Abstract
Importance
Obesity is prevalent among hospitalized children. Knowledge of the relationship between obesity and outcomes in hospitalized children will enhance nutrition assessment and provide opportunities for interventions.
Objective
To systematically review the existing literature concerning the impact of obesity on clinical outcomes in hospitalized children.
Evidence Acquisition
PubMed, Web of Science, and EMBASE databases were searched for studies of hospitalized children aged 2 to 18 years with identified obesity and at least 1 of the following clinical outcomes: all-cause mortality, incidence of infections, and length of hospital stay. Cohort and case-control studies were included. Cross-sectional studies, studies of healthy children, and those without defined criteria for classifying weight status were excluded. The Newcastle-Ottawa Scale was used to assess study quality.
Results
Twenty-eight studies (26 retrospective; 24 cohort and 4 case-control) were included. Of the 21 studies that included mortality as an outcome, 10 reported a significant positive relationship between obesity and mortality. The incidence of infections was assessed in 8 of the 28 studies; 2 reported significantly more infections in obese compared with nonobese patients. Of the 11 studies that examined length of stay, 5 reported significantly longer lengths of hospital stay for obese children. Fifteen studies (53%) had a high quality score. Larger studies observed significant relationships between obesity and outcomes. Studies of critically ill, oncologic or stem cell transplant, and solid organ transplant patients showed a relationship between obesity and mortality.
Conclusions and Relevance
The available literature on the relationship between obesity and clinical outcomes is limited by subject heterogeneity, variations in criteria for defining obesity, and outcomes examined. Childhood obesity may be a risk factor for higher mortality in hospitalized children with critical illness, oncologic diagnoses, or transplants. Further examination of the relationship between obesity and clinical outcomes in this subgroup of hospitalized children is needed.
The prevalence of obesity among children has reached epidemic proportions worldwide.1,2 Childhood obesity has been associated with insulin resistance, hypertension, and metabolic syndrome, as well as adult obesity and premature mortality.3 Obesity and excess body fat during acute illness in childhood may contribute to further morbidities. The inflammatory response to illness, surgery, and trauma triggers protein turnover to provide substrate for the catabolic response to stress, resulting in substantial loss of lean body mass,4 thereby increasing the relative proportion of fat mass. Obesity is associated with both inflammation and a weakened immune response.5 As a result, complications related to acute illness may be compounded by obesity.6
The relationships between obesity and clinical outcomes have been explored in adults with critical illness. Two meta-analyses concluded that obesity did not contribute to excess mortality in this group, although there were conflicting results about the effect of obesity on the duration of mechanical ventilation and hospital length of stay (LOS).7,8 Analyses of hospitalization patterns have suggested higher hospital charges and longer hospital stays for obese children.9,10 Several cross-sectional and cohort studies have suggested that obesity may contribute to respiratory complications in children.11–13 These findings provide important rationale for investigations of pediatric obesity outcomes. However, the findings may not be generalizable to acutely ill children, especially because unique short-term consequences may result from pediatric obesity.14
To our knowledge, a systematic review of the literature describing the impact of obesity on clinical outcomes in children has not been published. We explored the existing literature to address the question: do obese hospitalized children have a greater risk for mortality, more infectious complications, or a longer LOS when compared with children of normal weight? We hypothesized that children who are obese have poorer outcomes resulting from hospitalization for illness compared with those of normal weight.
METHODS
We included original studies of children, between 2 and 18 years of age, who were hospitalized for acute or chronic illness. Obesity was defined by standardized criteria (ie, percentiles or z scores for body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], weight for age, or weight for height, based on reference growth data from the World Health Organization, Centers for Disease Control/National Center for Health Statistics, or country-specific growth standards). Studies in which obesity was analyzed as a predictor for 1 or more measured clinical outcomes, namely, mortality, incidence of infections, and hospital LOS, were included. Studies not available in English were excluded. We excluded studies of ambulatory children; studies of exclusively adult or infant populations; studies without an explicit definition of obesity, measurement, or criteria for obesity and/or overweight status; and studies without a normal-weight comparison group. Cross-sectional studies, letters, and case reports were also excluded as they are not designed to answer etiologic questions.
