Abstract
Context
In the United States, levels of public participation in medical research in the era of Clinical and Translational Science Awards (CTSAs) are unknown.
Methods
In 2011, a household survey was administered to a sample of U.S. adults, asking whether they (and children <18 years old) had participated, or were aware of opportunities to participate, in medical research. Respondents living within 100 miles of CTSA sites were identified. Regression analyses of participation and awareness (PA) were performed, applying sampling weights to permit nationally representative inferences.
Results
Overall, 2,150 individuals responded (completion rate = 60%); 65% of adults and 63% of families with children resided within 100 miles of ≥1 CTSA location. Research participation rates were 11% among adults and 5% among children. Among nonparticipants, awareness rates were 64% among adults and 12% among parents of children. PA among adults was associated with higher income and education, older age, presence of chronic conditions, and living within 100 miles of four specific CTSA locations. For children, PA was associated with higher household income and parents’ chronic health conditions.
Conclusions
PA of medical research opportunities is substantially higher for adults than children. Higher PA levels near specific CTSAs merit investigation to identify their successful approaches.
Keywords: participation, subjects, adults, children, translational, awareness, clinical trials
Introduction
Advances in clinical science and medical care depend fundamentally on the participation of human subjects, including healthy volunteers and patients with specific conditions. Prior studies regarding research participation by the public appear chiefly within the domain of disparities, related to age, sex, and race/ethnicity.1, 2, 3, 4, 5
Research on such disparities, however, has largely omitted the larger perspective about population‐level participation in, and awareness about, medical research opportunities for adults and children. This perspective is critically important as the medical research enterprise contemplates growth, yet insufficient public participation threatens the completion of clinical trials.6 Previous estimates of public participation in medical research are 9–10% among adults and 4% among children, based on online sources.7, 8 We are not aware of any previously published, peer‐reviewed assessments of participation in medical research.
Public participation in medical research is particularly salient in the context of the considerable investment by the National Institutes of Health/National Center for Advancing Translational Sciences (NCATS) in Clinical and Translational Science Award (CTSA) sites around the country. A central premise and activity of CTSA sites is community engagement, through which academic centers inform and invite members of the public to participate in translational science initiatives.
Therefore, measures of public participation in—and awareness of—opportunities for medical research can serve as an objective measure of CTSA activities. To provide such a measure, we conducted a nationally representative household survey that asked respondents to indicate their prior participation in medical research and also their awareness about such research opportunities. Respondents with children were asked similar questions about their children. For adults and children separately, we used a composite measure of participation or awareness (PA) to assess whether proximity to a CTSA site is associated with these indicators of engagement.
Methods
Study design
We conducted a nationally representative, cross‐sectional, household survey of the U.S. population. The study was approved by the University of Michigan Medical School Institutional Review Board.
Sample
As part of the C.S. Mott Children,s Hospital National Poll on Children's Health (NPCH), a recurring online survey of parents and nonparents, we fielded the survey in January 2011. The NPCH is conducted in partnership with GfK and their web‐enabled KnowledgePanel, a probability‐based panel designed to be representative of the U.S. population, including cell‐phone only households.9 Initially, participants are chosen by a random selection of telephone numbers and residential addresses. Persons in selected households are then invited by telephone or by mail to participate in the web‐enabled panel. For those who agree to participate but do not already have Internet access, Knowledge Networks provides a laptop and ISP connection at no cost. Individuals who already have computers and Internet service are permitted to participate using their own equipment. Panelists then receive unique log‐in information for accessing surveys online, and are invited one or more times per month to participate in research.
For this survey, a unique sample was drawn from KnowledgePanel. The NPCH sample included oversampling of parents (adults with children 0–17 years living in the household), to ensure adequate representation of children. This NPCH/KnowledgePanel data collection method has served as the data source for several peer‐reviewed publications about health and health policy‐related issues.10, 11, 12, 13
Survey items
Questions about prior participation in medical research were drawn from the authors’ prior national survey of public research participation.7 New questions were developed by the authors for this study regarding awareness of opportunities to participate in medical research and the modes through which respondents had heard about research opportunities. Questions were pilot‐tested in November 2010 with a separate convenience sample of 100 KnowledgePanel members, to ensure that questions were clearly worded.
Survey administration
The final survey was fielded in January 2011, with 2,150 households responding (completion rate = 60%); 1,558 households included at least one child <18 years old.
