and have also been linked to mammalian cutaneous wound repair.33 These proteins bind to the frizzled (Fz) and lipoprotein receptor-related protein (LRP) family of receptors, with canonical Wnt signaling being transduced by β-catenin.34 Thorne et al. recently discovered that pyrvinium, an FDA-approved drug used to treat helminth infections, acts as a potent Wnt inhibitor.35 Applying this molecule in an in vivo murine model of wound healing resulted in increased cell proliferation, granulation tissue formation, and vascularity.36 Furthermore, pyrvinium promoted the engraftment and regenerative capacity of mesenchymal progenitor cells in vitro.11
A second prominent pathway that can be manipulated by small molecules to enhance wound healing involves the hypoxic tissue response, regulated by the transcription factor hypoxia-inducible factor 1 (HIF-1).37 HIF-1 includes an α-subunit that is degraded in the presence of oxygen and iron (Fe2+) by a family of enzymes known as prolyl hydroxylases (PHDs).38 Hypoxia impairs HIF-1α degradation, resulting in increased expression of several pro-regenerative proteins, including VEGF and stromal cell-derived factor-1 (SDF-1).39 Animal studies have demonstrated improved wound healing after HIF-1 pathway stimulation, mediated in part by its downstream effectors VEGF (neovascularization) and SDF-1 (progenitor cell homing).40,41 DFO, an FDA-approved iron chelator that has been in clinical use for decades, augments wound healing by inhibiting HIF-1α degradation and decreasing oxidative stress in the wound environment, leading to decreased tissue necrosis and improved wound healing.6,42
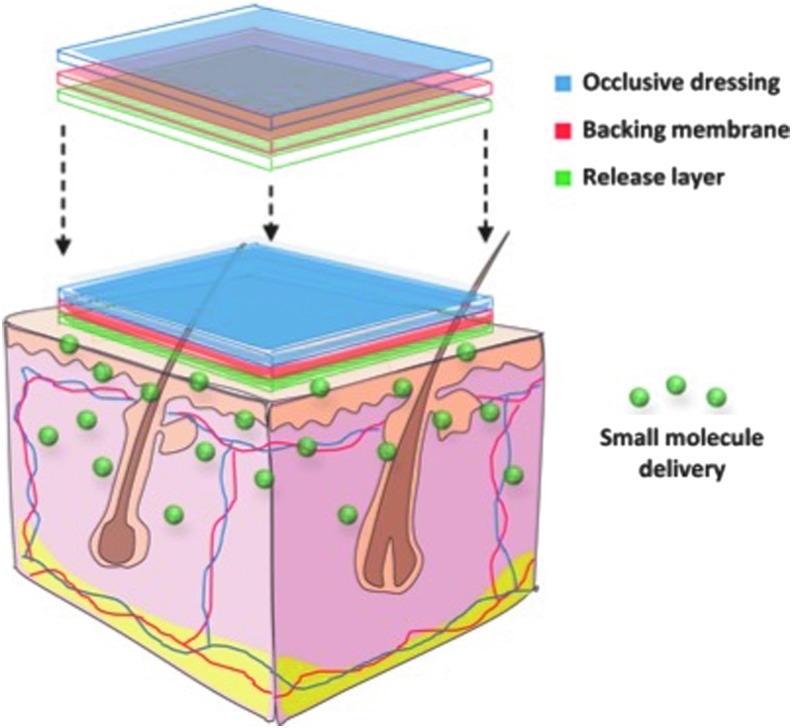
Acknowledging the benefits of sustained local small-molecule delivery, our laboratory has recently designed a transdermal delivery system containing DFO (Duscher D. et al., PNAS 2014, in press) (Fig. 3). This transdermal polymer patch overcomes the challenge of delivering hydrophilic DFO molecules through the normally impermeable stratum corneum and leads to significantly reduced ulcer formation in a preclinical model for pressure-induced wounds. These results suggest a possible novel approach to ulcer prophylaxis in high-risk patients, such as those who are bedbound or those with venous insufficiency. In addition, it provides a controlled release system and can be combined with other small molecules, such as pyrvinium, to enhance their delivery profile.
Figure 3.
