Abstract
Background
Major depressive disorder (MDD) is a highly recurrent condition, and improving our understanding of the abnormalities that persist in remitted MDD (rMDD) may provide insight into mechanisms that contribute to relapse. MDD has been characterized by reward learning deficits linked to dysfunction in frontostriatal regions. Although initial behavioral evidence of reward learning deficits in rMDD has recently emerged, it is unclear whether these reflect impairments in neural reward processing that persist into remission.
Methods
We examined behavioral reward learning and 128-channel event-related potentials (ERP) during a well-validated probabilistic reward task in 26 rMDD individuals and 34 never-depressed controls. Temporo-spatial principal components analysis (PCA) was used to separate overlapping ERP components, and group differences in neural activity in a priori regions were examined using low-resolution electromagnetic tomography (LORETA).
Results
Individuals with rMDD displayed reduced behavioral reward learning, as well as blunted ERP amplitude to reward feedback. Importantly, the reduction in ERP amplitude occurred at a PCA factor that peaked during the time at which phasic reward feedback-related signaling – hypothesized to originate in the striatum and project to the anterior cingulate cortex (ACC) – are thought to modulate scalp-recorded activity. Consistent with this, LORETA analyses revealed reduced activity in the ACC in the rMDD group, and this blunting correlated with poorer reward learning.
Conclusion
These findings suggest that the reward learning impairment observed in acute MDD persists into full remission and that these impairments may be attributable to abnormalities in the neural processes that support reward feedback monitoring, particularly within the ACC.
Keywords: major depression, anhedonia, reward learning, ERP, feedback-related negativity, anterior cingulate
Introduction
One of the most debilitating features of major depressive disorder (MDD) is its recurrent nature, with patients experiencing, on average, five to nine major depressive episodes (MDEs) in their lifetime [1, 2]. Understanding the neural abnormalities that persist between depressive episodes may provide insight into the mechanisms underlying increased relapse risk in those with remitted MDD (rMDD).
Impaired reward learning as a vulnerability factor for depression
Impaired reward learning has emerged as a key characteristic of MDD and may be a vulnerability marker in remitted individuals. Prior research has shown that individuals with acute MDD [3, 4], particularly those reporting anhedonia [5], show diminished reward responsiveness on reward learning paradigms, and the degree of impairment has been linked to the severity of depressive symptoms [3]. Although some studies report normalization of reward responsiveness following recovery [6], others have shown evidence of persistent impairments in remitted samples [7]. Evidence of hyposensitivity to reward has also been found in the non-depressed offspring of mothers with depression history [8, 9], and in adolescent girls who later go on to develop depression [10]. Collectively, these emerging findings indicate that blunted reward learning may be an endophenotype of MDD that exists in both symptomatic individuals as well as euthymic individuals who are at increased risk.
Neural signatures of reward processing
Event-related potentials (ERP) possess millisecond temporal resolution, and examining modulations in ERP waveforms provides an ideal means of probing the integrity of reward learning systems in remitted individuals. Of particular interest are ERP components that reflect the activity of the performance-feedback monitoring system. This system causes a negative deflection – known as the feedback-related negativity (FRN) – 200–300 ms following receipt of feedback, which is larger for outcomes that are worse than expected (for a review see [11]). Smaller amplitudes are observed following rewards, and although this has been referred to in prior research also as an FRN or a feedback-related positivity (FRP), it has been recently argued that the smaller amplitude results from a second reward positivity (RewP) that is superimposed on the FRN, rather than variation in the FRN per se [12, 13]. Therefore, in the current study we refer to the variation in FRN amplitude following reward feedback as the RewP.
Studies using ERP in conjunction with fMRI [14, 15] have linked variation in RewP amplitude to activation within the “brain reward pathway,” particularly the ventral striatum, anterior cingulate cortex (ACC), and midfrontal cortex. Pharmacological manipulation hypothesized to reduce phasic striatal dopaminergic (DA) responses has also been found to affect RewP and underlying ACC activation [16], suggesting that the RewP may provide an index of phasic reward signaling that originates in the striatum and projects to the ACC. Consistent with behavioral findings of blunted reward learning in MDD, reduced RewP amplitude has been observed in individuals with current MDD [17, 18] and offspring with a family history of depression [9]. Thus, examining the RewP during reward learning may pinpoint mechanisms contributing to reward processing deficits in remitted individuals. However, few studies have examined the RewP in remitted samples and none has done so in the context of reward learning. Therefore, a critical question remains as to whether reward processing deficits persist into remission.