Key variables
Knowledge Networks provided de‐identified data, along with Census‐based poststratification weights used to match the U.S. population distribution on gender, age, race/ethnicity, education, census region, and urban versus rural location.
Sociodemographic information for each household respondent was available from Knowledge Networks. Ages, self‐reported doctor‐diagnosed health conditions, and health status were reported by respondents for themselves and for their children.
CTSA proximity was based on the center of the respondent,s zip code area and the center of the zip code area of each CTSA site's central administrative office, for the 55 CTSA sites active in January 2011. If the respondent lived within 100 miles of one or more CTSA sites, then they were considered to have “proximity” to a CTSA. One hundred miles was selected by the authors as a plausible distance for most individuals and families to travel to access medical research opportunities. Given the location of several CTSA sites within 100 miles of each other, we assigned two or more CTSA sites to 10 “metro CTSAs” (in alphabetical order): Baltimore & Washington, Boston & Worcester, Chicago, Houston & Galveston, Los Angeles, Milwaukee & Madison, New York City & New Haven, Raleigh‐Durham‐Chapel Hill Research Triangle, San Diego, San Francisco Bay Area.
For CTSAs in comparatively less populated areas (Medical University of South Carolina, University of Alabama‐Birmingham, University of Arkansas‐Little Rock, University of Iowa, University of New Mexico), our sample included too few respondents (<20 per CTSA) to permit robust estimates. Respondents from these sites (n = 54) were excluded from the analysis, leaving an analytic sample of n = 2,096 (including 1,507 with at least one child <18 years old in the household).
Frequency distributions were calculated on all weighted items. Bivariate analyses of respondent PA versus the demographic variables of interest and CTSA proximity were performed using linear regression. Only statistically significant predictors from bivariate analyses (p < 0.05) were included in multivariate logistic regression analyses. All analyses were conducted with Stata 10 (Stata Corporation, College Station, TX, USA). All results reflect statistically weighted data to permit national inferences.
Results
Study sample
Characteristics of the nationally representative sample are presented in Table 1, for adult respondents and their children. Overall, 65% of adults and 63% of families with children lived within 100 miles of one or more CTSAs.
Table 1.
Characteristics of study sample regarding adults and children.*
| Adults (n = 2,096 households) | Children (n = 1,507 households) | |
|---|---|---|
| Age of respondent (years) | ||
| 18–29 | 18% | 22% |
| 30–44 | 29% | 54% |
| 45–64 | 29% | 23% |
| 65+ | 24% | 1% |
| Age of oldest child in household (years) | ||
| 0–5 | – | 24% |
| 6–11 | 30% | |
| 12–17 | 46% | |
| Race/Ethnicity | ||
| Non‐Hispanic white | 70% | 64% |
| Non‐Hispanic black | 10% | 11% |
| Hispanic | 13% | 18% |
| Non‐Hispanic other | 7% | 7% |
| Highest education level in household | ||
| Bachelor's degree or more | 29% | 30% |
| Annual household income | ||
| $100,000 or more | 12% | 14% |
| Adult has self‐reported, doctor‐diagnosed medical condition | ||
| Yes | 60% | 53% |
| Child health status** | ||
| Fair/poor | – | 19% |
| Proximity to CTSA | ||
| Resides 100 miles or less from CTSA | 65% | 63% |
*We have simplified the categorization of some variables to reflect the key distinctions identified in subsequent analyses.
**Reflecting the lowest health status of any child in the household.
Prior participation in medical research
When asked about participation in medical research at any point in time, 11% of adults and 5% of children indicated that they had participated.
Among adults who had participated, 58% indicated that they had done so by making special visits for research opportunities, and 36% had participated at specialty visits. Research participation was less common at primary care visits (19%), during hospital stays (13%), and at emergency medical visits (10%; percentages exceed 100% because respondents could endorse more than one location for participation).
Similarly, adults reported that children who had participated in research were most likely to report doing so in special visits (31%), followed by primary care (24%), hospital care (23%), and specialty care (20%) encounters. No respondents indicated that their children had participated in emergency care settings.
Awareness of opportunities to participate in medical research
Among adults who had not previously participated in medical research, 64% said they were aware of opportunities to participate. In contrast, 12% of parents whose children had not previously participated in medical research indicated that they were aware of opportunities for their children to participate.
Common sources of information about opportunities for participation in medical research were largely similar between adults and children (Table 2). In general, advertisements in print or broadcast media were cited more often than pamphlets in doctors’ offices and online advertisements.
