Abstract
Endothelial progenitor cells (EPCs) have the ability to form new blood vessels and protect ischemic tissues from damage. We previously reported that EPCs with low activity of aldehyde dehydrogenase (Alde-Low EPCs) possess the greater ability to treat ischemic tissues compared with Alde-High EPCs. The expression level of the hypoxia-inducible factors (HIFs), HIF-1α and HIF-2α, was found to be greater in Alde-Low EPCs than in Alde-High EPCs. However, the precise role of the HIF factors in the regulation of EPC activity remains obscure. In this study, we demonstrate a critical role of HIF-2α and its target gene CXCR4 for controlling the migratory activity of EPC to ischemic tissue. We found that coculture of Alde-High EPCs with microvesicles derived from Alde-Low EPCs improved their ability to repair an ischemic skin flap, and the expression of CXCR4 and its ligand SDF1 was significantly increased following the coculture. In Alde-Low EPCs, the expression of CXCR4 was suppressed by short hairpin RNA (shRNA)-mediated HIF-2α, but not HIF-1α downregulation. Chromatin immunoprecipitation assays showed that HIF-2α, but not HIF-1α, binds to the promoter region of CXCR4 gene. The CXCR4 shRNA treatment in Alde-Low EPCs almost completely abrogated their migratory activity to ischemic tissues, whereas the reduction of vascular endothelial growth factor (VEGF) showed much less effect. The CXCR4 overexpression in Alde-High EPCs resulted in a partial, but significant improvement in their repairing ability in an ischemic skin flap. Collectively, these findings indicate that the CXCR4/SDF-1 axis, which is specifically regulated by HIF-2α, plays a crucial role in the regulation of EPC migration to ischemic tissues.
Introduction
Endothelial progenitor cells (EPCs) were originally identified as a population of stem cells in human peripheral blood and are characterized by the expression of CD34, VEGFR-2, and CD133 markers [1]. EPCs circulate in the blood and possess the ability to differentiate into mature vascular endothelial cells (ECs), contribute to new vessels, and to aid in the regeneration of impaired blood vessels [2].
It has been suggested that bone marrow-derived EPCs localize within the site of EC damage and induce revascularization [3]. Indeed, after EPCs pass from bone marrow to peripheral circulation, they migrate to the sites at which endothelial injury and a hypoxic state occur within the tissue. Once there, EPCs promote angiogenesis with or without directly contributing to the formation of vessels in response to the physiologically distinct environment [4,5].
The mechanisms by which EPCs are able to induce recovery in damaged vessels and tissues are not fully understood. Several studies have demonstrated that EPC infusion exerts protective effects on hindlimb ischemia, myocardial infarction, and glomerular diseases in animal models [5]. Despite these benefits, the regenerative effects of EPC-based cell therapy remain to be clarified: whether EPCs directly contribute to and are incorporated in revascularization [6,7] or whether they favor reciprocal interaction due to a paracrine mechanism that occurs within ischemic tissues [8]. Recently, many studies have suggested that cells may also communicate through circular membrane fragments named microvesicles (MVs) [9]. It has been hypothesized that MVs released from EPCs may play an important role in the cell-to-cell communication of cytokines, growth factors, surface receptors, and nucleotides [10,11].
We previously demonstrated that aldehyde dehydrogenase 1 (ALDH1) activity was a useful marker for separating functional EPCs from the whole endothelial colony-forming cell population [12,13]. Alde-Low EPCs have been shown to promote significantly better wound healing in a mouse model of skin flap ischemia [12]. This improved wound healing was not observed with human umbilical vein endothelial cells (HUVECs) or Alde-High EPCs. Alde-Low EPCs were also found to grow faster, have a greater capacity to migrate to ischemic sites, and to be directly involved in new vessel formation. Moreover, the expression of hypoxia-inducible factor (HIF)-1α and -2α, under hypoxic conditions, was elevated in Alde-Low EPCs in comparison to Alde-High EPCs [12]. Among the HIF-targeting genes, we also found that under hypoxic conditions, the messenger RNA (mRNA) expression levels of vascular endothelial growth factor (VEGF) and CXCR4 were upregulated in Alde-Low EPCs in comparison to Alde-High EPCs.
The aim of the present study was to investigate how functional EPCs (Alde-Low EPCs) contribute to ischemic tissue repair and to clarify the key molecules that are involved in the recovery from tissue damage. In the present study, we investigated the crucial factors that are highly involved in ischemic tissue repair using two different types of EPCs such as Alde-Low and Alde-High EPCs. While the introduction of Alde-High EPC MVs derived from Alde-Low EPCs resulted in the full recovery of the ischemic tissue in the mouse model—a result which was similar to that observed with Alde-Low EPCs—the ischemic tissue was only partially repaired in the mice that were treated with Alde-High EPCs with CXCR4. These data demonstrate that CXCR4 has an important role in EPC migration at the ischemic site and that other factors might be involved in the tissue repair process.
Materials and Methods
The preparation of EPCs isolated by ALDH activity
Human full-term umbilical cord blood (UCB) samples were collected from umbilical cord veins with the permission of the local ethics authorities at the University of Tsukuba. Mononuclear cells from human UCB were separated by density gradient centrifugation after the depletion of hematopoietic cells. CD45−/CD31+ cells were then sorted using anti-CD45 and anti-CD31 antibodies (BioLegend) with a MoFlo (MoFlo XDP; Beckman Coulter). Cells were plated on a 25-cm2flask (Sumitomo Bakelite) with Iscove's modified Dulbecco's medium (IMDM; Life Technologies) with 10% fetal bovine serum (FBS; Life Technologies), 2 mg/mL l-glutamine (Life Technologies), 10 ng/mL hb-FGF (PeproTech), and 0.1% (vol/vol) penicillin–streptomycin (100 U/mL penicillin, 0.1 mg/mL streptomycin; Life Technologies) as described previously [12]. After more than 7 days, adherent cells began to grow and rapidly evolve to form colonies with tightly compact morphologies. Subsequently, DiI-Ac-LDL+/CD31+ cells were sorted (DiI-Ac-LDL; Molecular Probes) with a MoFlo. The culture of the sorted cells was continued in IMDM/10% FBS supplemented with b-FGF [12]. These cells displayed an EC-like morphology. Their ALDH activity was analyzed with an ALDEFLUOR ® (StemCell Technologies). The EPCs were then sorted into two fractions based on their level of ALDH expression (Alde-High EPCs and Alde-Low EPCs) using the MoFLo. The EPCs were labeled with the lentivirus-green fluorescent protein (GFP) (Sigma-Aldrich). Frozen cell stocks were prepared using the CELLBANKER (ZENOAQ) solution and stored in liquid nitrogen.
For hypoxic stimuli, EPCs were treated with or without desferrioxamine (DFO: 100 μM) for 6 h or cultured under hypoxic conditions (1% O2) for 6 h.