Isolating reward processing deficits in rMDD
The aim of this study was to examine whether reward learning deficits persist in individuals with rMDD and whether any deficits are associated with abnormalities in discrete aspects of reward processing, as indexed by ERPs. Given that key reward processing components (e.g., FRN, RewP, P300) overlap in time, a temporo-spatial principal components analysis (PCA) was used to improve the separation of overlapping component processes [19]. In line with prior evidence linking acute MDD with specific reductions in reward-related ERP amplitudes during the time frame of the RewP, we predicted that individuals with rMDD would also show specific blunting of the RewP during a probabilistic reward learning paradigm.
A second aim of this study was to identify neural sources of putative group differences in reward processing. Prior evidence has pointed strongly to the ACC as having a critical role in reward feedback monitoring, particularly when integration of reward probabilities is required [14, 20]. However recent meta-analyses indicate that, in addition to subcortical (e.g., striatal) regions and ACC, the posterior cingulate, insula and pre-supplementary motor area (pre-SMA) also show increased activation during reward-based tasks [21, 22]. Therefore, we first capitalized on the millisecond temporal resolution of ERP to isolate time periods in feedback processing that differed between controls and rMDD individuals, and then used a region-of-interest (ROI) approach with clusters defined on the basis of two recent meta-analyses of reward-related fMRI studies to identify potential group differences in activation within distinct regions of the reward circuit.
Methods and Materials
Participants
Forty-two never-depressed controls and 30 individuals with rMDD were recruited for this study. Controls were eligible if they were free of lifetime DSM-IV diagnoses, had no first-degree relatives with psychiatric illnesses, had a Beck Depression Inventory-II (BDI-II; [23]) score below 13, and had not used psychotropic medication in the past. rMDD subjects were eligible if they had experienced at least one MDE in the past 5 years, had been in remission from depression for at least 8 weeks as indicated by a score of 1 on the Depressed Mood and Anhedonia items from the Structured Clinical Interview for DSM-IV (SCID; [24]) and no SCID score above 2 for other MDD symptom items, were free of psychotropic medication (wash-out periods were applied), and had no lifetime DSM-IV diagnoses other than depression (substance abuse was allowed if in remission for at least 12 months, PTSD was allowed if in remission for at least 24 months, and other anxiety disorders were allowed if secondary to the MDD and in remission for at least 2 months).
Exclusion criteria for all subjects were: seizures, hypothyroidism, loss of consciousness longer than 2 minutes, or a positive urine screen for illicit drugs (Amedicheck CLIA-Waived 12-panel cup).
Procedure
The study consisted of two sessions. In the first, subjects were administered the SCID by Masters- or PhD-level clinical interviewers and completed the BDI-II. In the second session, participants performed a reward learning task while 128-channel ERPs were recorded. To ensure depressive symptom stability, the BDI-II was administered again at the second session.
Probabilistic reward task (PRT)
To probe reward learning, participants completed a 15-minute computer-based probabilistic reward task (PRT; [3, 25]). Rooted within signal detection theory, the PRT assesses participants’ propensity to modulate behavior based on reinforcement. The task consists of several trials in which cartoon faces are presented in the center of the monitor. Trials begin with a fixation cross (500 ms), followed by a face with no mouth. After a 500-ms delay, a short mouth (10 mm) or a long mouth (11 mm) is presented for 100 ms. Participants are instructed to indicate whether the short or long mouth was presented via keypress. The PRT included 2 blocks of 100 trials, and a total of 40 correct trials per block were followed by monetary reward feedback (“Correct!! You won 20 cents”). Participants were told to try and win as much money as possible and that not all correct trials would be rewarded. Long and short mouths were presented at equal frequency however, unbeknownst to participants, one of the mouth lengths (the ‘rich stimulus’) was rewarded three times more frequently than the other (the ‘lean stimulus’). As this was part of a larger study, half of the participants in each group were administered a version of the PRT where the length of the nose varied instead of the mouth.