The PubMed database from 1970 to April 2012, Web of Science from 1980 to 2012, and EMBASE from 1974 to 2012 were searched for prospective and retrospective observational cohort and case-control studies. Search terms used were obesity, body mass index, nutrition assessment, nutritional status, morbidity, mortality, infections, wound infections, cross infection, focal infection, bacterial infection, opportunistic infections, sepsis, systemic inflammatory response syndrome, respiratory distress, trauma, wounds and injuries, critical illness, and critical care. The primary electronic search strategy performed is outlined in Table 1. In addition, the suggested links and bibliographies of relevant manuscripts and retrieved articles meeting the inclusion criteria were hand searched.
Table 1.
PubMed Search Strategy
| Search No. | Querya | Items |
|---|---|---|
| 30 | Search 20, NOT 21; limits: humans, English, preschool children aged 2–5 y, children aged 6–12 y, adolescents aged 13–18 y, publication date from 1970 to 2012 | 951 |
| 29 | Search 20, NOT 21; limits: humans, preschool children aged 2–5 y, children aged 6–12 y, adolescents aged 13–18 y, publication date from 1970 to 2012 | 1079 |
| 28 | Search 20, NOT 21; limits: humans, preschool children aged 2–5 y, children aged 6–12 y, adolescents aged 13–18 y | 1088 |
| 24 | Search 20, NOT 21; limits: humans, infants aged 1–23 mo, preschool children aged 2–5 y, children aged 6–12 y, adolescents aged 13–18 y | 1096 |
| 22 | Search 20, NOT 21 | 4284 |
| 21 | Searches 14 and 19; limits: animals, editorial, letter, review, case reports, comment, in vitro | 49 |
| 20 | Searches 14 and 19 | 4333 |
| 19 | Search (“overweight/complications” [mesh] or “overweight/mortality” [mesh]) | 27 587 |
| 14 | Search (((“morbidity” [mesh]) or “mortality” [mesh]) or “infection” [mesh]) or “respiratory distress syndrome, adult” [mesh] | 1 076 667 |
NOT indicates the Boolean operator used in the search strategy of the PubMed database.
Relevant data were abstracted from each study, including study design, number and type of subjects, obesity criteria, and prevalence within each cohort, with the outcome data analyzed with respect to the 3 outcomes considered in the review question—mortality, incidence of infections, and LOS. Analytic features (comparison method and control for covariates), patient population, and the prospective vs retrospective nature of the data collections were also gathered for each outcome group. Results of multivariate analyses, where available, were prioritized for inclusion in the summary table. Studies were grouped by design and disease type for data abstraction (eTable, http://www.jamapeds.com). For the purposes of synthesizing study results, outcome data were tabulated and grouped according to the significance, or lack thereof, of the relationships between obesity and outcomes.
The quality of the research articles included in this review was assessed using the Newcastle-Ottawa Scale, a tool developed and partially validated for the purposes of evaluating nonrandomized studies used in systematic reviews and meta-analyses.7,15,16 One author (L.J.B.) conducted the quality assessments. A total score of 5 or less was considered low, 6 or 7was considered moderate, and 8 or 9 was deemed high quality.
RESULTS
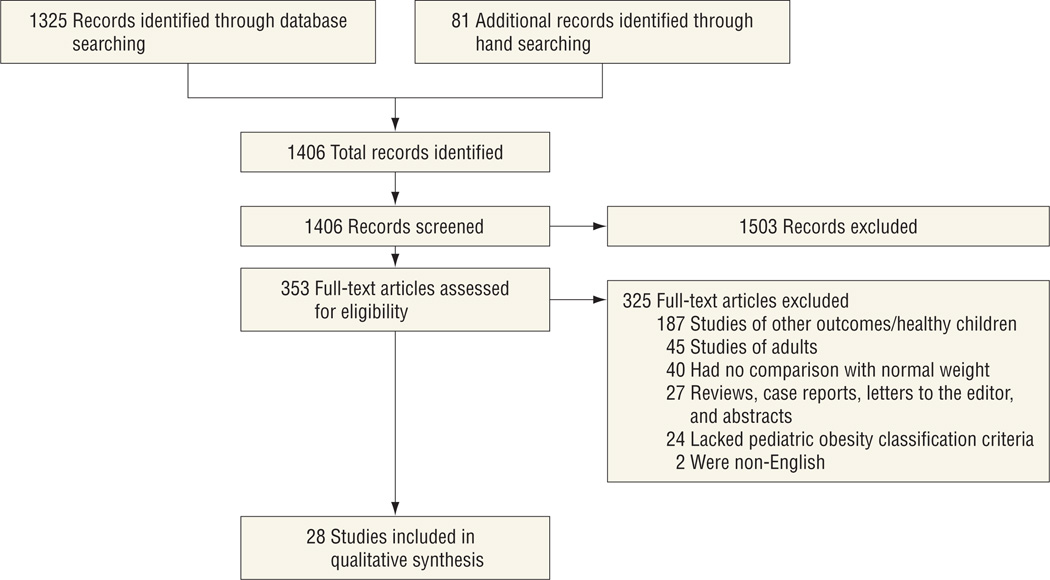
The results of the search and selection of studies are described in the Figure. All relevant full-text articles (n = 353) were assessed for inclusion criteria. There were 28 studies that met the inclusion criteria and were included in this review. Most of the studies were retrospective cohort (n = 23); 1 study was a prospective cohort. Of the 4 case-control studies, 1 was prospective and 3 were retrospective data collections. Summary data for each study are shown in the eTable and grouped by illness type or condition. Patients in 32% of the studies (n = 9) were categorized as having critical illness or trauma. Thirty-six percent of the studies (n = 10) included children with cancer or following hematopoietic stem cell transplantation, 14% (n = 4) were of those who underwent elective surgical procedures (orthopedic, adenotonsillectomy, and cholecystectomy), and the remaining 18% (n = 5) were of children who received solid organ transplants (1 renal, 3 cardiac, and 1 liver).