Transdermal small-molecule delivery for wound healing. Local sustained delivery of small molecules allows for prolonged diffusion across cell membranes, improving the ability for drugs to reach intracellular sites of action. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
RNAi therapies
Silencing gene expression through RNAi offers selective targeting of molecules that are difficult to regulate using growth factor or small-molecule-based therapies.43 Inhibiting gene expression at the post-transcriptional level, RNAi targets specific mRNA molecules for destruction. A recent in vitro study illustrated the therapeutic potential of RNAi by using siRNA to silence human keloid fibroblast expression of Smad2, a signal transducer in the transforming growth factor beta (TGF-β) pathway. Smad2 silencing led to decreased procollagen expression, suggesting a possible therapeutic use in treating hypertrophic and keloid scars.44 Multiple studies are now underway to further explore the potential for application of RNAi-based therapy in optimizing wound repair.19,45
The targeted delivery of RNAi in vivo is still limited due to high rates of degradation by ubiquitous RNases, difficulty in targeting of specific tissues and cells, and maintenance of long-term silencing.46–48 Developing a controlled and effective DDS is, therefore, crucial to realizing the full potential of these next-generation therapeutics. Modern technologies for the delivery of RNAi in vivo consist of the injection in saline solution, incorporation into liposomes, and nanoparticle-based delivery.45,46,48 Early challenges with RNAi therapy included a lack of sustained RNAi activity and low rates of cell uptake49; however, new evidence shows that these challenges can be partially overcome with the use of biomimetic scaffolds for cell and RNAi delivery.50 A collagen-based hydrogel scaffold containing siRNA-treated cells was injected subcutaneously in mice and showed sustained effects for approximately 9 days with high cell viability (>88%) and a low fraction of siRNA released into surrounding host tissue. These hydrogel scaffolds offer an exciting new platform for siRNA delivery in vivo.50
Another collagen-based scaffold, and the only current RNAi-based drug in clinical trials for wound healing, is RXi-109 (RXi Pharmaceuticals, Marlborough, MA). This drug aims at preventing scarring by employing a collagen/silicone membrane BDE, combined with trimethylchitosan (TMC) and siRNA to suppress the translation of connective tissue growth factor (CTGF), a downstream effector in the TGF-β1 pathway.45 This RNAi-BDE scaffold was shown to improve the viability of seeded fibroblasts and to suppress TGF-β1 expression for approximately 2 weeks in porcine models.19 In addition, it downregulated expression of collagen type I, collagen type III, and alpha-smooth muscle actin (α-SMA).19
Researchers have attempted to overcome the significant barriers faced by RNAi-based therapeutics, including targeting, retention, effect duration, bioavailability, and safety. Unfortunately, with very few clinical trials currently evaluating their use in wound healing, clinical translation has yet to be achieved. Newer therapies, such as RNAi, are associated with a significant regulatory burden impacting the translation of these technologies to clinical trials. As RNAi delivery methods are further refined, this modality may play an increasingly important role in regenerative medicine.
Summary
The significant disability and cost to society associated with chronic wounds highlight the inadequacy of our current therapeutic armamentarium. Emerging treatment options have embraced the need to address deficits in critical signaling pathways, cellular dysfunction, and impaired neovascularization associated with chronic wounds; however, these treatments are largely experimental or in very early development.