Behavioral and ERP data reduction
Behavioral data
PRT data were subject to a quality control assessment that is outlined in detail elsewhere [25] and in the Supplement. Briefly, trials where the reaction time (RT) was < 150 ms or > 1500 ms were excluded, as were remaining trials with RT falling ± 3SD from the mean. Next, signal detection analysis [26] was used to calculate response bias (the tendency to bias responding to the rich stimulus) and discriminability (the ability to distinguish between the mouth sizes) for each block of the PRT using the following formulae:
To compute response bias and discriminability for cases that had a zero in the formula, 0.5 was added to every cell in the matrix [27]. Mean accuracy (hit rates) and RT in response to rich and lean stimuli were also computed.
Scalp data
EEG was recorded using a 128-channel Hydrocel Geodesic Sensor Net system (Electrical Geodesics, Inc.) and sampled at 250 Hz (bandwidth, 0.1–100 Hz; impedances < 100 kΩ) referenced to the vertex electrode (Cz) at acquisition. Data processing occurred offline using BrainVision Analyzer 2.0 (Brain Products GmbH). Blinks, horizontal eye movement, and electrocardiogram were removed using independent components analysis [28] and corrupted channels were interpolated using a spherical spline interpolation [29]. Data were then re-referenced to the average reference and filtered (0.1–30 Hz). Epochs were extracted from −250 ms to 1050 ms around presentation of reward feedback following correct identification of the rich stimulus (segments from lean stimulus trials were not used because of the low number of usable segments). Epochs were visually inspected, had remaining artifacts removed, were baseline-corrected (−250 ms to 0 ms) and averaged. Additional information about the artifact rejection criteria used is presented in the Supplement.
The time-domain ERP elicited in response to reward feedback was decomposed using temporo-spatial PCA with the ERP PCA Toolkit [30]. In accordance with prior guidelines [31], temporal variance in the averaged ERP waveforms was first examined using temporal PCA and Infomax rotation. Based on the resulting scree plot, 12 temporal factors were retained for rotation. Spatial distributions of these factors were then examined using spatial PCA and Infomax rotation, with a separate spatial PCA being conducted for each temporal factor; 5 spatial factors were retained for each temporal factor. We focused analyses on the PCA components that showed a timing and topography most consistent with a possible RewP. There were three such components - Temporal Factor 4/Spatial Factor 1 (TF4/SF1), Temporal Factor 4/Spatial Factor 2 (TF4/SF2) and Temporal Factor 6/Spatial Factor 1 (TF6/SF1).
Source localization
Low-resolution electromagnetic tomography (LORETA; [32]) was used to estimate the intracerebral current density in a priori ROIs. The LORETA solution space was restricted to gray matter and hippocampus in the Talairach atlas and was divided into 2,394 voxels (7mm3). The ERP time window of interest for the LORETA analyses was chosen as the global field power peak (GFP) occurring 200–300 ms following reward feedback. GFP is reference-free and was computed as the spatial standard deviation of the brain electrical field; therefore, it reflects brain activity across the entire scalp. In the current study, we extracted mean activity around the GFP peak (248 ms ± 20 ms) following receipt of reward feedback.
Current density was then analyzed using an ROI approach. ROIs were defined based on two independent meta-analyses of fMRI studies examining neural activity in response to reward [21, 22]. Given that LORETA has lower spatial resolution than fMRI, it was not always possible to identify a LORETA voxel that exactly matched the peak reported in the fMRI meta-analyses. To circumvent this issue, a LORETA cluster was defined such that its center of gravity was closer to the fMRI coordinates than any single LORETA voxel, as done in prior work [33]. In cases where the two meta-analyses identified slightly different peak coordinates for the same regions, individual LORETA clusters were defined using the strategy outlined above and the mean center of gravity of the two clusters was calculated. A LORETA ‘meta-cluster’ was then defined whose center of gravity was closest to this mean. When defining the LORETA clusters, two parameters were emphasized: a) minimization of the distance between the fMRI peak coordinates and the LORETA ROI center of gravity, and b) similarity of size between the LORETA cluster and the ROI size in the fMRI meta-analyses. The LORETA voxels that contributed to each ROI are listed in the Supplement. ROI analyses were carried out using SPAMalize (http://brainimaging.waisman.wisc.edu/~oakes).