Figure.
Selection of studies that assessed the relationship between obesity and clinical outcomes in hospitalized children.
Studies varied in size from fewer than 100 subjects to nearly 10 000 subjects, with 15 single-center investigations and 13 multicenter reports. The quality scores for each domain of the Newcastle-Ottawa Scale are presented in Table 2. Six of the studies were considered low quality, 10 were considered moderate quality, and 12 were determined to be high quality based on their total scores. Univariate and multivariate analyses were used in most studies to allow adjustment for confounding variables such as admission diagnosis, illness severity, age, sex, and race/ethnicity. In 6 studies, no analysis or control for covariates were reported. Table 3 summarizes the data as classified by the 3 outcome categories—mortality, infections, and LOS—with size of the populations and quality assessment noted.
Table 2.
Quality Assessment of 28 Included Studies Using the Newcastle-Ottawa Scale15
| Stars From Newcastle-Ottawa Scale, No.a | |||
|---|---|---|---|
| Source | Selection | Comparability | Outcome |
| Cohort studies | |||
| Brown et al,17 2006 | 2 | 0 | 2 |
| Srinivasan et al,18 2010 | 4 | 2 | 3 |
| Goh et al,19 2013 | 3 | 2 | 3 |
| Carroll et al,20 2006 | 3 | 2 | 3 |
| Yu et al,21 2011 | 2 | 1 | 3 |
| Lange et al,22 2005 | 3 | 2 | 2 |
| Baillargeon et al,23 2006 | 2 | 2 | 3 |
| Barker et al,24 2011 | 3 | 2 | 3 |
| Hingorani et al,25 2011 | 3 | 0 | 3 |
| Rana et al,26 2009 | 2 | 0 | 3 |
| Patel et al,27 2010 | 3 | 2 | 3 |
| Garey et al,28 2010 | 2 | 0 | 2 |
| Hanevold et al,29 2005 | 3 | 2 | 3 |
| Kaufman et al,30 2009 | 3 | 2 | 3 |
| Dick et al,31 2010 | 3 | 2 | 3 |
| Rossano et al,32 2007 | 1 | 2 | 2 |
| Kaufman et al,33 2008 | 1 | 2 | 3 |
| Nafiu et al,34 2009 | 3 | 0 | 3 |
| Hijiya et al,35 2006 | 3 | 2 | 3 |
| Bulley et al,36 2008 | 2 | 2 | 2 |
| Fernandez et al,37 2009 | 3 | 2 | 3 |
| Pine et al,38 2011 | 3 | 2 | 3 |
| Kraft et al,39 2012 | 3 | 0 | 2 |
| White et al,40 2012 | 3 | 2 | 2 |
| Case-control studies | |||
| Morgan et al,41 2010 | 3 | 1 | 0 |
| Butturini et al,42 2007 | 3 | 2 | 3 |
| Linam et al,43 2009 | 3 | 2 | 2 |
| Fung et al,44 2010 | 2 | 2 | 3 |
Higher quality studies are reflected by higher numbers of stars in each category. The maximum possible star scores for each study criteria are 4 for selection, 2 for comparability, and 3 for outcome or exposure, for a total possible score of 9.
Table 3.
