Abstract
Purpose: Brilacidin (BRI), a novel defensin mimetic, was evaluated as an ocular anti-infective.
Methods: In vitro: Potency based on MIC90s was compared for 50 Staphylococcus aureus (SA), 50 Staphylococcus epidermidis (SE), and 25 each of Streptococcus pneumonia (SP), Streptococcus viridans (SV), Moraxella (MS), Haemophilus influenzae (HI), Pseudomonas aeruginosa (PA), and Serratia marcescens (SM). In vivo: Using established methods, ocular toxicity was graded with Draize testing. For efficacy testing, both corneas of 24 rabbits were infected with methicillin-resistant S. aureus (MRSA), whereas the corneal epithelium was removed in the left eye. After 4 h, 21 topical drops over 5 h were administered to 4 groups: BRI 0.5%, vancomycin (VAN) 5%, saline, and no treatment. The eyes were clinically graded and the corneas were harvested for colony counts.
Results: In vitro: Both SA and SE had the lowest minimum inhibitory concentrations among the bacterial groups. The MIC90s to BRI for SP, SV, MS, HI, PA, and SM were 4, 32, 256, 32, 16, and 128-fold higher, respectively, than SA and SE. In vivo: Draize testing determined BRI 0.5% to be minimally irritating. For abraded corneas, BRI was not statistically different from VAN for reducing MRSA. BRI was bactericidal. For intact corneas, VAN reduced more CFU than BRI. BRI reduced CFU in abraded corneas more than intact corneas suggesting poor corneal penetration.
Conclusions: BRI has Gram-positive in vitro activity; topical BRI 0.5% was minimally irritating; and BRI 0.5% was equally efficacious as VAN in a MRSA keratitis model when the corneal epithelium was removed.
Introduction
Brilacidin (BRI) (formerly known as PMX30063, PolyMedix, Inc., Radnor, PA) is a novel anti-infective in a new class of defensin mimetics that is being developed for the treatment of eye infections.1 BRI is a nonpeptidic analogue that mimics the structural properties of a naturally occurring defensin. Defensins are part of the innate immune system that serves as a first line of defense against microbes on the ocular surface. BRI acts primarily on the bacterial cell membrane by depolarization in a similar manner to daptomycin.2 A few key features of BRI are: (1) broad-spectrum antibacterial activity, (2) unlikely development of antibacterial resistance, (3) activity against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin (VAN)-resistant Enterococcus, and (4) activity against stationary phase bacteria. BRI, listed on the Cellceutix website, is currently in preclinical development by Cellceutix (Beverly, MA) as an ocular antimicrobial1 (http://cellceutix.com/brilacidin-ocular/; last accessed September 14, 2015) and has indicated promise in the treatment of acute bacterial skin structure infections in a phase 2 clinical trial (http://cellceutix.com/cellceutix-to-start-brilacidin-phase-3-program-in-absssi/#sthash.MRPHE92F.dpbs; last accessed on September 14, 2015). We are reporting our original testing of BRI as contracted by PolyMedix, Inc., which no longer holds the rights to this peptide mimetic.
The goals of the current study were to evaluate the in vitro antimicrobial activity of BRI against a number of clinically relevant ocular pathogens. Ocular toxicity was evaluated against normal and bacterially infected eyes using established methods.3–6 Ocular toxicity in normal eyes was evaluated using Draize testing.3,4 A rabbit model of fluoroquinolone-resistant and MRSA keratitis5,6 was used to assess: (1) the potential toxic effects of topical BRI therapy, (2) the antibacterial efficacy of topical BRI, and (3) penetration of topical BRI through the corneal epithelium. This rabbit keratitis model was not implemented to test for any specific treatment indication, but rather to evaluate the in vivo antibacterial efficacy of a novel agent in comparison to a commonly used standard of therapy anti-infective.