The preparation of MVs
The medium of the subconfluent EPCs was changed to EBM-2 (Lonza) with 0.25% bovine serum albumin (Sigma). After 12 h, the supernatants were collected and centrifuged at 1,000g for 20 min. Cell-free supernatants were ultracentrifuged at 100,000g for 60 min at 4°C. MV pellets were washed with phosphate-buffered saline (PBS) and recentrifuged at 100,000g for 60 min at 4°C. A MoFLO was used to purify and isolate MVs, which have pore size of 100 − 200 μm. The isolated MVs were then transfected to EPCs (1 × 104 cells). The collected MVs were stained with PKH26 Red Fluorescent dye (Sigma) and the effects of transfection were evaluated.
Quantitative RT-PCR
RNA was isolated from cultured cells or MV samples using an RNeasy Mini Kit (Qiagen). Total RNA (1 μg) was reverse transcribed using an RT-PCR Kit (TOYOBO). Complementary DNA (cDNA) was analyzed using a GeneAmp 7500 Fast Real-Time PCR System (Life Technologies) using the SYBR Green reagent (TOYOBO). The expression levels of the target genes were analyzed with the ΔΔCt method. The primers used for the polymerase chain reaction (PCR) were as follows: VEGF (5′-AGATGAGCTTCCTACAGCACAAC; 3′-AGGACTTATACCGGGATTTCTTG), CXCR4 (5′-CTGTGACCGCTTCTACCCCAATGACTT; 3′-CCAAGGAAAGCATAGAGGATGGGGTTC), KDR (5′-AGTGTGGAGGACTTCCAGGGAGGAAAT; 3′-GGCCAAGCTTGTACCATGTGAGGTTCT), SDF-1 (5′-TGAGAGCTCGCTTTGAGTGA; 3′-CACCAGGACCTTCTGTGGAT), VCAM-1 (5′-GTAAGCTGCAAGGTTCCTAGCGTGT; 3′-GCTGACCAAGACGGTTGTATCTCTG), Glut-1 (5′-ACTGCTCAAGAAGACATGGAGAC; 3′-ATTTACAAGTTGGCTTGTCCAGA), TGF-β (5′-AGAGCTCCGAGAAGCGGTACCTGAACCC; 3′-GTTGATGTCCACTTGCAGTGTGTTATCC), HIF-1α (5′-TTACCGAATTGATGGGATATGAG; 3′-TCATGATGAGTTTTGGTCAGATG), Col IV (5′-AGGGCCAGCCTGGCCTGCCAGGACTTCC; 3′-TCACCCTTAGAGCCTGTGATTCCTGGAG), and β-actin (5′-GTGCGTGACATTAAGGAGAAGCTGTGC; 3′-GTACTTGCGCTCAGGAGGAGCAATGAT).
Animal studies
C57BL/6J mice were purchased from Japan SLC, Inc. The mice were treated in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All animal studies were performed after receiving approval from the Institutional Animal Experimental Ethics Committee of the University of Tsukuba.
The mice were anesthetized with Avertin and the back skin was shaved. To create the ischemic region, a rectangular-shaped incision (3 × 2 cm) was made on the dorsal skin with only the proximal end remaining attached to the circulation, thus creating a proximal to distal ischemic gradient [6]. Immunosuppression was induced by an intraperitoneal injection of cyclosporin-A (20 mg/kg/day) (Wako) from 2 days before the assay [7]. Following surgery, GFP-labeled EPCs (5 × 105 cells/mouse) were injected into the tail vein. The results are expressed as the average area of necrotic skin as a percentage of the total flap area 7 days after the surgery/injection. An in vivo migration assay was carried out 24 h after the EPC injection. To detect GFP-labeled EPCs, frozen flap skin tissue sections were analyzed by fluorescence microscopy. The results are expressed as the average number of cells that were seen as per high-power field.
Western blotting
Sample cells, which were treated with or without DFO (100 μM) for 6 h, were harvested and suspended in low salt buffer [10 mM HEPES, 10 mM KCl, 1 mM dithiothreitol, 0.1 mM EDTA, protease inhibitor cocktail (PIC; Roche Diagnostics), and 1% Nonidet P-40] and nuclear pellets were collected by centrifugation. The nuclear pellets were suspended in high salt buffer (20 mM HEPES, 400 mM NaCl, 1 mM dithiothreitol, and 1 mM EDTA, PIC); after centrifugation, a nuclear extract was obtained. These nuclear extract samples were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel by electrophoresis and transferred onto PVDF membrane (Millipore). An immunoblotting analysis was performed as previously described [12]. Anti-human HIF-2α antibody [14], anti-human HIF-1α antibody (Novus Biologicals), and anti-Lamin B antibody (Santa Cruz Biotechnology, Inc.) were used for the immunoblotting assay. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Invitrogen) or goat anti-mouse IgG (Life Technologies) was used as the secondary antibody, and enhanced chemiluminescence (GE Healthcare Biosciences) was used for detection.
Overexpression and the shRNA treatment of target genes
The Alde-High EPCs were infected using cell-free retroviral supernatant from PT67 packaging cells (Clontech) producing MSCV-hCXCR4-IRES-EGFP or MSCV-IRES-EGFP (control virus) with 8 mg/mL polybrene. After 24 h, the medium was changed to fresh virus-free medium, and cells were expanded for 4–6 days. The GFP-positive cells were then sorted using a MoFlo and then expanded for the subsequent experiments. MSCV-hVEGF-PGK-puro retroviruses were used to promote VEGF overexpression in Alde-High EPCs. Puromycin (2 μg/mL) was used to select infected EPCs.
To downregulate the target genes, we used the shRNA MISSION lentiviral transduction system (Sigma-Aldrich) according to the manufacturer's protocol. The infected cells were selected with a puromycin resistance method. Puromycin (2 μg/mL) was used to select the infected EPCs.
Chromatin immunoprecipitation assay
For each assay, 5 × 106 Alde-Low EPCs were fixed with 1% formaldehyde for 10 min at room temperature. After being washed with PBS containing 1 μM PIC (Roche Diagnostics), the EPCs were treated with hypotonic solution (5 mM HEPES, 85 mM KCl, 0.5% NP-40, and 1 μM PIC). Nuclei were collected after centrifugation at 14,000g for 5 min, then lysed with a lysis buffer (50 mM Tris-HCl, [pH 8.1], 10 mM EDTA, 1% SDS, and 1 μM PIC). After the fragmentation of DNA by sonication, immunoprecipitation reactions were performed using a rotating mixer at 4°C with 1 μg/mL anti-HIF-1α antibody or anti-HIF-2α antibody (Novus Biologicals). Normal mouse IgG or rabbit IgG was used as a negative control to verify the specificity of the reaction. After incubation with the antibody, reaction mixtures were incubated with preblocked protein A agarose beads (Calbiochem) at 4°C for 1 h and the precipitated complexes were collected by centrifugation at 3,000g for 5 min. These complexes were washed three times with the wash buffer (0.25 mM LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, and 10 mM Tris-HCl [pH 8.1]) and eluted from the protein A agarose beads with the elution buffer (1% SDS and 0.1 M NaHCO3). DNA–protein complexes were denatured by incubation at 65°C for 4 h. DNA fragments were purified with phenol/chloroform, and resuspended in the TE buffer. The following primers were used for the PCR: forward, 5′-TTCTTCAACCTAATTTCTGATTCGTGC-3′ and reverse, 5′-ATCACTAGGAACTTGCACAGAATCAC-3′.