Relationship Between Obesity and Clinical Outcomes in Hospitalized Children
| Studies Reporting an Association of Clinical Outcomes With Obesity, No.a | ||||||
|---|---|---|---|---|---|---|
| Mortality | Infection | Length of Stay | ||||
| Disease Group | Yes (n = 32 761) |
No (n = 8842) |
Yes (n = 492) |
No (n = 5022) |
Yes (n = 3185) |
No (n = 6013) |
| Mean NOS score | 7.3 | 6.4 | 5.5 | 5.8 | 7.0 | 5.7 |
| Critical care/trauma | 34b | 24b | 19c | 32c | 44b | 24b |
| 35d | 19c | 36c | 43b | 19c | ||
| 32c | 32c | |||||
| 36c | 36c | |||||
| 30c | ||||||
| Oncology/HSCT | 21b | 26b | 42d | |||
| 18b | 23b | |||||
| 29d | 31b | |||||
| 20d | 17d | |||||
| 37d | ||||||
| Elective surgical procedures | 40c | 41d | 39c | |||
| 38c | ||||||
| Solid organ transplant | 25b | 27b | 27b | 28d | 27b | |
| 22b | 33c | 33c | ||||
| 28d | 28d | |||||
Abbreviations: HSCT, hematopoietic stem cell transplantation; NOS, Newcastle-Ottawa Scale.
Significant results indicated by number of reference citation and study quality as assessed by NOS score.
High quality (score, 8–9).
Low quality (score, ≤5).
Moderate quality (score, 6–7).
MORTALITY
Of the 21 studies investigating the relationship between obesity and mortality,* 10 studies reported obesity as a significant predictor of death† through multivariate modeling by logistic regression or a Cox proportional hazards model. One half of the cancer and stem cell transplant studies (n = 5) found obesity to be a significant predictor of mortality.22,24,36,40,42 None of the elective surgery studies (n = 4) investigated mortality as an outcome,28,34,43,44 probably owing to the low mortality rates associated with these procedures. In 11 studies, there were no significant associations between obesity and mortality.‡ Study quality, as assessed by the Newcastle-Ottawa Scale, was higher, on average, among studies reporting obesity as a significant predictor of mortality. In 3 studies, the incidence of mortality was compared by weight group only,17,26,41 with no analysis for covariates. Only 1 study was prospective.18 Most studies were large cohort and multicenter studies. Most analyses controlled for covariates and confounders, although the retrospective data collection limited the availability of relevant confounding data in several studies.
INFECTIONS
Eight studies (29%), all including surgical patients, investigated the effect of obesity on the incidence of infectious outcomes.17,25,26,30,32,33,39,43 Populations studied included trauma patients, patients with osteosarcoma, and 3 studies of heart transplant recipients.30,32,33 A significant association between obesity and the incidence of infections was reported in 2 of the 8 studies, including 1 study of critically ill trauma patients17 and 1 study of patients who underwent spinal fusion surgery.43 The remaining 6 studies found no significant associations between obesity and infectious outcomes, although 3 of these studies did not report detailed data on the actual incidence of infections in the cohorts.26,32,33 The average quality score was similar among all studies that analyzed for relationships between obesity and infections. One study reported a significantly higher risk for surgical site infections in obese patients compared with nonobese control subjects, which persisted in a multivariate regression model.43 Two multicenter studies25,30 and 1 large single-center study26 were among those that did not find significant relationships between obesity and the risk for infections.
HOSPITAL LOS
Six studies of critically ill/trauma patients17,19,20,27,39,41 and 3 including patients who underwent elective surgery28,34,44 investigated the relationship between obesity and LOS. Two of the critical illness studies20,27 reported a significant relationship between LOS and obesity. Of the 3 studies of elective surgery patients that examined hospitalization time, 2 found that obesity was significantly related to a lengthier hospital course.34,44 Two solid organ transplant studies compared LOS among weight categories. One found significantly longer LOS in obese patients,33 and the other found no difference in LOS according to weight category.30 A similar discrepancy existed with the 2 studies of patients with burns27,39; however, only 1 of these adjusted for covariates in the analysis.27 As assessed by the Newcastle-Ottawa Scale, quality scores were higher, on average, among studies reporting obesity as a significant contributor to LOS (n = 5). Four of the 6 studies that reported no significant association between obesity and LOS were of low quality. None of the studies of children with cancer or following hematopoietic stem cell transplantation explored LOS systematically.