Statistical analyses
Behavioral data
Response bias and discriminability were analyzed using Group (control, rMDD) × Block (block 1, block 2) analyses of covariance (ANCOVAs), entering mean BDI-II score (averaged across sessions) as a covariate (see Results). For accuracy and RT, a second within-subjects variable Stimulus (rich, lean) was added.
PCA factors
A Group (control, rMDD) × PCA factor (TF4/SF1, TF4/SF2, TF6/SF1) ANCOVA was used to determine whether there were differences in the mean amplitude of the scalp-recorded PCA factors after controlling for BDI-II scores.
LORETA ROIs
For each ROI, an ANCOVA with Group as the between-subjects factor was used to determine whether there were group differences in levels of reward-related neural activity within the LORETA ROIs after controlling for BDI-II scores.
Results
Sample
Thirty-four controls and 26 rMDD individuals had PRT data that met quality control criteria. Demographic and clinical characteristics are shown in Table 1. Although the mean BDI-II scores for both groups were well below clinical threshold, the rMDD group had significantly higher scores at both screening and at the time of EEG recording. Therefore, these two BDI-II scores were averaged and the mean score used as a covariate in all subsequent analyses.
Table 1.
Demographic and clinical characteristics
| Controls (n = 34) | rMDD (n = 26) | test value | p value | |
|---|---|---|---|---|
| Female, N (%) | 23 (67.6) | 19 (73.1) | χ2 = 0.21 | .65 |
| Age (yrs) | 29.8 (10.9) | 30.6 (14.0) | t = −0.25 | .81 |
| Years of education post-high school | 4.6 (2.4) | 4.5 (2.1) | t = 0.24 | .81 |
| BDI-II | 0.9 (1.9) | 2.0 (1.8) | t = −2.25 | .03 |
| Number of prior MDEs | N/A | 2.3 (1.6) | N/A | |
| Time in remission (months) | N/A | 23.4 (13.4) | N/A | |
| Past comorbidities, N (%) | 0 (0.0) | 8 (30.8)a |
Values are in mean (SD) unless otherwise indicated (%).
rMDD=remitted major depressive disorder; yrs=years; BDI-II=Beck Depression Inventory-II total score; MDEs=major depressive episodes;
Substance-related (n=8), post-traumatic stress disorder (n=2), panic disorder (n=1); 3 participants had two of these past comorbidities.
Behavioral Data
Response bias
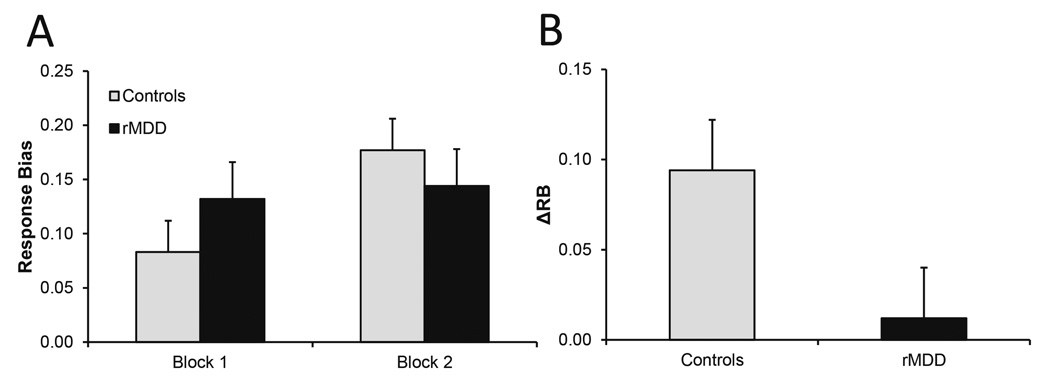
A trend-level Group × Block interaction emerged F(1,57) = 3.33, p = .07, ηp2 = .06 (Fig. 1A), indicating that groups tended to differ in their reward learning (Fig. 1B). Bonferroni-corrected follow-up tests showed that the response bias of healthy controls increased significantly from block 1 to block 2 (p = .002) but did not differ across blocks for rMDD participants (p = .72)a.