Methods
In vitro BRI MIC testing
Bacterial test isolates
The minimum inhibitory concentrations (MICs) (25 ocular isolates each) of ciprofloxacin-susceptible S. aureus (CSSA), ciprofloxacin-resistant S. aureus (CRSA), ciprofloxacin-susceptible Staphylococcus epidermidis (CSSE), ciprofloxacin-resistant S. epidermidis (CRSE), Streptococcus pneumonia (SP), Streptococcus viridans group (SV), Moraxella species (including Moraxella catarrhalis) (MS), Haemophilus influenzae (HI), Pseudomonas aeruginosa (PA), Serratia marcescens (SM), and 2 controls (SA ATCC 26670 and Escherichia coli D31) were determined to BRI (PMX30063), using a modified broth-dilution method by Hancock. (http://cmdr.ubc.ca/bobh/methods/MODIFIEDMIC; last accessed September 14, 2015). The deidentified ocular isolates were collected from patients with keratitis and endophthalmitis. All isolates were frozen at −80°C in a clinical bank, as stocks, for validation testing of antibiotics.
Inoculum preparation
Subcultures (from −80°C stocks) of CSSA, CRSA, CSSE, CRSE, SP, SV, PA, SM, SP, and controls (SA ATCC 26670 and E. coli D31) were plated onto trypticase soy agar supplemented with 5% sheep blood [Becton Dickinson and Company (BD), Sparks, MD] for 18 h at 37°C in a 6% CO2 incubator. HI was subcultured on chocolate II agar (BD). A 1 μL loop (disposable, 22-363-598; Fisher Scientific, Waltham, MA) of the subcultures were inoculated into 5 mL of Mueller-Hinton broth (MH) (Hardy Diagnostics, Santa Maria, CA) and incubated at 37°C overnight on a shaker set at 250 rpm. In a similar manner, SP, SV, and SM (plus controls) were prepared except 2% lysed horse red cells (HBC) were supplemented to the MH. Likewise, HI was prepared in Haemophilus Test Medium (HTM) (Remel, Lenexa, KS). The final inoculums were created by diluting turbid shaken cultures to a 0.5 McFarland standard (∼1 × 108 CFU/mL)5,6 in respective broths (MH, HBC, HTM), and further diluting to create a final inoculum for testing at 5 × 105 CFU/mL for MH (50 μL to 5 mL) and 1 × 106 CFU/mL (HBC and HTM) (100 μL to 5 mL).
BRI preparation
All susceptibility testing was performed by adding 90 μL of bacterial inoculum to 10 μL of 10× serial concentrations of BRI into rows of a 96-well polypropylene plate. Each plate could test 8 bacterial isolates with each row containing a positive growth control and 11 serial dilutions. The diluent of BRI contained 0.01% acetic acid and 0.2% bovine serum albumin. Polypropylene tubes, pipettes, and pipette tips were used to avoid binding of BRI. A 1% stock of BRI was diluted to 1,280 μg/mL and this was serially diluted 2-fold for 10 times to provide testing concentrations. PA and SM were tested starting at 128 μg/mL and ending at 1.25 μg/mL. All other bacteria were tested starting at 64 μg/mL and ending at 0.625 μg/mL. BRI was provided by PolyMedix, Inc.
MIC determination
As noted, 90 μL of inoculum was added to the 10 μL of the 10× BRI concentrations in the prepared plates. The plates were placed on a shaker for 15 min to distribute the BRI with the bacterial inoculum. The plates were incubated for 24 h at 37°C in a CO2 incubator. The 2 control bacteria were included in testing to assure the concentration of BRI. The control MICs as indicated by PolyMedix, Inc., for SA ATCC 26670 and E. coli D31 were 0.098 and 0.78 μg/mL, respectively.
MICs were determined visually by locating the lowest concentration of BRI that inhibited visible bacterial growth (pellet formation). The MICs were tabulated for each bacterial group and reported as MIC50, MIC90, and a range from the lowest to highest MIC. The MIC at the 13th rank was deemed as the MIC50 and MIC at the 23rd rank was deemed as the MIC90. The potencies of the MIC90s based on the lowest MICs were compared among the bacterial groups. Any potency difference greater than or equal to 4-fold was considered significant.7,8
In vivo animal testing
The present study conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research, and was approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC Protocol #0701145A-1, “The In vivo Evaluation of Biomimetics as Topical Ocular Antibiotics”).