Table 2.
Sources of information about opportunities for medical research endorsed by the public
| Most common sources | Proportion identifying as source for information about medical research* |
|---|---|
| Adults | |
| Television commercial | 53% |
| Radio commercial | 46% |
| Newspaper advertisement | 44% |
| Online/web advertisement | 22% |
| Pamphlet at doctor's office | 21% |
| Children | |
| Radio commercial | 36% |
| Newspaper advertisement | 32% |
| Television commercial | 29% |
| Pamphlet at doctor's office | 25% |
| Online/web advertisement | 18% |
*Proportions sum to >100% in each age group because respondents could endorse more than one source.
Association of CTSA proximity with PA
With PA as a composite outcome, there was broad variation across CTSAs. For adults (Figure 1), PA ranged from 46% to 97% across 30 CTSA sites we considered (20 stand‐alone CTSA sites, plus 10 metro CTSAs indicated by “m” suffixes in the site labels). This range of levels of PA reported by respondents in proximity to CTSAs spanned the level of PA (66%) reported by respondents living beyond 100 miles of any CTSA. Year of CTSA award was not associated with PA levels. Two metro CTSAs had PA rates in the top tercile, while four metro CTSAs appeared in the bottom tercile.
Figure 1.
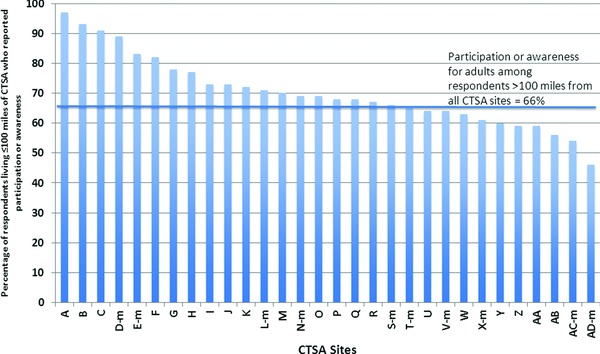
Proportion of adults in sample living in proximity (≤100 miles) to CTSA sites who report prior participation in, or awareness of, opportunities for medical research.
The letter “m” after a CTSA designation indicates a metro CTSA of ≥2 CTSA sites; see Methods for details.
For children, PA rates among households in proximity to CTSAs ranged from 9% to 32%, compared with 4% among households beyond 100 miles of any CTSA (Figure 2). With CTSA labels maintained from Figure 1 to Figure 2, it is evident the public is participating and perceiving opportunities differently for adults versus children. As in the adult analysis, for children's research PA two metro CTSA sites appeared in the top tercile of PA rates while four metro CTSAs appeared in the bottom tercile. Yet, several CTSA sites fell in different terciles than for the comparison of PA by CTSAs for adults. Again, year of CTSA award was not associated with PA levels.
Figure 2.
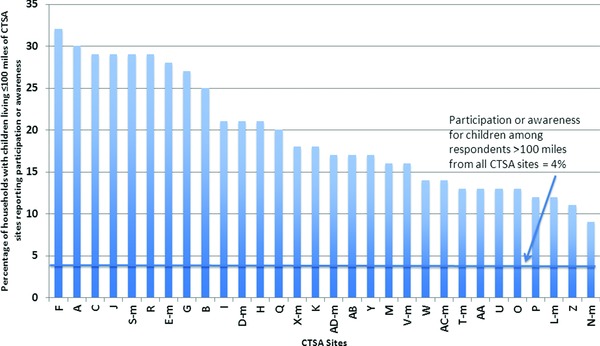
Proportion of households with children living in proximity (≤100 miles) to CTSA sites who report prior participation in, or awareness of, opportunities for medical research.
The letter “m” after a CTSA designation indicates a metro CTSA of ≥2 CTSA sites; see Methods for details. CTSA labels in this figure are consistent with labels in Figure 1, to facilitate comparisons.
Multivariate analysis of factors associated with participation and awareness
Based on bivariate analyses for adult PA, respondent age, race/ethnicity, household income, education, and chronic conditions were included in multivariate analyses (Table 3). Older age (especially 65 years and older) and higher education and income were associated with higher odds of PA, as was the presence of a chronic health condition for the adult. Proximity to each of the four specific CTSA sites—those four with highest PA rates in unadjusted analyses (Figure 1)—was associated with significantly higher odds of PA, controlling for the sociodemographic factors in the model. Otherwise, proximity to CTSA sites was not associated with higher or lower PA than living more than 100 miles from any CTSA site.