COMMENT
To our knowledge, this is the first systematic review to appraise the existing literature concerning the relationship between obesity and important clinical outcomes in hospitalized children. We summarized studies with diverse relationships between obesity and 3 clinically important outcomes: mortality, incidence of infections, and hospital LOS. Compared with studies finding no relationship between obesity and adverse clinical outcomes, studies describing a higher risk for mortality and longer LOS in obese children compared with normal-weight children were generally higher quality studies with adjustment for covariates. The incidence of infections was described in fewer studies overall, with smaller populations and lower study quality, suggesting that no conclusion about this outcome can be proposed.
Based on the large number of studies that were identified and reviewed for eligibility (Figure; n = 353), there is great interest in this topic of investigation; however, studies are often limited to small cohorts, otherwise healthy children, or nutritional outcomes. Studies meeting the inclusion criteria for this review encompassed a variety of patients with acute illness, chronic conditions, and surgical interventions, but they did not capture all of the common reasons for hospitalization of children. The limited number of available studies, heterogeneity of populations, analysis methods, obesity groupings, and measured outcomes made it difficult to combine results for a meaningful conclusion.
All but 2 of the evaluated studies18,44 were retrospective in nature. The value of retrospective data is limited owing to the historical nature of the collection efforts. There can be bias in sample selection because the targeted population may not have had the specific data collected in the past. Several studies21,22,26,41 included in this review consisted of samples that were subsets of a population, most often owing to lack of data recorded on key variables, notably height and weight.
The criteria for defining obesity and how patients were grouped also varied; 9 studies grouped patients dichotomously as obese or nonobese, but the remaining 19 defined more than 2 groups by weight status. Specification of criteria for obesity designation based on published standards was required for inclusion in this review. However, different criteria and cutoff points were reported. Two studies did not specifically identify the growth data set for the sample’s calculated percentiles. Differences in obesity classification may also confound results of observational studies. Bias may be introduced during the selection of obesity cutoff points, particularly with a retrospective design, in an effort to better reflect a proposed relationship. Standardized, meaningful criteria across age groups and transparent reporting of data may help clarify sample descriptions. Globally, overweight and obesity status may be defined as greater than 2 and greater than 3 standard deviations, respectively, from the World Health Organization standard BMI median,45,46 commonly referred to as BMI z scores.
Accurate weights and heights must be available for calculation of BMI. One study included in this review18 relied on a calculated median value of height for age to determine BMIs used in the analysis, while 2 studies19,20 did not obtain or estimate height and used weight for age as obesity criteria. Lack of height measures in acute care settings is common and may lead to less accurate growth assessment. Criteria for defining obesity varied in the studies included in this review. Moreover, the use of different national reference norms and cutoff criteria may reduce the generalizability of international data comparisons.45 Therefore, a uniform definition for obesity across populations is needed to advance the epidemiologic study of obesity-related outcomes. The use of BMI z scores as continuous variables for the analysis of large, multicenter cohorts of children may help differentiate the magnitude of obesity with associated outcomes, although the limitations of the comparative data are acknowledged.47
The investigation of mortality in children is challenging owing to its infrequent occurrence. The possibility of publication bias exists because nonsignificant findings may be perceived as uninteresting to report. Studies with mortality as an outcome are more common with chronic diseases, such as cancer, or with critical illness. Several studies that found obesity predictive of mortality were conducted in children with chronic disease. Although results were mixed, the large number of patients in studies suggesting obesity was a significant predictor of mortality implicates the presence of a persistent relationship, particularly when studies controlled for covariates. Therefore, there is a suggestion that the risk for mortality is elevated in obese children with prolonged illnesses. This is consistent with the overall implication that obese adults have a shortened life span; however, perhaps in these ill children, the effect of obesity is enhanced by the length of illness.
Morbidity for the purposes of this review was limited to the incidence of infections, which in severely ill children are likely to be important indicators for overall treatment success or failure.48–50 Physiological effects of obesity on immune function have been described in animal models and proposed by human associations.51 Obesity is linked to chronic inflammation and a defective, inattentive immune response.5 While the findings of this review suggest no clear association between obesity and the incidence of infections, sample sizes and the number of studies investigating infectious outcomes were small. A carefully controlled, small study of children who underwent spinal fusion found obesity to be a significant and independent risk factor for surgical site infections (odds ratio, 3.1; 95% CI, 1.1–9.1; P = .04) in a multivariable regression model including hypothermia, antibiotic prophylaxis, and an underlying health status score.43 Conversely, a large multicenter study of heart transplant recipients30 found no significant association between obesity and the incidence of infection and accounted for more than half of the total patients reviewed with this finding. This particular analysis compared wasted, normally nourished, and obese groups, but it did not investigate the effect of confounders or covariates on the incidence of infections. The total number of patients studied for which no significant association between obesity and infections was found was much larger than those that did find an association; however, the skew of the single large cohort without covariate analysis precludes any conclusions from generalization to the larger population of ill children.