Draize testing for ocular toxicity determination
Before administering any topical compound to an animal eye, it is our practice to determine any immediate adverse reaction and delayed reaction. Ocular toxicity was evaluated with 5 concentrations (1%, 0.5%, 0.25%, 0.1%, and 0.01%) of BRI, formulated in Tris-buffered saline (TBS; provided by PolyMedix, Inc.) using the Draize scoring system in the NZW rabbit ocular irritation model.3 The Draize Scale grades for toxicity to: cornea (opacity-degree of density, area involved), iris, and conjunctiva (redness, chemosis, and discharge). Two rabbits were tested for each BRI concentration and TBS (vehicle control). Rabbits were treated in both eyes with (37 μL) topical drops every 30 min for 3 h (7 total doses). Rabbits were evaluated in a masked fashion for ocular toxicity by Jerold S. Gordon, MD, an ophthalmologist with specialty training in corneal and external disease. Ocular toxicity was evaluated using the Draize scoring system after treatment on day 0 for acute toxicity and on day 4 posttreatment for any delayed toxicity. Maximum mean total scores (MMTS) were calculated and eye irritation was classified.4
Toxicity, efficacy, and anti-infective penetration determination
The anti-infective efficacy of BRI 0.5%, VAN 5.0%, and saline (SAL) were compared in the treatment of a fluoroquinolone-resistant, methicillin-resistant S. aureus (FQrMRSA) infection in the NZW rabbit keratitis model with or without intact corneal epithelium to evaluate: (1) drug toxicity, (2) bacterial reduction, and (3) drug penetration. The BRI 0.5% was provided by PolyMedix, Inc.; the VAN 5% was prepared as a patient topical clinical medication by the University of Pittsburgh Medical Center Central Pharmacy, Pittsburgh, PA; and the injectable nonpreserved SAL (Baxter Healthcare Corp., Deerfield, IL) was purchased through a commercial source.
Protocol
(A) A stock keratitis isolate of FQrMRSA (K950) (methicillin resistance based on disk diffusion) (BRI MIC was 0.5 μg/mL; VAN MIC was 2.0 μg/mL) was subcultured on 5% sheep blood agar and incubated at 37°C in 6% CO2 overnight. The next morning, the FQrMRSA strain was suspended in sterile trypticase soy broth to a 0.5 McFarland Standard,5–7 containing ∼5 × 108 CFU/mL of bacteria. The absorbance of the suspension was measured at 650 nm using a Beckman DU-70 spectrophotometer. 5 × 108 CFU/mL of bacteria produced an optical density (OD) of 0.07 OD. This concentration was appropriately diluted in sterile trypticase soy broth to provide the inoculum of 1,000 CFU/eye in 25 μL. Colony counts were performed on the inoculum to determine the actual CFU inoculated.
(B) The rabbits were systemically anesthetized with intramuscular injections of ketamine (40 mg/kg) and xylazine (4 mg/kg) in the rear flank. Topical anesthesia (0.5% proparacaine) was applied to each cornea before proptosing the globe with a dacron-tipped applicator. The corneal epitheliums of the left corneas of 24 NZW rabbits (separated into duplicate trials of 12 rabbits each) were abraded using an Amoils epithelial scrubber (Innovative Excimer Solutions, Inc., Ontario, Canada), while the epitheliums in the right eyes remained intact. The comparison of the data between the 2 eyes would determine the ability of BRI to penetrate the corneal epithelium into the infected corneal stroma. The corneas were intrastromally injected with 1,000 CFU of FQrMRSA. The rabbits were immediately treated with analgesia in the form of intramuscular injections of ketoprofen, 1.5 mg/kg.
(C) The rabbits were separated into 4 groups (n = 6 total; n = 3 per trial): (1) BRI 0.5%, (2) VAN 5%, (3) SAL, and (4) no treatment (euthanized at the time of the onset of treatment for baseline CFU). VAN was used as standard therapy for FQrMRSA at the highest concentration used for topical application. This high concentration is known to be highly irritating to the eye during treatment and was chosen in this study to provide a dose that presented with acceptable toxicity. Four hours after FQrMRSA challenge, topical treatment of 1 drop every 15 min for 5 h (21 total doses) was initiated.