In vitro migration assay
EPCs (5 × 104 cells) were seeded onto transwells (6.5 mm, 8 μm pore; BD Biosciences) in IMDM supplemented with 0.5% FBS. IMDM/0.5% FBS with recombinant human SDF-1 (200 ng/mL; R&D Systems) was added to the lower chamber. The assays were performed under different oxygen tensions (20% or 5%) for 6 h, and nonmigrated cells were wiped away from the top surface of the membrane. Cells that adhered to the undersurface of the membrane were stained with Diff-Quik staining solution (International Reagents) and then counted using an inverted microscope.
Statistical analysis
Data were statistically analyzed using Student's t-test or a one-way analysis of variance as appropriate. Data are presented as the mean ± standard deviation.
Results
Alde-High EPCs with MVs derived from Alde-Low EPCs possess the ability to repair wounds
Alde-High EPCs and Alde-Low EPCs were derived from the same UCB samples and separated with fluorescence-activated cell sorting on the basis of ALDH activity, as previously reported. To characterize the cell types, we investigated whether Alde-High EPCs could attain similar characteristics to Alde-Low EPCs through exterior influences.
MVs are released from the cell surface and are able to transfer mRNAs and microRNAs between cells. The release of MVs from the cell surface increases cell growth activity, reduces apoptosis, and protects from cellular injury [15,16].
We first isolated MVs derived from Alde-Low EPCs and transfected them into Alde-High EPCs. In contrast to Alde-Low EPCs, Alde-High EPCs have not been shown to promote wound healing.
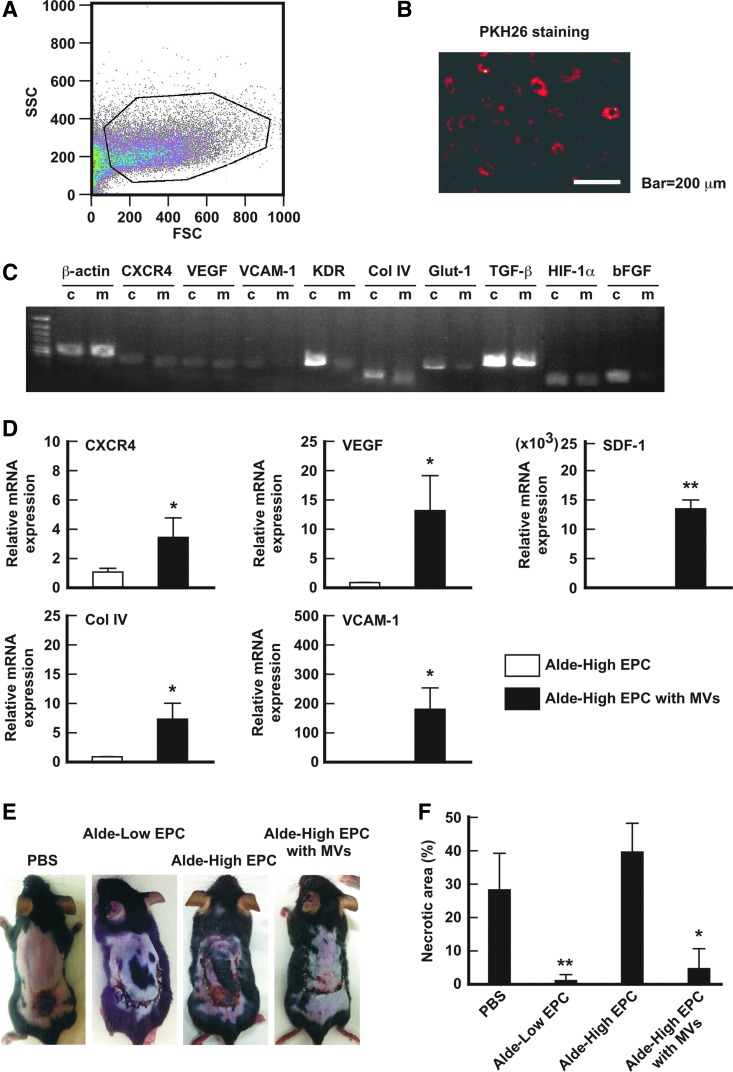
The isolation of MVs from Alde-Low EPCs was performed with reference to previous reports and evaluated by examining the expression of angiogenic genes in the MVs. As shown in Fig. 1C, PKH26-labeled MVs, which were isolated from Alde-Low EPCs, were incorporated in Alde-High EPCs by coculture for 12 h. The expression of MVs in relation to the angiogenic genes was analyzed; most of the examined genes were positively expressed similar to those in their parent cells (Alde-Low EPCs) (Fig. 1B).
FIG. 1.
MVs derived from Alde-Low EPCs can improve the angiogenic activity of Alde-High EPCs. (A) MVs derived from Alde-Low EPCs were isolated by FACS after centrifugation. (B) MVs derived from Alde-Low EPCs were stained with PKH-26 (red fluorescent) and transfected to cells. After 24 h, the cells were observed under fluorescence microscope. Scale bar: 200 μm. (C) The analysis of mRNA levels of the indicated genes in MVs and parental cells by an RT-PCR. c, Alde-Low EPCs; m, MVs derived from Alde-Low EPCs. (D) CXCR4, VEGF, SDF1, ColIV, and VCAM-1 mRNA expression were analyzed in Alde-High EPCs that were transfected with MVs (white bar) in comparison to Alde-High EPCs (black bar) under normoxic conditions. The expression level detected in Alde-High EPCs was normalized to a value of 1 as the standard for each factor. *P < 0.05, **P < 0.01. (E) Skin incisions were created on the dorsal skin of mice. PBS alone, Alde-Low EPCs, Alde-High EPCs, or Alde-High EPCs with MVs were injected to the mice. The effects of the EPCs in the recovery from ischemia were analyzed on day 7 after surgery. (F) The necrotic regions in the four different types of mice (n = 3 in each) were measured. Note that the area of necrosis in the mice that were injected with Alde-High EPCs with MVs was similar to that in mice that were injected with Alde-Low EPCs. *P < 0.05, **P < 0.01. EPCs, endothelial progenitor cells; FACS, fluorescence-activated cell sorting; mRNA, messenger RNA; MVs, microvesicles; PBS, phosphate-buffered saline; RT-PCR, reverse transcription-polymerase chain reaction; VEGF, vascular endothelial growth factor. Color images available online at www.liebertpub.com/scd
We then examined the expression of angiogenic genes in Alde-High EPCs that were cocultured with MVs derived from Alde-Low EPCs. The expression of the CXCR4 chemokine receptor, which is important for migration at the wound site, and its ligand SDF-1, were found to be highly elevated in the transfected Alde-High EPCs.