Less than half of the included studies systematically measured the relationship between obesity and hospitalization time.§ The results were nearly evenly split between a significant relationship (n = 5) and no significant relationship (n = 6) between obesity and LOS. Most analyses included patients with short-term hospital stays, which may be less influenced by the chronic effects of obesity. Institutional and protocol specifications of treatment, as well as the extended course often incurred by patients with chronic diseases such as cancer, may preclude unbiased studies of LOS. Larger numbers of patients were included in studies that found no significant relationship between obesity and LOS. However, only 1 of these studies controlled for covariates compared with 3 of the 5 studies that found a significant relationship between obesity and LOS. All but 1 of the studies that analyzed for LOS were single-center cohorts, an effect that could be specific to an institution, therefore, may limit the generalizability of the findings.
Limitations of this review include the inconsistency of methods and obesity groupings, as well as the variety of reported study outcomes and populations included. Body mass index often correlates with body composition in large groups, but reliance on BMI percentile cutoffs to assess the influence of obesity on outcomes may further dilute its association with changes in body fat.52 Consistent criteria for identifying excessive body fat in children would help investigators clarify its relationship with clinical outcomes. Among large groups of sick children, BMI z score is likely the most practical currently available marker of excessive body fat because it accounts for age, weight, and height, and it allows the analysis of a specific numeric indicator.
The relationship between obesity and clinical outcomes across the spectrum of childhood illnesses remains inconclusive. Current research is limited by the heterogeneity of populations studied, definitions for obesity, and reported outcomes. This review suggests there may be a relationship between obesity and mortality among children with critical illness, cancer, stem cell transplants, or solid organ transplants. Further study of this relationship is needed. Knowledge of the immediate impact of childhood obesity on important clinical outcomes, such as those investigated in this review, will highlight the relevance of nutrition assessment during acute and chronic illness. Well-designed, large, prospective, multicenter studies of the relationships between pediatric morbidity, mortality, and nutritional outcomes, such as obesity, will provide valuable information to plan appropriate medical and nutritional interventions in acute and chronic care settings.
Supplementary Material
Acknowledgments
Funding/Support: Dr Duggan’s work was supported in part by grant K24HD058795 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Author Contributions: Study concept and design: Bechard, Rothpletz-Puglia, Touger-Decker, and Mehta. Acquisition of data: Bechard. Analysis and interpretation of data: Bechard, Duggan, and Mehta. Drafting of the manuscript: Bechard. Critical revision of the manuscript for important intellectual content: All authors. Obtained funding: Duggan. Administrative, technical, and material support: Bechard and Duggan. Study supervision: All authors.
Conflict of Interest Disclosures: None reported.
Online-Only Material: The eTable is available at http://www.jamapeds.com.