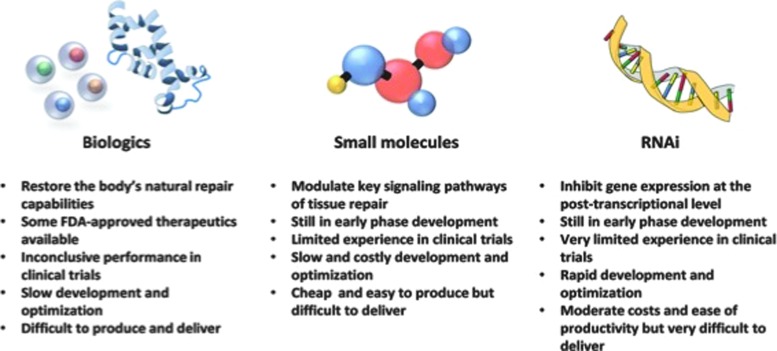
Bioactive dressings and scaffolds, growth factor and cell-based therapies, small-molecule delivery, and RNAi therapies do not, unfortunately, independently replicate the precise spatiotemporal gradients of molecules and factors present during wound healing (Fig. 4). BSEs offer an encouraging approach to wound therapy through volume replacement, wound coverage, and a provisional matrix for cell engraftment. However, imperfect processing techniques and rejection of allogenic tissues and constructs should be overcome before clinical implementation. Growth factor-based therapy is exciting in principle, but studies are inconclusive regarding their clinical benefit, potentially due to the complexity and dynamic nature of growth factor expression during the response to injury. Stem cell-based therapies are limited by the capacity of cells to survive and function in a harsh wound environment, while small-molecule-based treatments rely on efficient, targeted, and sustainable delivery systems. Finally, RNAi enables post-transcriptional regulation of specific gene targets, but is limited by its propensity for degradation by native RNAses and its ability to be delivered effectively. Integration of multiple therapeutic approaches, including stem cell, BSE, and small-molecule and/or siRNA technology, may offer improved treatment modalities for the care of chronic wounds.
Figure 4.
Therapeutic approaches: pros and cons. Key features of recent advances in biological, small-molecule, and RNA interference (RNAi)-based therapeutics. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Abbreviations and Acronyms
- α-SMA
alpha-smooth muscle actin
- BDE
bilayer dermal equivalent
- BSE
biological skin equivalent
- CTGF
connective tissue growth factor
- DDS
drug-delivery system
- DFO
deferoxamine
- ECM
extracellular matrix
- EGF
epidermal growth factor
- Fe2+
iron
- FGF
fibroblast growth factor
- Fz
frizzled
- HIF-1
hypoxia-inducible factor 1
- HIF-1α
hypoxia-inducible factor 1-alpha
- LRP
lipoprotein receptor-related protein
- PHD
prolyl hydroxylases
- rhVEGF
recombinant human VEGF
- RNAi
RNA interference
- rPDGF-BB
recombinant platelet-derived growth factor BB
- SDF-1
stromal cell-derived factor-1
- siRNA
small interfering RNA
- TGF-β
transforming growth factor beta
- TMC
trimethylchitosan
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
The authors would like to thank Michael S. Hu and Graham G. Walmsley for their assistance in the ideation of this critical review and Arnetha J. Whitmore and Melanie Rodrigues for their extensive proofreading and editing of this article. Funding for wound-healing research in our laboratory has been provided by the National Institutes of Health (R01-DK074095, R01-EB005718, and R01-AG025016 to G.C.G.), the Hagey Family Endowed Fund in Stem Cell Research and Regenerative Medicine, The Oak Foundation, and the National Library of Medicine (#LM0077033 to M.J.).
Take-Home Messages.
• Chronic wounds represent a significant public health burden, and require the development of new, effective therapies.
• Multiple therapeutic modalities, such as growth factors and stem cells (biologic), small molecules, and RNAi, have shown promising results in improving wound healing in preclinical trials.
• Despite recent advances, challenges in retention and duration of therapeutic effect in the harsh wound environment limit clinical implementation.
• New DDSs, including cell- or sealant-sprays, hydrogel scaffolds, nanoparticles, and transdermal DDSs, improve the delivery and efficacy of biological, small-molecule, and RNAi-based therapies.
• Integration of multiple therapeutic approaches and delivery systems appears necessary to effectively treat chronic wounds in the clinical setting.
Author Disclosure and Ghostwriting
No competing financial interest exists. The content of this article was expressly written by the authors listed. G.C.G. is listed on the following patent assigned to Stanford University: “Topical and Transdermal Delivery of HIF-1 Modulators to Prevent and Treat Chronic Wounds” (# 20100092546) and “Intelligent Biodegradable Pullulan Regenerative Matrix for Tissue Engineering” (#12/932,736). G.C.G. and D.D. are listed on the patent “Efficient stem cell delivery into biomaterials using a novel capillary driven encapsulation technique” (#61/994,340) assigned to Stanford University. A.J.W., Z.N.M., V.W.W., J.A.B., and M.J. have no potential conflicts of interest, affiliations, or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed here.