Table 3.
Adjusted odds of composite participation in and awareness (PA) about medical research for adults and for children
| Adjusted odds of PA for adults (95% CI) | Adjusted odds of PA for children (95% CI) | |
|---|---|---|
| Age of respondent (years) | ||
| 18–29 | 1.00 | (p > 0.05 in bivariate) |
| 30–44 | 1.62 (1.07–2.45) | |
| 45–64 | 1.58 (0.99–2.54) | |
| 65+ | 2.06 (1.21–3.49) | |
| Race/Ethnicity | ||
| Non‐Hispanic white | 1.00 | (p > 0.05 in bivariate) |
| Non‐Hispanic black | 0.78 (0.46–1.34) | |
| Hispanic | 0.86 (0.53–1.42) | |
| Non‐Hispanic other | 1.03 (0.52–2.01) | |
| Respondent education | ||
| Less than a bachelor's degree | 1.00 | 1.00 |
| Bachelor's degree or more | 2.28 (1.29–4.04) | 1.27 (0.62–2.60) |
| Annual household income | ||
| <$100,000 | 1.0 | 1.0 |
| $100,000 or more | 2.34 (1.27–4.28) | 2.00 (1.09–3.67) |
| Chronic condition (adult) | ||
| No | 1.0 | 1.0 |
| Yes | 1.56 (1.14–2.14) | 1.77 (1.24–2.53) |
| CTSA proximity* | ||
| >100 miles from any CTSA | 1.0 | 1.0 |
| 100 miles or less from… | ||
| Site D‐m | 5.80 (1.81–18.6) | (no sites significantly different from reference group) |
| Site B | 6.72 (1.76–25.7) | |
| Site C | 7.49 (2.14–26.2) | |
| Site A | 14.6 (2.89–74.0) |
Based on bivariate analyses of child PA, household education, income, and presence of a chronic condition for the parent respondent were entered into multivariate analyses. Of note, race/ethnicity and children,s own health status were not associated with children,s PA in bivariate analyses and therefore were not included in multivariate models. In adjusted analyses (Table 3), odds of children,s PA were associated with higher household income, but parental education was not a factor. Presence of a chronic condition in the parent respondent was also associated with higher odds of children,s PA. In contrast to adults, children,s PA was not associated with proximity to any specific CTSA sites.
Comment
This study presents the first known nationally representative measure of public participation of and awareness about medical research opportunities in the era of the federal CTSA program. As the first such study, it begins to fill gaps in our collective knowledge about public engagement regarding medical research at the national level. These gaps concern overall levels of PA for adults and children, current sociodemographic factors that continue to be associated with research engagement, and the relationship of CTSAs to public engagement in their early years.
Overall levels of participation and awareness regarding medical research
Whether research participation by 11% of adults and 5% of children appears high or low depends entirely on context. There is a limited set historical benchmarks for these levels, measured in 2008 at 9–10% for adults and 4% for children using similar methods.7, 8 From the perspective of investigators whose research efforts are hindered by underenrollment, participation rates at this level merit major engagement efforts. Given national attention to medical research and its benefits for clinical care, it is remarkable that there is no estimate—nor any current means to estimate—the need for research participants on a national, regional, or local basis. Even a data source such as ClinicalTrials.gov that serves as a repository for trial information does not provide data about enrollment success rates.
Yet, consider the following estimates based on these latest national findings: 11% of adults of all ages amount to more than 20 million experienced research participants; 5% of children total more than 3 million who have previously participated. Even if only half of these individuals wish to participate again, more than 10 million adults and children would be potentially available. Is it possible that the aggregate subject need in the United States for currently open medical research studies exceeds 10 million individuals? Likely not, in which case this finding suggests that awareness of opportunities or a match of research needs with potential participant characteristics, rather than a public willingness to participate, is a key limiting factor in enrollment.
Another enrollment challenge is that studies carry a steadily shifting set of eligibility criteria, as scientific understanding evolves, studies focus on specific diseases and funding priorities shift. Changing criteria for eligibility drive the imperative for program initiatives to inform the public about new opportunities to participate in medical research. In this study, the awareness of research opportunities among nonparticipants was fivefold higher for adults than for children. Such a broad gap may reflect a similarly greater number of open studies for adults than for children. Alternatively, the difference may reflect varying communication strategies for adult research versus children's research. Regardless of the reason, low awareness rates among parents about children's research present a major challenge to near‐term success.