(D) Immediately after treatment, the eyes were clinically evaluated (FSM) by slit lamp for discharge, conjunctival/scleral injection, limbal injection, corneal infiltrate, and iritis. A total clinical score for each treatment was calculated and the groups were nonparametrically compared with Kruskal–Wallis (K-W) multiple comparisons (True EPISTAT, Round Rock, TX).
(E) One hour after the final dose, the animals were euthanized, and the corneas were harvested and homogenized for standard colony counts.
(F) As similar results were seen within the duplicate trials the data were combined for statistical analysis. The CFU +1 were log10 transformed and nonparametrically analyzed with K-W for multiple comparisons and Mann–Whitney (MiniTab, State College, PA) for two-sample comparison. A 3 log10 decrease in median CFU between treatment and onset denoted a bactericidal effect.5,6
Nonparametric analysis was performed because the data may not conform to a t- or standard distribution with possible zero colony counts. Graded data such as clinical scores should always be analyzed with nonparametric analysis. With nonparametric analysis, a median is calculated instead of a mean and standard deviation. The analysis may indicate no differences, but this may be based on numbers that do not provide a high power of analysis.
Results
In vitro susceptibility testing of BRI
Table 1 details the descriptive statistics [MICs (μg/mL)] of ocular bacterial pathogens (n = 25 per bacterial group) to BRI. Both SA and SE have the lowest MICs among the bacterial groups tested based on the MIC90s. Of the 50 SA, 13 (26%) were methicillin-resistant with a MIC50, a MIC90, and a range of 0.25, 0.5, and 0.125–1.0 μg/mL, respectively. The potency was equivalent to the SA (including MRSA) and SE groups. The MIC90s for SP and SV were 4-fold and 32-fold higher than SA and SE. The MIC90s for MS, HI, PA, and SM were 256-, 32-, 16-, and 128-fold higher, respectively, than SA and SE. Based on a 4-fold difference, BRI was more potent for Gram-positive bacteria (except SV) than Gram-negative bacteria. The MICs for the control organisms (SA ATCC 26670 and E. coli D31) were within a 2-fold difference of their benchmark MICs for validating the experimental concentrations.
Table 1.
Descriptive Statistics (Minimum Inhibitory Concentrations (μg/mL)) of Ocular Bacterial Pathogens (n = 25 per bacterial group) to Brilacidin (PMX30063)
| Ocular bacterial pathogen | MIC50 | MIC90 | MIC range | Potency at MIC90 level |
|---|---|---|---|---|
| Gram-positive bacteria | ||||
| Ciprofloxacin susceptible Staphylococcus aureus | 0.25 | 0.25 | 0.125–0.5 | 0 |
| Ciprofloxacin resistant Staphylococcus aureus | 0.25 | 0.5 | 0.125–1 | 2 |
| Ciprofloxacin susceptible Staphylococcus epidermidis | 0.125 | 0.25 | 0.03125–0.25 | 0 |
| Ciprofloxacin resistant Staphylococcus epidermidis | 0.125 | 0.25 | 0.03125–0.25 | 0 |
| Streptococcus pneumonia | 1 | 1 | 0.5–128 | 4 |
| Streptococcus viridans | 4 | 8 | 1–32 | 32 |
| Gram-negative bacteria | ||||
| Moraxella species | 4 | 64 | 0.5–128 | 256 |
| Haemophilus influenzae | 8 | 8 | 2–32 | 32 |
| Pseudomonas aeruginosa | 4 | 4 | 0.5–8 | 16 |
| Serratia marcescens | 8 | 32 | 0.25–32 | 128 |
Potency–An equal or greater than 4-fold difference depicts lower potency at the MIC90 level. Potency less than 4 would indicate equivalence.
MIC, minimum inhibitory concentration.