About the Authors
Alexander James Whittam, BA, is a research scientist whose interests include skin and soft tissue regeneration as well as stem cell-based therapies for diabetic and aged wound healing. Zeshaan N. Maan, MBBS, MS, MRCS, is a postdoctoral research fellow investigating cellular and molecular mechanisms of mammalian regeneration. Dominik Duscher, MD, is a postdoctoral research fellow whose research interests include stem cell-based therapies for diabetic and aged wound healing. Victor W. Wong, MD, is a plastic surgery resident at Johns Hopkins with research interests in cutaneous wound healing and tissue engineering. Janos A. Barrera, BS, is a medical student whose research interests include the discovery and translation of new methods for promoting tissue regeneration. Michael Januszyk, MD, is a research fellow currently pursuing a PhD in Biomedical Informatics whose research interests focus on wound healing and adult stem cell heterogeneity. Geoffrey C. Gurtner, MD, is the Johnson and Johnson Professor of Surgery and Materials Science Engineering at Stanford University. He also serves as the Associate Chairman for Research in the Department of Surgery. Dr. Gurtner runs an NIH and DoD funded laboratory that seeks to elucidate the human response to injury for the promotion of tissue repair and regeneration.
References
- 1.Telgenhoff D, Shroot B. Cellular senescence mechanisms in chronic wound healing. Cell Death Differ 2005;12:695–698 [DOI] [PubMed] [Google Scholar]
- 2.Redelings MD, Lee NE, Sorvillo F. Pressure ulcers: more lethal than we thought? Adv Skin Wound Care 2005;18:367–372 [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 6.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci U S A 2009;106:13505–13510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 8.Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci 2008;97:2892–2923 [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Loftus AL, Mulley G, Jenkins AT. A thin film detection/response system for pathogenic bacteria. J Am Chem Soc 2010;132:6566–6570 [DOI] [PubMed] [Google Scholar]
- 10.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012;25:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saraswati S, Deskins DL, Holt GE, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, increases engraftment and inhibits lineage commitment of mesenchymal stem cells (MSCs). Wound Repair Regen 2012;20:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hom DB, Goding GS, Jr., Price JA, Pernell KJ, Maisel RH. The effects of conjugated deferoxamine in porcine skin flaps. Head Neck 2000;22:579–584 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Chan HF, Leong KW. Advanced materials and processing for drug delivery: the past and the future. Adv Drug Deliv Rev 2013;65:104–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiser JR, Saltzman WM. Controlled release for local delivery of drugs: barriers and models. J Control Release 2014;190:664–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu Y, Yu D, Wang P, Xu J, Li D, Ding M. Nanotechnology promotes the full-thickness diabetic wound healing effect of recombinant human epidermal growth factor in diabetic rats. Wound Repair Regen 2010;18:499–505 [DOI] [PubMed] [Google Scholar]
- 16.Jerome D. Advances in negative pressure wound therapy: the VAC instill. J Wound Ostomy Continence Nurs 2007;34:191–194 [DOI] [PubMed] [Google Scholar]
- 17.Zimmerlin L, Rubin JP, Pfeifer ME, Moore LR, Donnenberg VS, Donnenberg AD. Human adipose stromal vascular cell delivery in a fibrin spray. Cytotherapy 2013;15:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong VW, Rustad KC, Glotzbach JP, et al. Pullulan hydrogels improve mesenchymal stem cell delivery into high-oxidative-stress wounds. Macromol Biosci 2011;11:1458–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Ma L, Liang J, Zhang B, Teng J, Gao C. RNAi functionalized collagen-chitosan/silicone membrane bilayer dermal equivalent for full-thickness skin regeneration with inhibited scarring. Biomaterials 2013;34:2038–2048 [DOI] [PubMed] [Google Scholar]
- 20.Ho YP, Grigsby CL, Zhao F, Leong KW. Tuning physical properties of nanocomplexes through microfluidics-assisted confinement. Nano Lett 2011;11:2178–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galiano RD, Tepper OM, Pelo CR, et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 2004;164:1935–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanft JR, Pollak RA, Barbul A, et al. Phase I trial on the safety of topical rhVEGF on chronic neuropathic diabetic foot ulcers. J Wound Care 2008;17:30–32, 34–37 [DOI] [PubMed] [Google Scholar]