REFERENCES
- 1.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 3.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond) 2011;35(7):891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 5.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 6.Kline AM. Pediatric obesity in acute and critical care. AACN Adv Crit Care. 2008;19(1):38–46. doi: 10.1097/01.AACN.0000310750.85663.ae. [DOI] [PubMed] [Google Scholar]
- 7.Hogue CW, Jr, Stearns JD, Colantuoni E, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009;35(7):1152–1170. doi: 10.1007/s00134-009-1424-5. [DOI] [PubMed] [Google Scholar]
- 8.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 9.Woolford SJ, Gebremariam A, Clark SJ, Davis MM. Incremental hospital charges associated with obesity as a secondary diagnosis in children. Obesity (Silver Spring) 2007;15(7):1895–1901. doi: 10.1038/oby.2007.224. [DOI] [PubMed] [Google Scholar]
- 10.Woolford SJ, Gebremariam A, Clark SJ, Davis MM. Persistent gap of incremental charges for obesity as a secondary diagnosis in common pediatric hospitalizations. J Hosp Med. 2009;4(3):149–156. doi: 10.1002/jhm.388. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama N, Segawa T, Ida H, et al. Bimodal effects of obesity ratio on disease duration of respiratory syncytial virus infection in children. Allergol Int. 2011;60(3):305–308. doi: 10.2332/allergolint.10-OA-0252. [DOI] [PubMed] [Google Scholar]
- 12.Ginde AA, Santillan AA, Clark S, Camargo CA., Jr Body mass index and acute asthma severity among children presenting to the emergency department. Pediatr Allergy Immunol. 2010;21(3):480–488. doi: 10.1111/j.1399-3038.2009.00911.x. [DOI] [PubMed] [Google Scholar]
- 13.Plessa E, Diakakis P, Gardelis J, Thirios A, Koletsi P, Falagas ME. Clinical features, risk factors, and complications among pediatric patients with pandemic influenza A (H1N1) Clin Pediatr (Phila) 2010;49(8):777–781. doi: 10.1177/0009922810368558. [DOI] [PubMed] [Google Scholar]
- 14.Reilly JJ, Methven E, McDowell ZC, et al. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–752. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Tugwell P. The Newcastle- Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed April 16, 2012]; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 16.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 17.Brown CV, Neville AL, Salim A, Rhee P, Cologne K, Demetriades D. The impact of obesity on severely injured children and adolescents. J Pediatr Surg. 2006;41(1):88–91. doi: 10.1016/j.jpedsurg.2005.10.012. discussion 88–91. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan V, Nadkarni VM, Helfaer MA, Carey SM, Berg RA. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Childhood obesity and survival after in-hospital pediatric cardiopulmonary resuscitation. Pediatrics. 2010;125(3):e481–e488. doi: 10.1542/peds.2009-1324. [DOI] [PubMed] [Google Scholar]
- 19.Goh VL, Wakeham MK, Brazauskas R, Mikhailov TA, Goday PS. Obesity is not associated with increased mortality and morbidity in critically ill children. JPEN J Parenter Enteral Nutr. 2013;37(1):102–108. doi: 10.1177/0148607112441801. [DOI] [PubMed] [Google Scholar]
- 20.Carroll CL, Bhandari A, Zucker AR, Schramm CM. Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7(6):527–531. doi: 10.1097/01.PCC.0000243749.14555.E8. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Feng Z, Uyeki TM, et al. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clin Infect Dis. 2011;52(4):457–465. doi: 10.1093/cid/ciq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange BJ, Gerbing RB, Feusner J, et al. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293(2):203–211. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]
- 23.Baillargeon J, Langevin AM, Lewis M, et al. Obesity and survival in a cohort of predominantly Hispanic children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2006;28(9):575–578. doi: 10.1097/01.mph.0000212985.33941.d8. [DOI] [PubMed] [Google Scholar]
- 24.Barker CC, Agovi MA, Logan B, et al. Regimen-Related Toxicity Writing Committee, Center for International Blood and Marrow Transplant Research. Childhood obesity and outcomes after bone marrow transplantation for patients with severe aplastic anemia. Biol Blood Marrow Transplant. 2011;17(5):737–744. doi: 10.1016/j.bbmt.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hingorani P, Seidel K, Krailo M, et al. Body mass index (BMI) at diagnosis is associated with surgical wound complications in patients with localized osteosarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2011;57(6):939–942. doi: 10.1002/pbc.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rana AR, Michalsky MP, Teich S, Groner JI, Caniano DA, Schuster DP. Childhood obesity: a risk factor for injuries observed at a level-1 trauma center. J Pediatr Surg. 2009;44(8):1601–1605. doi: 10.1016/j.jpedsurg.2008.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel L, Cowden JD, Dowd D, Hampl S, Felich N. Obesity: influence on length of hospital stay for the pediatric burn patient. J Burn Care Res. 2010;31(2):251–256. doi: 10.1097/BCR.0b013e3181d0f549. [DOI] [PubMed] [Google Scholar]