In vivo testing of BRI
Draize testing of normal eyes
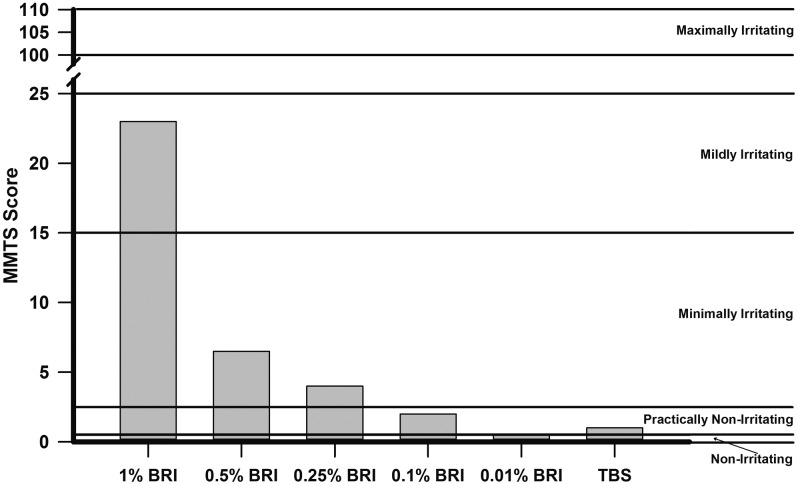
Figure 1 details the results of the Draize testing demonstrating the MMTS of rabbit eyes treated with several concentrations of BRI and its vehicle TBS. BRI demonstrated dose-dependent ocular toxicity after 7 topical instillations (every 30 min for 3 h) in the NZW rabbit ocular toxicity model. BRI 1% was determined to be Mildly Irritating (23.0), BRI 0.5% (6.5), and BRI 0.25% (4.0) were determined to be Minimally Irritating, while BRI 0.1% (2.0) and TBS (1.0) were determined to be Practically Nonirritating and 0.01% BRI (0.5) was determined to be Nonirritating based on their MMTS values.4
FIG. 1.
The results of the Draize testing demonstrating the maximum mean total scores (MMTS) of rabbit eyes treated with several concentrations of brilacidin (BRI) and its vehicle Tris-buffered saline (TBS). The MMTS irritation scores are classified as follows: 0.0–0.5 Nonirritating; 0.6–2.5 Practically Nonirritating; 2.6–15.0 Minimally Irritating; 15.1–25.0 Mildly Irritating; 25.1–50.0 Moderately Irritating; 50.1–80.0 Severely Irritating; 80.1–100.0 Extremely Irritating; and 100.1–110.0 Maximally Irritating.
Rabbits treated with BRI 1% wiped their eyes immediately upon instillation after the second dose and continued for every dose thereafter. This behavioral reaction suggested that BRI 1% appeared to be irritating to the eyes upon instillation. BRI 1% also demonstrated corneal (median score = 2.5) and iris toxicity (median score = 7.5). One rabbit treated with BRI 0.25%, vocalized shortly after the 5th dose. There were no acute reactions by the rabbits (flinching, immediate wiping of eyes, vocalization, hopping to rear of cage) upon instillation of the other doses (0.5%, 0.25%, 0.1%, and 0.01%). There was no prolonged or delayed toxicity (4 days after drops) demonstrated in any treatment group (all total scores were equal to zero).
Toxic effects of topical BRI therapy in FQrMRSA-infected eyes
For corneas with intact epithelium, the median total clinical scores (range) for VAN 5% (8.0, 6.5–9.5) and SAL (7.7, 7.0–10.5), were not different, but statistically lower than BRI 0.5% (11.0, 9.5–12.5) (K-W). This would indicate that topical BRI may be more irritating than VAN 5% for treating a corneal infection. For corneas with abraded epithelium, the median total clinical score for SAL (7.0, 5.5–9.5) was statistically lower than VAN 5% (9.0, 8.0–10.5) and BRI 0.5% (9.75, 9.0–11.0) (K-W). In contrast, this may indicate that BRI 0.5% would not be more irritating than VAN 5% in the treatment of a corneal ulcer.