Angiogenic factor, VEGF, and adhesion molecules, Col IV and VCAM-1, were also upregulated in the transfected Alde-High EPCs in comparison to the nontransfected Alde-High EPCs. An in vivo assay using a mouse model of skin flap ischemia clearly demonstrated that transfected Alde-High EPCs had a wound repair ability that was similar to that of Alde-Low EPCs (necrotic area: PBS, 40.6% ± 8.2%; Alde-Low EPCs, 1.3% ± 2.3%; Alde-High EPCs, 39.0% ± 8.9%; Alde-High EPCs with MVs, 4.8% ± 6.3%; Fig. 1E, F). Interestingly, HUVEC-transfected Alde-Low EPC-derived MVs showed less recovery from necrosis than the transfected Alde-High EPCs (data not shown).
Taken together, these results indicate that Alde-High EPCs possess a similar phenotype to Alde-Low EPCs from the point of the functional characteristics of EPCs in relation to neovascularization, suggesting that Alde-High EPCs would be a useful tool for investigating the molecular mechanisms underlying the angiogenic properties of Alde-Low EPCs.
HIF-2α, but not HIF-1α is a crucial factor for the expression of CXCR4 in EPCs
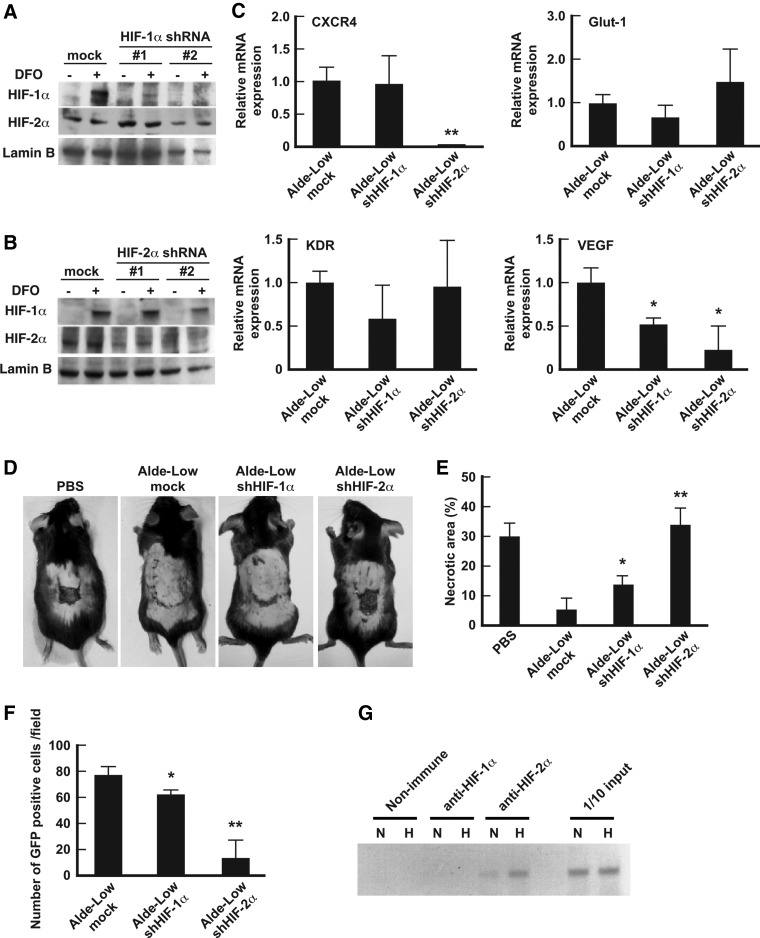
We previously found that under hypoxic conditions the expression of HIF-1α and HIF-2α were highly upregulated in Alde-Low EPCs in comparison to Alde-High EPCs [12]. To investigate the differences in the roles of these HIF factors in EPCs, the shRNAs of the HIFs were used to differentially decrease their expression. A western blotting analysis clearly showed that HIF-1α and HIF-2α were restrained by their respective shRNAs at levels of below 20% (Fig. 2A, B). We subsequently examined the expression levels of the target genes of HIF-1α and HIF-2α in the HIF shRNA-treated cells after exposure to 1% O2 for 6 h (Fig. 2C). VEGF mRNA expression was suppressed by both HIF-1α shRNA and HIF-2α shRNA [Alde-Low EPCs with HIF-1α shRNA, 0.51 ± 0.11-fold decrease, P < 0.05 (n = 3); Alde-Low EPC with HIF-2α shRNA, 0.23 ± 0.28-fold decrease, P < 0.05 (n = 3)]. Of note, CXCR4 mRNA expression was suppressed by HIF-2α shRNA, but not by HIF-1α shRNA [0.06 ± 0.03-fold decrease, P < 0.01 (n = 3)]. Collectively, the results indicate that HIF targeting genes are regulated differently by HIFs in EPCs and that further experiments are required to elucidate the mechanisms that underlie the differences in their regulation in EPCs.
FIG. 2.
The analysis of the involvement of HIF-1α and HIF-2α in the repair of ischemic tissue. (A, B) The protein expression levels of HIF-1α (A) and HIF-2α (B) were analyzed by western blotting in mock Alde-Low EPCs (control), Alde-Low EPC transfected with shHIF-1α RNA, or Alde-Low EPC transfected with shHIF-2α RNA. The control cells and the two types of transfected cells (#1 and #2) were treated with (+) or without (−) 100 μM DFO for 6 h before the analysis. (C) The mRNA expression level of the indicated genes in the control cells, the Alde-Low EPCs with HIF-1α shRNA, and the Alde-Low EPCs with HIF-2α shRNA were measured using an RT-PCR. Note that the CXCR4 expression was downregulated by HIF-2α shRNA, but not by HIF-1α shRNA. *P < 0.05, **P < 0.01. (D) Skin incisions were created on the dorsal skin of mice. PBS only, mock Alde-Low EPCs, Alde-Low EPCs with HIF-1α shRNA, or Alde-Low EPCs with HIF-2α shRNA were injected into the mice. The effects of the EPCs on the repair of ischemic tissue were analyzed on day 7 after surgery. (E) The necrotic regions in the four types of mice (n = 3 in each) were measured. Note that in the ability to repair ischemic tissue was lost in mice that were injected with Alde-Low EPCs with HIF2α shRNA. *P < 0.05, ** P < 0.01. (F) The number of migrated GFP+ cells was measured. Note that the number of GFP+ cells at the site of skin flap was decreased in the mice that were injected with Alde-Low EPCs with HIF-2α shRNA. *P < 0.05, **P < 0.01. (G) A ChIP assay was performed to examine binding to the hypoxia response element (HRE) region of the CXCR4 promoter under normoxic (N) and hypoxic conditions (H). Note that HIF-2α, but not HIF-1α binds to CXCR4 promoter under both conditions. ChIP, chromatin immunoprecipitation; DFO, desferrioxamine; GFP, green fluorescent protein; HIF, hypoxia-inducible factor; shRNA, short hairpin RNA.