Fig. 1.
Group differences in (A) response bias and (B) reward learning [= ΔRB = Response Bias(Block 2) - Response Bias(Block 1)].
Discriminability
There was no Group × Block interaction or main effect of Group for discriminability (all ps > .05), indicating that response bias findings were not due group differences in task difficulty.
Results from analyses of secondary variables (accuracy and RT) are presented in the Supplement.
Scalp ERP data
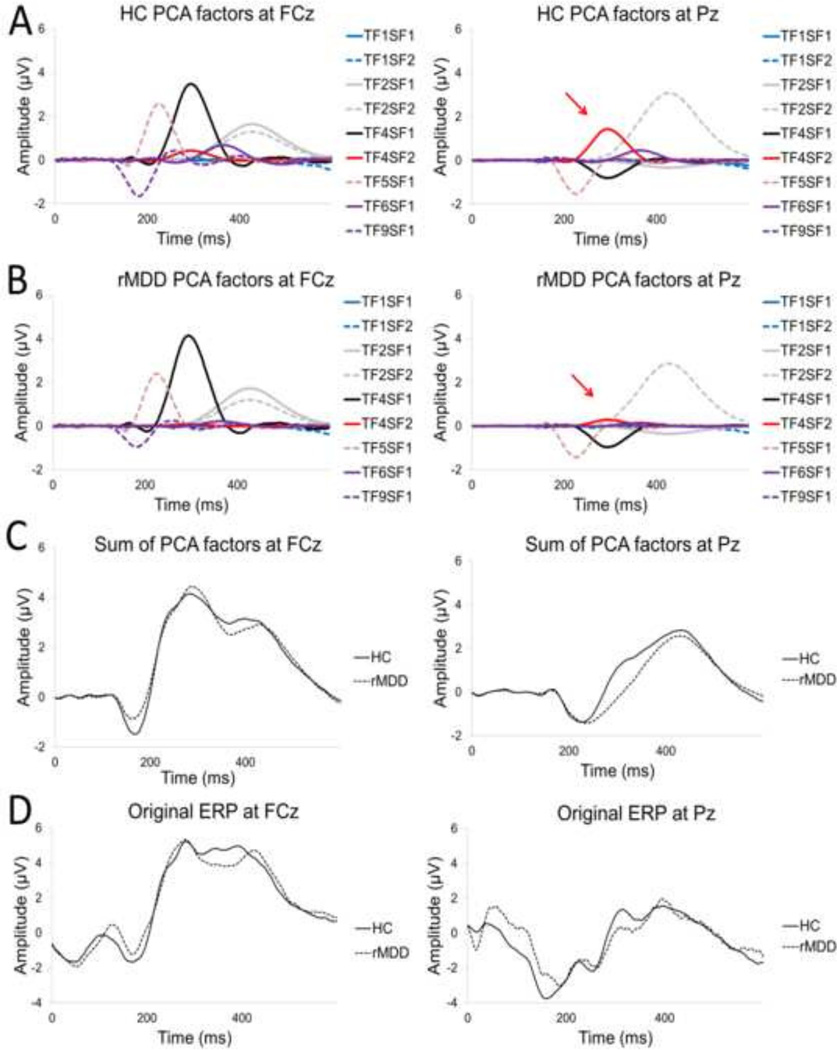
ERP data for 5 healthy controls and 4 rMDD subjects was discarded due to excessive noise artifacts, leaving a final sample of 29 controls and 22 rMDD subjects with usable ERP data. Fig. 2 shows how the original ERP waveforms at frontocentral (FCz) and parietal (Pz) sites were decomposed into temporo-spatial factors using PCA analysis. In addition to the waveforms with morphologies consistent with the RewP, for completeness, Fig. 2 also shows waveforms for earlier (< 200 ms) and later (> 300 ms) components.
Fig. 2.
Individual principal components analysis (PCA) factors in the healthy control (A) and remitted depressed (rMDD) groups (B), sum of all PCA factors (C), and the original event-related potential (ERP) waveforms (D). Panels on the left are at the fronto-central site (FCz) and panels on the right are at the parietal site (Pz). Arrows indicate PCA factor TF4/SF2, which was significantly reduced in the rMDD group relative to healthy controls.