Anti-infective efficacy of topical BRI
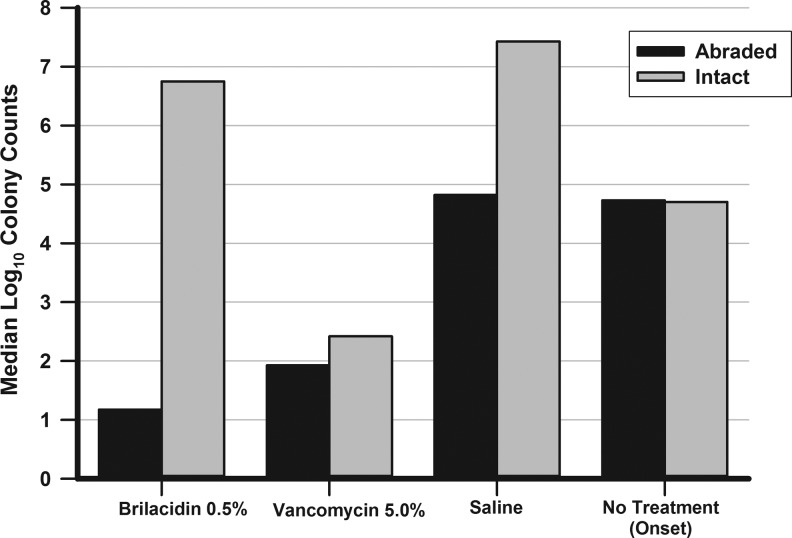
The inocula injected into the corneas contained 1,272 CFU/cornea of FQrMRSA for the first trial and 1,082 CFU/cornea for the second trial. Figure 2 depicts the comparison of colony counts in corneas with abraded versus intact epithelium after topical therapy. For intact corneas (median log10 converted CFU in parentheses; range), VAN 5% (2.4, 1.7–3.7) significantly reduced CFU compared with BRI 0.5% (6.8, 5.3–7.2), which demonstrated a slight but significant decrease versus SAL (7.4, 5.9–7.6) (K-W). Based on onset (4.7, 4.3–4.85), neither VAN nor BRI demonstrated a bactericidal effect (3 log10 decrease).
FIG. 2.
The comparison of colony counts of fluoroquinolone-resistant, methicillin-resistant Staphylococcus aureus in corneas with abraded versus intact epithelium after topical therapy.
For abraded corneas, VAN 5% (1.9, 1.4–2.4) and BRI 0.5% (1.17) were not different in the reduction of CFU, but were less than SAL (4.8, 3.1–6.5) (K-W). However, only BRI 0.5% demonstrated a defined bactericidal effect (3.6 log10 reduction) compared to median baseline CFU (4.9). VAN 5% demonstrated a 2.86 log10 reduction in CFU compared to median baseline.
Topical penetration of BRI through the corneal epithelium
BRI 0.5% reduced CFU in abraded corneas significantly more than in intact corneas (P = 0.005, M-W) suggesting that the corneal epithelium acts as a barrier for penetration. There was no difference in CFU in abraded and intact corneas with VAN (P = 0.13, M-W) suggesting penetration through the corneal epithelium.
Discussion
A novel anti-infective must be able to meet certain criteria before acceptance for patient care. Topical anti-infective therapy is obviously different from systemic therapy, but both routes need to meet issues regarding toxicity, efficacy, and tissue penetration. We present an independent study in which the original data were generated for a previous company (PolyMedix, Inc.), after which the rights to BRI were obtained by another company (Cellceutix), which plans to develop it clinically. Our purpose was to present the data without any current designation of financial interest.
With regard to BRI toxicity, we were able to demonstrate that BRI 0.5% was minimally irritating to normal rabbit tissue, but not necessarily more than infected tissue treated with BRI 0.5% or VAN 5%. Some clinicians believe that minimal toxicity allows better drug penetration into the infected tissue, while others believe that toxicity could interfere with the clinical picture. It appears that the toxicity of BRI 0.5% would be a judgement call made by the attending physician. Although Draize testing is well established and accepted, the FDA (Food and Drug Administration) may require more stringent toxicity testing (Hackett–McDonald) before final clinical trial approval.9 For the purpose of this study, Draize testing provided the necessary information to allow an initial animal evaluation of BRI.