To investigate how HIFs are involved in the functional role of Alde-Low EPCs in vivo, we analyzed our mouse model of skin flap ischemia. Seven days after the injection of Alde-Low EPCs into the tail vein, there was a reduction in the size of the necrotic area (2.5% ± 5.3%, Fig. 2D, E). However, Alde-Low EPCs with HIF-1α shRNA showed a reduced ability to repair the necrotic area [13.2% ± 2.81%, P < 0.05 (n = 3) vs. control Alde-Low EPC]. Remarkably, when Alde-Low EPCs with HIF-2α shRNA were injected, wound repair was significantly impaired and large necrotic areas were observed [34.1% ± 5.15%, P < 0.01 (n = 4) vs. control Alde-Low EPCs]. Further supporting this finding, a significantly lower number of GFP-labeled HIF-2α shRNA Alde-Low EPCs was detected at the site of the skin flap in comparison to mice that were injected with HIF-1α shRNA Alde-Low EPCs [control Alde-Low EPCs, 76.8 ± 5.9; Alde-Low EPCs with HIF-1α shRNA, 61.2 ± 4.6, P < 0.05 (n = 3); Alde-Low EPCs with HIF-2α shRNA, 13.5 ± 14.6 cells per field, P < 0.01 (n = 3), Fig. 2F]. These results strongly suggest that CXCR4 is a crucial factor for cell migration and that in EPCs, the expression of CXCR4 is regulated by HIF-2α. Finally, we examined how the expression of CXCR4s is regulated in EPCs by HIF-2α using a chromatin immunoprecipitation (ChIP) assay. The ChIP assay clearly demonstrated that HIF-2α, but not HIF-1α binds to CXCR4 promoter region, indicating that HIF-2α is a key factor in the regulation of CXCR4 expression in EPCs (Fig. 2G). HIF-1α may also be involved in the migratory activity of EPCs; however, the effect was less than that of HIF-2α, which suggests the possible existence of a separate mechanism that is related to EPC migration from the CXCR4/SDF-1 axis.
CXCR4 is a crucial factor in the migration of EPCs
We found that CXCR4 expression is profoundly regulated by HIF-2α, whereas VEGF expression is regulated by both HIF-1α and HIF-2α (Fig. 3). We next investigated the functional associations of CXCR4 and VEGF with the repair of ischemic tissue in our mouse model of skin flap ischemia. Alde-Low EPCs with CXCR4 shRNA or VEGF shRNA were prepared and used to examine the involvement of these genes in EPC migration.
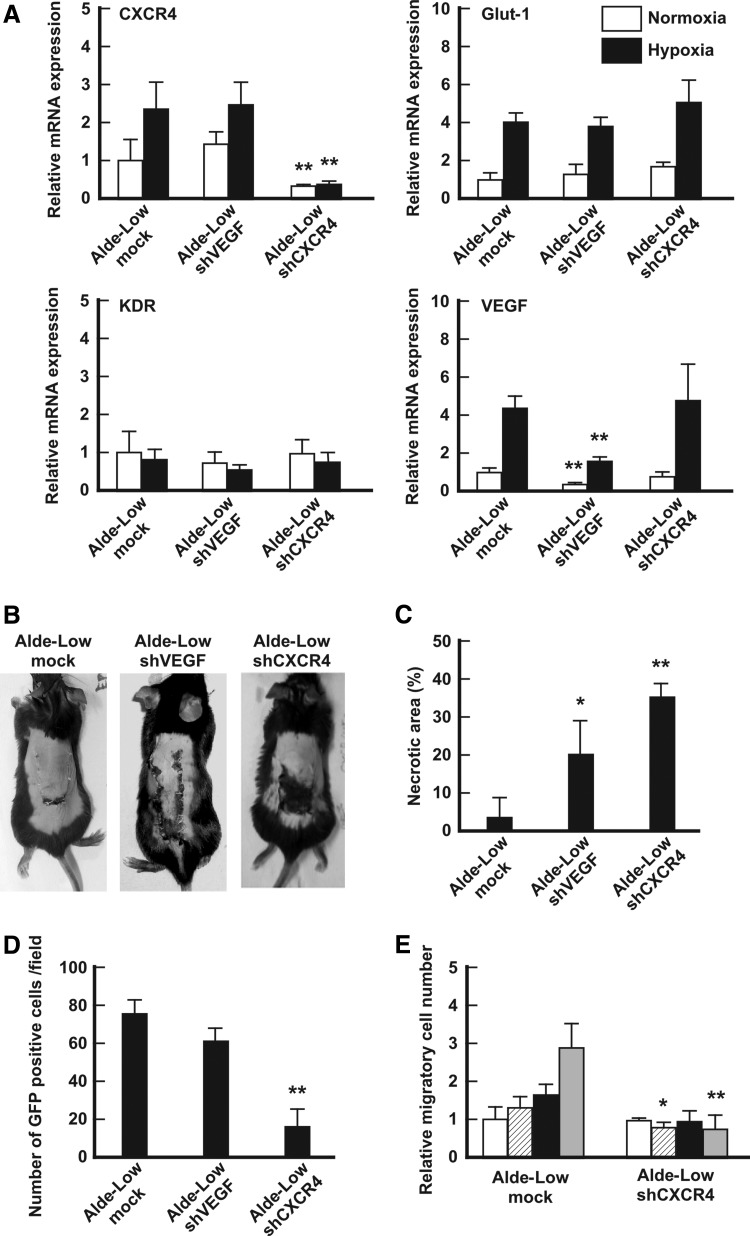
FIG. 3.
Analyses of the involvement of CXCR4 and VEGF in the repair of ischemic tissues. (A) The mRNA expression levels of the indicated genes in mock Alde-Low EPCs, Alde-Low EPCs with VEGF shRNA, or Alde-Low EPCs with CXCR4 shRNA were analyzed by a qPCR [white bar: normoxic conditions; black bar: hypoxic conditions (1% O2 for 6 h)]. The expression levels that were detected in the mock Alde-Low EPCs under normoxic conditions were normalized to a value of 1 as the standard for each gene. **P < 0.01. (B) Skin incisions were created on the dorsal skin of mice. Mock Alde-Low EPCs, Alde-Low EPCs with VEGF shRNA, or Alde-Low EPCs with CXCR4 shRNA were injected into the mice. The effect of EPCs on the repair of ischemic tissue was analyzed on day 7 after surgery. (C) The necrotic regions in the mice that were injected with three different types of EPCs (n = 3 in each) were measured. *P < 0.05, **P < 0.01. (D) The number of migrated GFP-labeled Alde-Low EPCs at the site of the skin flap was counted. **P < 0.01. (E) An in vitro transwell culture assay was performed to analyze migration. The migratory ability of Alde-Low EPCs with CXCR4 shRNA was analyzed and compared with that of the mock Alde-Low EPCs in the presence of SDF-1. White bar, 20% O2 without SDF-1; white striped bar, 20% O2 with SDF-1; black solid bar, 5% O2 without SDF-1; gray bar, 5% O2 with SDF-1. *P < 0.05, **P < 0.01. qPCR, quantitative PCR.