A significant Group × PCA factor interaction emerged F(2,96) = 4.16, p = .047, ηp2 = .08. Bonferroni-adjusted post hoc tests showed that group differences emerged for factor TF4/SF2 (p = .03). This positive-going centro-parietal PCA factor peaked 294 ms following reward feedback, and was blunted in the rMDD group relative to controls.
LORETA data
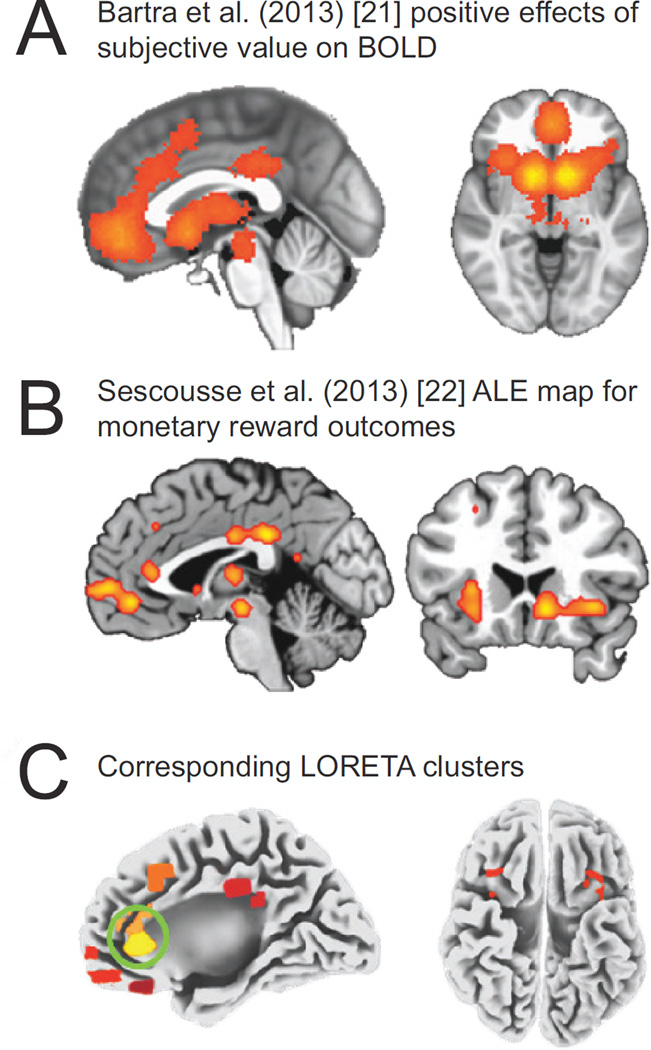
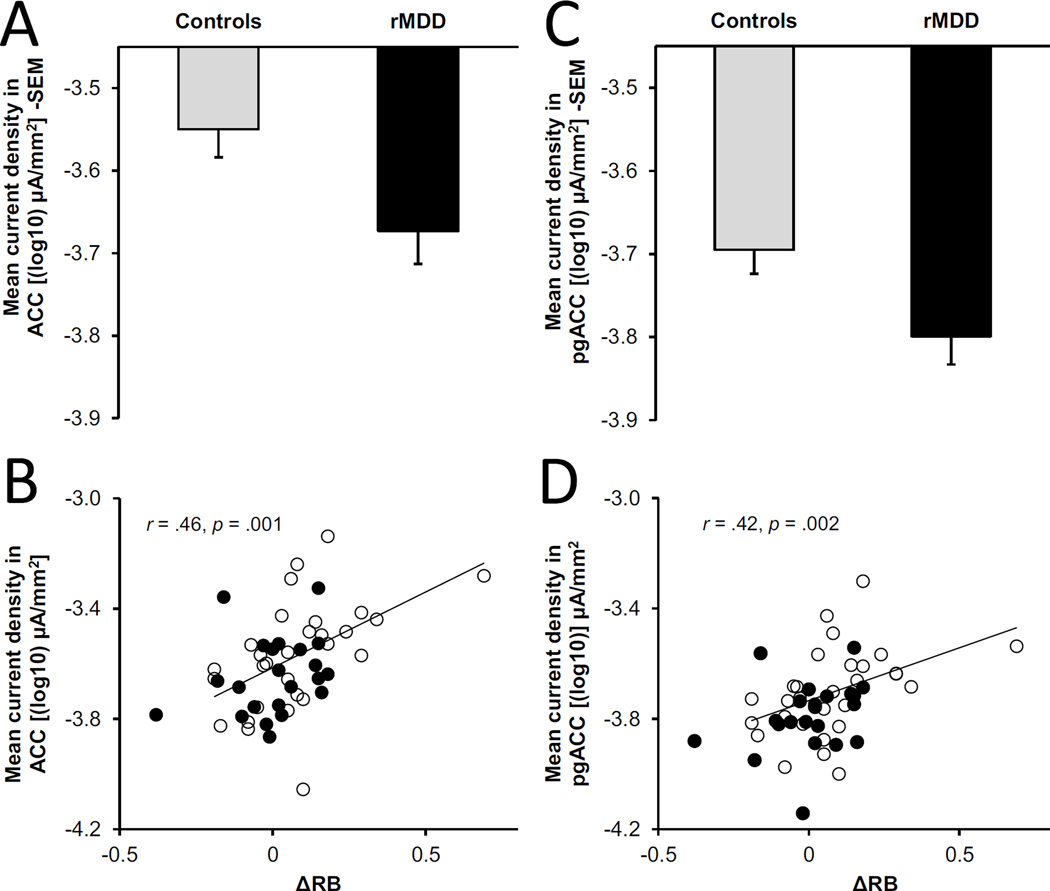
Eight ROIs were chosen from the fMRI meta-analyses and used in LORETA analyses. Table 2 and Fig. 3C show the coordinates and spatial extent of the ROIs defined. For reference, Fig. 3A and 3B show the ROIs from the meta-analyses from which our LORETA ROIs were defined. Specifically, Fig. 3A displays significant clustering for regions with greater fMRI blood oxygen level-dependent (BOLD) activation for more rewarding outcomes in the meta-analysis by Bartra and colleagues [21]. Fig. 3B shows the activation likelihood estimation (ALE) map highlighting the brain regions most consistently activated by monetary rewards in the meta-analysis by Sescousse and colleagues [22]. Analyses showed a main effect of Group for the ACC meta-cluster F(1,48) = 4.92, p = .03, ηp2 = .09, and the