Factors associated with research engagement
Amid these challenges, this study offers some positive news about engagement of the public in research. Over the last several decades, several investigators have described major disparities in medical research by sex and race/ethnicity.1, 2, 3, 4, 5, 6 However, those disparities are not evident in this national analysis of PA at the population level, for either adults or children. Instead, we observed disparities related to income and education—two factors that are less immediately visible than sex and race/ethnicity but nonetheless merit the same attention and effort that have helped reduce sex‐ and race/ethnicity‐related disparities in research participation in recent years.
To our knowledge, this is the first study to find a difference in research participation and awareness by presence of chronic conditions for adults. On one hand, we would be surprised not to find this association for adults, given the predominant focus in medical research on therapeutic modalities. On the other hand, our finding that parents’ chronic conditions (and not children's own health status) are associated with higher odds of PA for children was unexpected. This may suggest that adults with chronic conditions are generally more aware of communication about research opportunities and the limitation of current disease management options, or that parents with chronic conditions may be more inclined to consider medical research for their children. These possibilities deserve further exploration as researchers work to engage parents around opportunities for their children.
CTSA sites and research engagement
The CTSA program, in its 5th year with 55 separate sites at the time this study was conducted, represents an unprecedented effort at the national level to leverage the strengths of many institutions toward greater accomplishments in translational and clinical science. CTSA accomplishments will take many forms; without sufficient participation of the public, however, the promise of translational programs will be limited.6 In this study, the first to attempt to measure the possible population‐level impact of CTSA sites in encouraging PA regarding medical research for adults and children, the results are mixed.
For adults, CTSA sites differed twofold in the levels of PA for respondents within 100 miles. When adjusted for relevant sociodemographic and health factors, four sites stood apart from other consortium programs, and clearly distinct from no CTSA proximity at all. Importantly, the year of CTSA award was not associated with PA—suggesting that PA is not strictly related to time and opportunities to connect with community members and organizations. Rather, these four sites may be conducting their outreach, communication, and engagement in unique ways from which other sites can learn; their strategies may have preceded their CTSA status.
For children, the impact of CTSA sites is not as evident. In fact, when controlled for sociodemographic factors, proximity to CTSA sites was no different in terms of PA from living >100 miles from a CTSA. Possible explanations for this finding, which merit further investigation, include that levels of PA for children are generally so low across the country that no CTSA site is distinct in this sample size. In addition, CTSA sites may have prioritized and had more investigators dedicated to adult‐oriented than child‐oriented research and community engagement efforts within their first 5 years.
This study also raises questions about public engagement in urban areas with multiple active CTSA sites—identified here as metro CTSAs. It is reasonable to expect that academic institutions in major metropolitan areas would leverage resources such as outreach and thereby facilitate high levels of PA. However, that is not what we found: among the 10 metro CTSAs we specified, only two rated in the top tercile for adult PA. Only one other metro CTSA rated in the top tercile for children's PA. These findings suggest that public engagement efforts may need to be better coordinated among sites within metro CTSAs, in order to identify opportunities for synergy.
In particular, metro CTSAs may provide economies of scale in offering services such as participation‐related transportation services and child care that can mitigate income‐related challenges that individuals and families face. Metro CTSAs may also find efficiencies in advertising to boost public awareness about trial opportunities, and in merging their databases of available research opportunities and individuals who have expressed interest in participating in future research initiatives. With such strengths, metro CTSAs are especially well suited have the potential to address many of the recognized barriers to recruitment and retention of research subjects in clinical trials, including communication with the public and consolidation of infrastructure.14 However, natural institutional boundaries among academic centers must be overcome in order to maximize benefits for the public through the CTSA model.
Limitations
As a cross‐sectional survey, this study has certain limitations. It was not possible to explore the reasons underlying respondents’ responses in greater depth. In addition, consistent with the nature of surveys, there is the potential for response bias, which may have led to the participation of people with a particular interest in medical research. However, because the survey covered a number of pediatric topics beside medical research and did not mention research in the invitation, it is unlikely that the majority of respondents had a specific interest in this topic. Furthermore, the findings in this study—while statistically significant—are associations and cannot be interpreted as causal.