CXCR4 mRNA expression in Alde-Low EPCs with CXCR4 shRNA showed a 0.25 ± 0.01-fold decrease [P < 0.01 (n = 3)] in comparison to the control under normoxic conditions [1.00 ± 0.59-fold (n = 3)] and a 0.33 ± 0.01-fold decrease [P < 0.01 (n = 3)] under hypoxic conditions [control: 2.39 ± 0.43-fold (n = 3)]. On the other hand, VEGF mRNA expression in Alde-Low EPCs with VEGF shRNA showed a 0.37 ± 0.09-fold decrease [P < 0.01 (n = 3)] in comparison to the control under normoxic conditions [control: 1.00 ± 0.30-fold, (n = 3)] and a 1.55 ± 0.18-fold decrease [P < 0.01 (n = 3)] under hypoxic conditions [control: 3.82 ± 0.37-fold, (n = 3)] (Fig. 3A). The expression levels of the other HIF target genes (Glut-1 and KDR) in the Alde-Low EPCs with these CXCR4/VEGF shRNAs did not differ from the control (data not shown).
We then investigated the involvement of these genes in the repair of ischemic tissue in our mouse model of skin flap ischemia. Alde-low EPCs that were transfected with CXCR4 shRNA totally lost their ability to repair the ischemic sites. In contrast, Alde-low EPCs that were transfected with VEGF shRNA showed a slight suppression in their ability to repair ischemic tissue [necrotic area: control Alde-Low EPCs, 3.0% ± 5.1%; Alde-Low EPCs with VEGF shRNA, 21.2% ± 9.1%; Alde-Low EPCs with CXCR4 shRNA, 35.5% ± 3.5% (n = 3 each)] (Fig. 3B, C).
CXCR4 is a chemokine receptor that is strongly associated with the migration of stem cells, including EPCs [16]. To analyze the involvement of CXCR4 in the migration of EPCs, we performed two assessments. First, the number of migrated cells at ischemic sites was assessed in response to Alde-Low EPCs with GFP transfected with each shRNA (Fig. 3D). We observed a 75% reduction in the number of GFP-positive cells following the injection of Alde-low EPCs with CXCR4 shRNA in comparison to the control [control Alde-Low EPCs, 76.8 ± 5.9 cells per field; Alde-Low EPCs with CXCR4 shRNA, 16.3 ± 10.3 cells per field, P < 0.01 (n = 3)]. When transfected with VEGF shRNA, the number of GFP-positive cells was not significantly different from that in the control Alde-Low EPCs [control Alde-Low EPCs, 76.8 ± 5.9 cells per field; Alde-Low EPC with VEGF shRNA, 61.1 ± 7.2 cells per field (n = 3)].
Second, we performed an in vitro cell migration assay, which clearly showed that the migratory ability of Alde-Low EPCs was suppressed under hypoxic conditions in the presence of SDF-1 in the EPCs that were transfected with CXCR4 shRNA in comparison to the control Alde-Low EPCs [control Alde-Low EPCs: 2.95 ± 0.57 vs. Alde-Low EPCs with CXCR4 shRNA: 0.81 ± 0.28-fold, P < 0.01 (n = 4); Fig. 2E].
Collectively, these data demonstrated that the expression of CXCR4 was a crucial factor in the successful repair of ischemic tissue by Alde-Low EPCs in this model. Taken together, the results indicate that the CXCR4/SDF-1 axis promoted the migration of EPCs to the ischemic tissue in our mouse model of skin flap ischemia.
The effects of CXCR4 and VEGF gene overexpression in Alde-High EPCs
We subsequently examined whether the capacity of Alde-High EPCs to regenerate ischemic tissue was improved after the transfection of CXCR4 or VEGF genes. As shown in Fig. 1, Alde-High EPCs are useful for determining the key molecules that play a functional role in Alde-Low EPCs in wound repair.
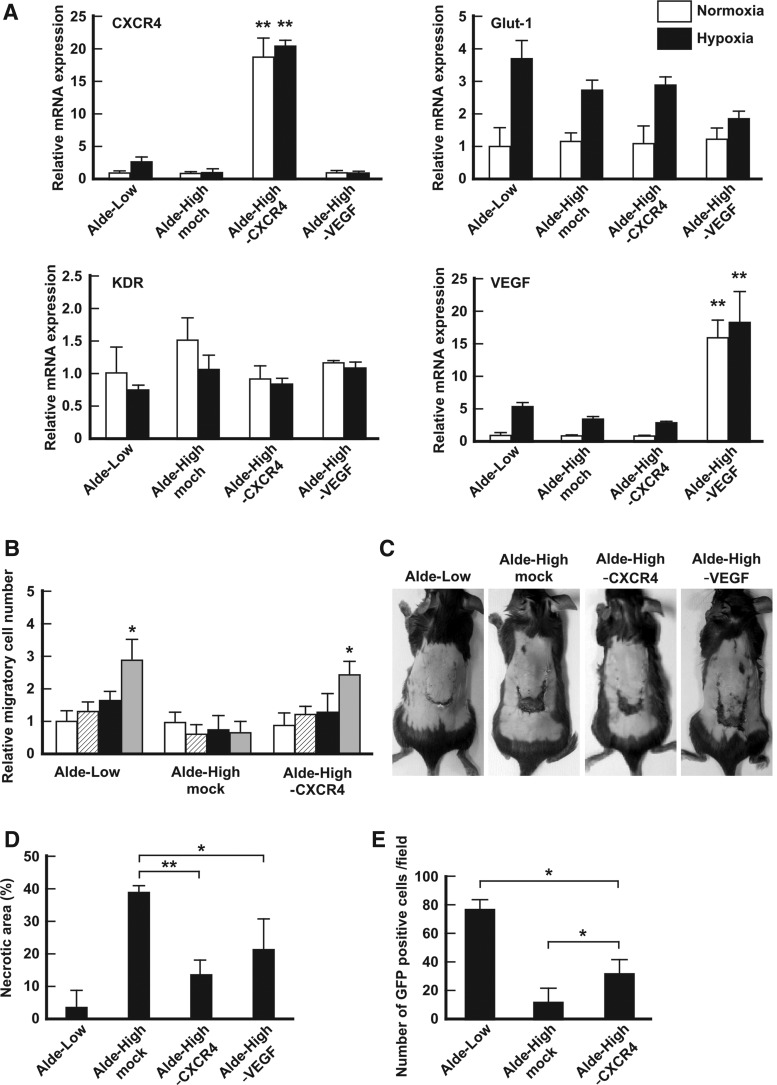
An reverse transcription-polymerase chain reaction (RT-PCR) clearly demonstrated the successful transfection of the target genes in Alde-High EPCs [CXCR4 overexpression: 18.6 ± 3.1-fold expression (n = 3); VEGF overexpression: 16.4 ± 2.0-fold expression (n = 3); Fig. 4A]. An in vitro migration assay demonstrated that Alde-High EPCs, which were transfected with CXCR4, had a migratory ability that was similar to that of Alde-Low EPCs in the presence of SDF-1 under 5% O2 conditions [Alde-Low EPCs, 2.95 ± 0.57-fold; Alde-High EPCs, 0.82 ± 0.18-fold; Alde-High EPCs with CXCR4 overexpression, 2.52 ± 0.41-fold (n = 3); Fig. 4B].