The efficacy of BRI 0.5% has been evaluated using a FQrMRSA keratitis model in comparison with VAN. We compared the topical treatment of infected corneas with intact and abraded corneal epithelium. In the clinical situation, an abraded corneal epithelium would be a better representation of a bacterial corneal ulcer, and topical application of BRI 0.5% was efficacious in this setting. Based on the treatment of a bacterial infiltrate with an intact corneal epithelium, BRI 0.5% was less efficacious. Topical VAN was equally efficacious in corneas with both abraded and intact epithelium.
In vitro MIC data and anti-infective penetration need to be discussed concurrently.
The efficacy data indicate that topical BRI 0.5% can reach corneal levels (0.25 μg/mL), in eyes with abraded corneal epithelium, to eliminate FQrMRSA. We do not know if the levels are sufficient to treat other bacteria with significantly higher MICs that infect the cornea. It would be advantageous to know the optimal cornea concentrations that can be achieved in the human cornea.
At this point, BRI 0.5% appears to be a narrow-spectrum staphylococcal anti-infective. There appears to be no distinction between MSSA and MRSA. Additional rabbit keratitis testing of Streptococcus species and Gram-negatives (i.e., P. aeruginosa) needs to be evaluated to extend BRI 0.5% as a broad-spectrum anti-infective. The treatment indications, based on the current study, could support clinical trials for keratitis and conjunctivitis. The poor penetration of BRI 0.5% through the corneal epithelium probably would render the anti-infective as an inadequate choice for surgical prophylaxis.
In summary, BRI 0.5% is a narrow-spectrum anti-infective that is minimally irritating and appears to penetrate the corneal epithelium poorly. Testing should be extended to determine anti-infective concentration in the ocular tissues for the treatment of streptococcal and Gram-negative bacteria. Our laboratory is independent in any recommendation for the future development of BRI.
Acknowledgments
The authors are grateful to the Pennsylvania Lions Club, The Eye and Ear Foundation of Pittsburgh, PA, The National Institutes of Health Core Grant P30 EY008098, and The Unrestricted Grant from Research to Prevent Blindness, New York, NY, for continued financial support. The project was initially funded by PolyMedix, Inc. The authors have not received financial support from Cellceutix, the company that owns the rights to BRI.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Novack G.D. Eyes on new product development: trabecular meshwork. J. Ocul. Pharmacol. Th. 30:83–84, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Mensa B., Howell G.L., Scott R., and DeGrado W.F. Comparative mechanistic studies of brilacidin, daptomycin, and the antimicrobial peptide LL16. Antimicrob. Agents Chemother. 58:5136–5145, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draize J.H., Woodward G., and Calvery H.O. Methods for the study of irritation and toxicity of articles applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 82:377–390, 1944 [Google Scholar]
- 4.Kay J.H., and Calandra J.C. Interpretation of eye irritation tests. J. Soc. Cos. Chem. 13:281–289, 1962 [Google Scholar]
- 5.Kowalski R.P., Romanowski E.G., Yates K.A., and Gordon Y.J. Lomefloxacin is an effective treatment for experimental bacterial keratitis. Cornea. 20:306–308, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Romanowski E.G., Mah F.S., Yates K.A., Kowalski R.P., and Gordon Y.J. The successful treatment of gatifloxacin-resistant Staphylococcus aureus keratitis with Zymar (gatifloxacin 0.3%) in a NZW rabbit model. Am. J. Ophthalmol. 139:867–877, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Mather R., Karenchak L.M., Romanowski E.G., and Kowalski R.P. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am. J. Ophthalmol. 133:463–466, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Stroman D.W., Djacs J.J., Cupp G.A., and Schlech B.A. In vitro and in vivo potency of moxifloxacin and moxifloxacin ophthalmic solution 0.5%, a new topical fluoroquinolone. Surv. Ophthalmol. 50:S16–S31, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Hackett R.B., and McDonald T.O. Eye irritation. In: Marzulli F.N. and Maibach H.I., eds. Dermatotoxicology. 4th ed. New York, NY: Hemisphere Publishing Company; 1991; p. 749–815 [Google Scholar]