- 28.Garey CL, Laituri CA, Keckler SJ, et al. Laparoscopic cholecystectomy in obese and non-obese children. J Surg Res. 2010;163(2):299–302. doi: 10.1016/j.jss.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Hanevold CD, Ho PL, Talley L, Mitsnefes MM. Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics. 2005;115(2):352–356. doi: 10.1542/peds.2004-0289. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman BD, Chuai S, Dobbels F, Shaddy RE. Wasting or obesity at time of transplant does not predict pediatric heart transplant outcomes: analysis of ISHLT pediatric heart transplant registry. J Heart Lung Transplant. 2009;28(12):1273–1278. doi: 10.1016/j.healun.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Dick AA, Perkins JD, Spitzer AL, Lao OB, Healey PJ, Reyes JD. Impact of obesity on children undergoing liver transplantation. Liver Transpl. 2010;16(11):1296–1302. doi: 10.1002/lt.22162. [DOI] [PubMed] [Google Scholar]
- 32.Rossano JW, Grenier MA, Dreyer WJ, et al. Effect of body mass index on outcome in pediatric heart transplant patients. J Heart Lung Transplant. 2007;26(7):718–723. doi: 10.1016/j.healun.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman BD, Nagle ML, Levine SR, et al. Too fat or too thin? body habitus assessment in children listed for heart transplant and impact on outcome. J Heart Lung Transplant. 2008;27(5):508–513. doi: 10.1016/j.healun.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Nafiu OO, Green GE, Walton S, Morris M, Reddy S, Tremper KK. Obesity and risk of peri-operative complications in children presenting for adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2009;73(1):89–95. doi: 10.1016/j.ijporl.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Hijiya N, Panetta JC, Zhou Y, et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood. 2006;108(13):3997–4002. doi: 10.1182/blood-2006-05-024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulley S, Gassas A, Dupuis LL, et al. Inferior outcomes for overweight children undergoing allogeneic stem cell transplantation. Br J Haematol. 2008;140(2):214–217. doi: 10.1111/j.1365-2141.2007.06900.x. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez CV, Anderson J, Breslow NE, et al. National Wilms Tumor Study Group/Children’s Oncology Group. Anthropomorphic measurements and event-free survival in patients with favorable histology Wilms tumor: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52(2):254–258. doi: 10.1002/pbc.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pine M, Wang L, Harrell FE, Jr, et al. The effect of obesity on outcome of unrelated cord blood transplant in children with malignant diseases. Bone Marrow Transplant. 2011;46(10):1309–1313. doi: 10.1038/bmt.2010.312. [DOI] [PubMed] [Google Scholar]
- 39.Kraft R, Herndon DN, Williams FN, Al-Mousawi AM, Finnerty CC, Jeschke MG. The effect of obesity on adverse outcomes and metabolism in pediatric burn patients. Int J Obes (Lond) 2012;36(4):485–490. doi: 10.1038/ijo.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White M, Murphy AJ, Hallahan A, Ware RS, Fraser C, Davies PS. Survival in overweight and underweight children undergoing hematopoietic stem cell transplantation. Eur J Clin Nutr. 2012;66(10):1120–1123. doi: 10.1038/ejcn.2012.109. [DOI] [PubMed] [Google Scholar]
- 41.Morgan OW, Bramley A, Fowlkes A, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5(3):e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25(15):2063–2069. doi: 10.1200/JCO.2006.07.7792. [DOI] [PubMed] [Google Scholar]
- 43.Linam WM, Margolis PA, Staat MA, et al. Risk factors associated with surgical site infection after pediatric posterior spinal fusion procedure. Infect Control Hosp Epidemiol. 2009;30(2):109–116. doi: 10.1086/593952. [DOI] [PubMed] [Google Scholar]
- 44.Fung E, Cave D, Witmans M, Gan K, El-Hakim H. Postoperative respiratory complications and recovery in obese children following adenotonsillectomy for sleep-disordered breathing: a case-control study. Otolaryngol Head Neck Surg. 2010;142(6):898–905. doi: 10.1016/j.otohns.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 45.de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes. 2010;5(6):458–460. doi: 10.3109/17477161003615583. [DOI] [PubMed] [Google Scholar]
- 46.Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 47.Butte NF, Garza C, de Onis M. Evaluation of the feasibility of international growth standards for school-aged children and adolescents. J Nutr. 2007;137(1):153–157. doi: 10.1093/jn/137.1.153. [DOI] [PubMed] [Google Scholar]
- 48.Stoll BJ, Gordon T, Korones SB, et al. Late-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129(1):63–71. doi: 10.1016/s0022-3476(96)70191-9. [DOI] [PubMed] [Google Scholar]
- 49.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271(20):1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 50.Milliken J, Tait GA, Ford-Jones EL, Mindorff CM, Gold R, Mullins G. Nosocomial infections in a pediatric intensive care unit. Crit Care Med. 1988;16(3):233–237. doi: 10.1097/00003246-198803000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 52.Demerath EW, Schubert CM, Maynard LM, et al. Do changes in body mass index percentile reflect changes in body composition in children? data from the Fels Longitudinal Study. Pediatrics. 2006;117(3):e487–e495. doi: 10.1542/peds.2005-0572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.