FIG. 4.
Alde-High EPCs with CXCR4 overexpression partially rescue ischemic tissue. (A) The mRNA expression levels of the indicated genes in mock Alde-Low EPCs (control), mock Alde-High EPCs (control), Alde-High EPC-CXCR4 overexpression, and Alde-High EPC-VEGF overexpression were analyzed using a qPCR [white bar: normoxic conditions; black bar: hypoxic conditions (1% O2 for 6 h)]. The expression level of the mock Alde-Low EPCs under normoxic conditions was normalized to a value of 1 as the standard for each gene. **P < 0.01. (B) The migration ability of EPCs was analyzed with an in vitro transwell culture assay. The migratory ability of Alde-Low-EPCs (positive control), mock Alde-High EPCs, or Alde-High EPCs with CXCR4 overexpression in the presence of SDF-1 was investigated. White bar, 20% O2 without SDF-1; striped bar, 20% O2 with SDF-1; black bar, 5% O2 without SDF-1; gray bar, 5% O2 with SDF-1. *P < 0.05. (C) Skin incisions were created on the dorsal skin. Alde-Low EPCs, mock Alde-High EPCs, Alde-High EPCs with CXCR4 overexpression, or Alde-Low EPCs with VEGF overexpression were injected into the mice. The effects of EPCs on the repair of ischemic tissue were analyzed on day 7 after surgery. (D) The necrotic regions in the four types of mice (n = 3, each) were measured. Note that the recovery of ischemic tissue was superior in mice that were injected with Alde-High EPCs with CXCR4 overexpression to that in mice that were injected with Alde-High EPCs. *P < 0.05, **P < 0.01. (E) The number of migrated cells at the site of the skin flap was measured. Note the difference in the number of migrated cells in the mice that were injected with Alde-High EPCs with CXCR4 overexpression and the mice that were injected with Alde-Low EPCs. *P < 0.05.
We then examined whether CXCR4 and VEGF-transfected Alde-High EPCs possessed the wound repair ability in our mouse model of skin flap ischemia. The sizes of the necrotic areas expressed as a percentage of the total flap area were as follows: 3.0% ± 5.1%, 38.9% ± 2.1% in Alde-High EPCs, 13.1% ± 5.1% in the Alde-High EPCs with CXCR4 gene transfection (P < 0.01 vs. Alde-High EPCs), and 21.4% ± 9.1% in the Alde-High EPCs with VEGF gene transfection (Fig 4C, D; n = 3). These results clearly indicate that even when CXCR4 is expressed in Alde-High EPCs, their capacity to repair the ischemic tissue was still inferior to that in Alde-Low EPCs.
When we analyzed the number of transfected cells that were present at the site of the skin flap, we found that there were significantly more CXCR4-expressing Alde-High EPCs than there were normal Alde-High EPCs (Fig. 3E). However, the number of migrated CXCR4-expressing Alde-High EPCs was still lower than the number of Alde-Low EPCs [Alde-Low EPCs, 76.8 ± 5.9; Alde-High EPC, 11.3 ± 10.9; CXCR4-expressing Alde-High EPCs, 31.8 ± 9.4 cells per field, P < 0.05 (n = 3); Fig 4E]. These results indicate that the migratory effect related to CXCR4 was not sufficient to achieve the repair of the ischemic site, suggesting that there may be other factors involved in the wound repair ability of Alde-Low EPCs.
Discussion
EPCs that are harvested according to their cell surface markers show a hierarchy that is related to their growth activity [17]. We previously demonstrated that EPCs could be further separated into Alde-Low EPCs and Alde-High EPCs based on their aldehyde dehydrogenase (Alde) activity [12]. The ability of Alde-Low EPCs to repair ischemic tissue is superior to that of Alde-High EPCs; however, the molecular mechanisms that prove these differences have not been elucidated. To characterize the differentiating states of each type of EPC, we transfected Alde-High EPC with MVs isolated from Alde-Low EPCs. As expected, the wounds in our mouse model of skin flap ischemia showed a full recovery after treatment with Alde-High EPCs that were transfected with MVs derived from Alde-Low EPCs. This finding suggests that the differentiating states of Alde-Low EPCs and Alde-High EPCs are highly similar and that the comparison of these EPCs would enable us to investigate the molecular mechanisms that underlie their association with recovery from ischemia.
It has been reported that the direct intravenous injection of MVs derived from EPCs can improve revascularization after ischemic damage in a mouse model of hindlimb ischemia [18]. We also injected MVs (derived from Alde-Low EPCs) into the tail vein in a mouse model of skin flap ischemia. After treatment, however, the necrotic area did not significantly differ to that in the untreated control mice (data not shown). We cannot rule out the possibility that MVs alone are ineffective in repairing ischemic tissue, possibly because of the distance of the injection site from the site of skin flap. Further detailed analyses will be necessary to confirm the effectiveness of MVs by using a different ischemic model or a different transplantation method. In addition, it is speculated that the molecular composition of MVs derived from EPCs might change according to the physiological state. Indeed, the production of MVs was found to be enhanced after appropriate stimulation: in EPCs, hypoxia enhanced the production of MVs carrying miR-126 and miR-296 [19]. In this study, we investigated the effects of MVs released from Alde-Low EPCs under normal culture conditions. The results observed with MVs derived from Alde-Low EPCs might have been different in association with neovessel formation or angiogenesis regulated by miR functions or others [20,21], if we harvested MVs from EPCs cultured under different conditions.