Finally, while the national scope of this study is its chief strength, our sample size ultimately limited our ability to distinguish the PA levels in the population proximal to certain CTSAs from each other, and to measure PA levels in robust manner for five CTSAs in less heavily populated areas. Despite these limitations, we were able to identify four CTSA sites that appear to have significantly higher adult PA rates in the populations living proximal to them. It is possible that even more sites could be identified as significantly different from the reference group with a larger sample size.
Conclusions
These limitations notwithstanding, we believe that this study provides uniquely informative findings that may serve as a helpful guide in the near term for facilitating greater public engagement through translational research efforts in general, and via CTSA programs specifically. Ultimately, repeated measures of public participation and awareness regarding medical research for adults and children will provide one of the most illustrative ways to gauge the success of the translational research era.
Access to Data
Authors Davis, Clark, Butchart, and Singer had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding
This research was funded by the Michigan Institute for Clinical and Health Research (MICHR; NCRR UL1RR024986) and the University of Michigan Health System.
Acknowledgments
The authors are grateful for the support of the University of Michigan Health System and the Department of Pediatrics and Communicable Diseases at the University of Michigan, which support the C.S. Mott Children's Hospital National Poll on Children's Health.
References
- 1. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race‐, sex‐, and age‐based disparities. JAMA. 2004; 291: 2720–2726. [DOI] [PubMed] [Google Scholar]
- 2. Corbie‐Smith G, Thomas SB, George DMM St.. Distrust, race, and research. Arch Intern Med. 2002; 162: 2458–2463. [DOI] [PubMed] [Google Scholar]
- 3. Gifford AL, Cunningham WE, Heslin KC, Andersen RM, Nakazono T, Lieu DK, Shapiro MF, Bozzette SA. Participation and research and access to experimental treatments by HIV‐infected patients. N Engl J Med. 2002; 346: 1373–1382. [DOI] [PubMed] [Google Scholar]
- 4. Smith YR, Johnson AM, Newman LA, Greene A, Johnson TRB, Rogers JL. Perceptions of clinical research participation among African American women. J Women‘s Health. 2007; 16: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wendler D, Kington R, Madans J, Van Wye G, Christ‐Schmidt H, Pratt LA, Brawley OW, Gross CP, Emanuel E. Are racial and ethnic minorities less willing to participate in health research? PLoS Medicine. 2006; 3: e19 Doi:10.1371/journal.pmed.0030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins FS. The importance of clinical trials. NIH Medline Plus. Summer 2011. Available at: http://www.nih.gov/about/director/articles/medlineplus_07262011.pdf. Accessed November 11, 2011.
- 7. Davis MM, Singer DC, Butchart A, Clark SJ. Children in research: many parents willing if risk of harm is small C.S. Mott Children‘s Hospital National Poll on Children‘s Health, University of Michigan; Vol 3, Issue 5, May 2008. Available at: http://www.med.umich.edu/mott/research/chearChildrenInResearch.html. Accessed November 11, 2011. [Google Scholar]
- 8. Young K. Medical research studies struggle to recruit women. Society for Women‘s Health Research. Available at: http://www.womenshealthresearch.org/site/News2?page=NewsArticle&id=7593. Accessed August 27, 2012.
- 9. Dennis JM. Summary of KnowledgePanel(R) Design. 2010; . http://www.knowledgenetworks.com/ganp/reviewer‐info.html Accessed August 17, 2011.
- 10. Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010; 125: 654–659. [DOI] [PubMed] [Google Scholar]
- 11. Dempsey AF, Singer DC, Clark SJ, Davis MM. Parents’ views on three shot‐related visits: implications for use of adolescent vaccines like human papillomavirus vaccine. Acad Pediatrics. 2009; 9: 348–352. [DOI] [PubMed] [Google Scholar]
- 12. Woolford SJ, Clark SJ, Gebremariam A, Davis MM, Freed GL. To cut or not to cut: physicians ‘perspectives on referring adolescents for bariatric surgery. Obes Surg. 2010: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tarini BA, Goldenberg A, Singer D, Clark SJ, Butchart A, Davis MM. Not without my permission: parents ‘willingness to permit use of newborn screening samples for research. Public Health Genomics. 2010; 13: 125–130. [DOI] [PubMed] [Google Scholar]
- 14. Probstfield JL, Frye RL. Strategies for recruitment and retention of participants in clinical trials. JAMA. 2011; 306: 1798–1799. [DOI] [PubMed] [Google Scholar]