- 23.Smiell JM, Wieman TJ, Steed DL, Perry BH, Sampson AR, Schwab BH. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regeneration 1999;7:335–346 [DOI] [PubMed] [Google Scholar]
- 24.Rees RS, Robson MC, Smiell JM, Perry BH. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study. Wound Repair Regen 1999;7:141–147 [DOI] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. Regranex (beclapermin) Postmarket Drug Safety Information for Patients and Providers. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHealthcareProfessionals/ucm072121.htm 2008. (accessed August28, 2014)
- 26.Senet P, Vicaut E, Beneton N, Debure C, Lok C, Chosidow O. Topical treatment of hypertensive leg ulcers with platelet-derived growth factor-BB: a randomized controlled trial. Arch Dermatol 2011;147:926–930 [DOI] [PubMed] [Google Scholar]
- 27.McCarty SM, Percival SL. Proteases and delayed wound healing. Adv Wound Care 2013;2:438–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 2011;9:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rennert RC, Rodrigues M, Wong VW, et al. Biological therapies for the treatment of cutaneous wounds: phase III and launched therapies. Expert Opin Biol Ther 2013;13:1523–1541 [DOI] [PubMed] [Google Scholar]
- 30.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A 2012;18:806–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NIH: Phase I, Arteriocyte Magellan MAR01 Therapy—Compartment Syndrome and Battlefield Trauma. http://clinicaltrials.gov/ct2/show/NCT01837264?term=magellan&rank=1 (last accessed September22, 2014)
- 32.Ekenseair AK, Kasper FK, Mikos AG. Perspectives on the interface of drug delivery and tissue engineering. Adv Drug Deliv Rev 2013;65:89–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci 2013;70:2059–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamai K, Semenov M, Kato Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000;407:530–535 [DOI] [PubMed] [Google Scholar]
- 35.Thorne CA, Hanson AJ, Schneider J, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol 2010;6:829–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One 2010;5:e15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev 1998;8:588–594 [DOI] [PubMed] [Google Scholar]
- 38.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468–472 [DOI] [PubMed] [Google Scholar]
- 39.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature medicine 2004;10:858–864 [DOI] [PubMed] [Google Scholar]
- 40.Wetterau M, George F, Weinstein A, et al. Topical prolyl hydroxylase domain-2 silencing improves diabetic murine wound closure. Wound Repair Regen 2011;19:481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest 2007;117:1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Botusan IR, Sunkari VG, Savu O, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A 2008;105:19426–19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavrilov K, Saltzman WM. Therapeutic siRNA: principles, challenges, and strategies. Yale J Biol Med 2012;85:187–200 [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Z, Wang Z, Shi Y, et al. Modulation of collagen synthesis in keloid fibroblasts by silencing Smad2 with siRNA. Plastic Reconstr Surg 2006;118:1328–1337 [DOI] [PubMed] [Google Scholar]
- 45.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater 2013;12:967–977 [DOI] [PubMed] [Google Scholar]
- 46.Gary DJ, Puri N, Won YY. Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release 2007;121:64–73 [DOI] [PubMed] [Google Scholar]
- 47.Song E, Zhu P, Lee SK, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol 2005;23:709–717 [DOI] [PubMed] [Google Scholar]
- 48.Dykxhoorn DM, Palliser D, Lieberman J. The silent treatment: siRNAs as small molecule drugs. Gene Ther 2006;13:541–552 [DOI] [PubMed] [Google Scholar]
- 49.Krebs MD, Jeon O, Alsberg E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J Am Chem Soc 2009;131:9204–9206 [DOI] [PubMed] [Google Scholar]
- 50.Ma Z, Yang C, Song W, Wang Q, Kjems J, Gao S. Chitosan hydrogel as siRNA vector for prolonged gene silencing. J Nanobiotechnol 2014;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]