As described above, one of the key functions of EPCs in vivo is related to environmental stimuli, such as hypoxia. Under hypoxic conditions, EPCs, which are derived from the bone marrow, migrate through the peripheral blood to the sites of ischemia, where EPCs promote or directly contribute to the formation of new vessels [22]. Hypoxia-inducible factors, HIF-1α and HIF-2α, are known to respond to hypoxia, to form a heterodimer with Arnt and to transactivate the target genes [23]. It has been reported that these factors do not replenish each function, as shown by a knockout mouse study, and that the functional roles of these two factors are not fully understood [24]. In the present study, we found that HIF-1α and HIF-2α were both important in the transactivation of VEGF, whereas CXCR4 expression was profoundly regulated by HIF-2α in Alde-Low EPCs. Thus, the ability of Alde-Low EPCs that were transfected with HIF-2α shRNA to repair ischemic tissue was clearly reduced. Consequently, the size of the necrotic area was increased in comparison to the mice that were transfected with Alde-Low EPC with HIF-1α shRNA. In fact, it has been shown that the expression level of SDF-1 that is secreted at the site of the skin flap is dependent on a low concentration of oxygen [6]. We also found that a high level of SDF-1 was expressed in Alde-High EPC MVs derived from Alde-Low EPCs. It suggests that in addition to inflammatory cells, SDF-1 receptor, CXCR4-expressing EPCs would be the first candidate cells to be recruited to the ischemic sites in our mouse model of skin flap ischemia. Alternatively, the CXCR4/SDF-1 axis might have survival/antiapoptotic effects on EPCs that enhance their engrafting capability in an autocrine manner [6].
We previously found that Alde-Low EPCs were incorporated into newly formed neovessels, indicating that transplanted EPCs contribute to the revascularization of ischemic tissue at the site of the skin flap rather than through the promotion of neoangiogenesis. In the case of mesenchymal stem cells (MSCs), we reported that, in addition to Alde-Low EPCs, transplanted adipose tissue-derived MSCs (AT-MSCs) effectively repaired the perfusion of the blood flow in a mouse model of hindlimb ischemia, indicating that AT-MSC promoted neoangiogenesis through a paracrine mechanism [25]. The migrated EPCs can secrete SDF-1 themselves to recruit the additional migration of CXCR4-positive EPCs or inflammatory cells to the site of ischemic tissues, suggesting that transplanted and migrated EPCs may function through a paracrine mechanism.
Taken together, these results indicate that while both HIF-1α and HIF-2α have an important role in the recovery from ischemia, the role of HIF-2α in association with EPCs would be crucial during the process of ischemic tissue repair. Importantly, in the present study, we did not observe similar or increased numbers of migrated EPCs at the site of the ischemic skin flap, even when CXCR4 or VEGF were overexpressed in the Alde-High EPCs. Nevertheless, the ischemic tissue fully recovered after the transplantation of the control Alde-Low EPCs or the MV-transfected Alde-High EPCs. Deregibus et al. described the contribution of MV-derived antiapoptotic protein Bcl-xL in target ECs [10], and hypothesized that an antiapoptotic mechanism would be involved in the migration of EPCs and in their survival at the site of ischemia.
In conclusion, we proved the possible role of CXCR4 expressed on Alde-Low EPCs in the process of ischemic tissue repair. While CXCR4 is profoundly regulated by HIF-2α, another candidate gene, VEGF is regulated by HIF-1α and HIF-2α in Alde-Low EPCs. To specify the target molecules in each step of recovery from ischemia, further investigation will be needed to clarify the complicated mechanisms that underlie the tissue repair process. The precise analysis of the function of EPCs would provide new evidence that could be applied to stem cell therapy for patients with tissue ischemia of various etiologies.
Acknowledgment
This work was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA. and Finkel T. (2003). Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600 [DOI] [PubMed] [Google Scholar]
- 2.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME. and Gurtner GC. (2005). Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105:1068–1077 [DOI] [PubMed] [Google Scholar]
- 3.Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B. and Case J. (2009). Endothelial progenitor cells: identity defined? J Cell Mol Med 13:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang M, Wang B, Wang C, He B, Fan H, Guo TB, Shao Q, Gao L. and Liu Y. (2008). Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem 103:321–334 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T. and Kawamoto A. (2004). Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol 287:572–579 [DOI] [PubMed] [Google Scholar]
- 6.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP. and Gurtner GC. (2004). Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10:858–864 [DOI] [PubMed] [Google Scholar]
- 7.Pesce M, Orlandi A, Iachininoto MG, Straino S, Torella AR, Rizzuti V, Pompilio G, Bonanno G, Scambia G. and Capogrossi MC. (2003). Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res 93:e51–e62 [DOI] [PubMed] [Google Scholar]
- 8.Alev C, Ii M. and Asahara T. (2011). Endothelial progenitor cells: a novel tool for the therapy of ischemic diseases. Antioxid Redox Signal 15:949–965 [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A. and Ratajczak MZ. (2006). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20:1487–1495 [DOI] [PubMed] [Google Scholar]
- 10.Deregibus MC, Cantaluppi V, Calogero R, Iacono ML, Tetta C, Biancone L, Bruno S, Bussolati B. and Camussi G. (2007). Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110:2440–2448 [DOI] [PubMed] [Google Scholar]
- 11.Gu S, Zhang W, Chen J, Ma R, Xiao X, Ma X, Yao Z. and Chen Y. (2014). EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS One 9:e85396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagano M, Yamashita T, Hamada H, Ohneda K, Kimura K, Nakagawa T, Shibuya M, Yoshikawa H. and Ohneda O. (2007). Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood. Blood 110:151–160 [DOI] [PubMed] [Google Scholar]
- 13.Critser PJ, Kreger ST, Voytik-Harbin SL. and Yoder MC. (2010). Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res 80:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita M, Ohneda O, Yamashita T, Takahashi S, Suzuki N, Nakajima O, Kawauchi S, Ema M, Shibahara S, et al. (2003). HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J 22:1134–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 20:1053–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Zhu D, Huang L, Zhang J, Bian Z, Chen X, Liu Y, Zhang CY. and Zen K. (2012). Argonaute 2 complexes selectively protect the circulating microRNAs in cell-secreted microvesicles. PLoS One 7:e46957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D. and Yoder MC. (2004). Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104:2752–2760 [DOI] [PubMed] [Google Scholar]
- 18.Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, Deregibus MC, Tetta C, Segoloni GP. and Camussi G. (2012). Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol 25:75–85 [DOI] [PubMed] [Google Scholar]
- 19.Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, Tetta C. and Camussi G. (2012). Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82:412–427 [DOI] [PubMed] [Google Scholar]
- 20.Würdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, Weissleder R, Breakefield XO. and Krichevsky AM. (2008). miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell 14:382–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R. and Olson EN. (2008). The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell 15:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoenig MR, Bianchi C, Rosenzweig A. and Sellke FW. (2008). Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Curr Mol Med 8:754–767 [DOI] [PubMed] [Google Scholar]
- 23.Ohneda O, Nagano M. and Fujii-Kuriyama Y. (2007). Role of hypoxia-inducible factor-2alpha in endothelial development and hematopoiesis. Methods Enzymol 435:199–218 [DOI] [PubMed] [Google Scholar]
- 24.Ryan HE, Lo J. and Johnson RS. (1998). HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J 17:3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura K, Nagano M, Salazar G, Yamashita T, Tsuboi I, Mishima H, Matsushita S, Sato F, Yamagata K. and Ohneda O. (2014). The role of CCL5 in the ability of adipose tissue-derived mesenchymal stem cells to support repair of ischemic regions. Stem Cells Dev 23:488–501 [DOI] [PMC free article] [PubMed] [Google Scholar]