pregenual ACC (pgACC) cluster F(1,48) = 4.87, p = .03, ηp2 = .09. For both clusters, reward-related neural activity was diminished in the rMDD group relative to controls. Furthermore, reduced activity in the ACC meta-cluster (r = .46, p = .001, N = 51) and the pgACC cluster (r = .42, p = .002, N = 51) correlated with poorer reward learning on the PRT (Fig. 4).
Table 2.
Summary of regions-of-interest (ROI) entered in the analyses
| fMRI Meta-analysesa | LORETA approximationb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | ROI source | Side | x | y | z | x | y | z | Voxels | Vectorc |
| Posterior OFC | Sescousse | Leftd | −25 | 35 | −18 | −25.75 | 34.50 | −16.50 | 4 | 0.90 |
| vmPFC | Batra | Middle | 2 | 46 | −8 | 1.20 | 46.40 | −8.80 | 5 | 0.89 |
| Sescousse | Right | 12 | 60 | 0 | 12.75 | 60.75 | −0.75 | 4 | 1.06 | |
| Meta-cluster | Middle | 6.98 | 53.58 | −4.78 | 5 | |||||
| pgACC | Sescousse | Middlee | −5 | 38 | 7 | −5.33 | 38.00 | 8.00 | 3 | 0.33 |
| ACC | Batra | Middle | −2 | 28 | 28 | −1.25 | 29.25 | 27.25 | 4 | 1.46 |
| Sescousse | Right | 8 | 36 | 24 | 8.67 | 35.67 | 24.33 | 3 | 0.75 | |
| Meta-cluster | Middle | 3.71 | 32.46 | 25.79 | 4 | |||||
| PCC | Batra | Middle | −4 | −30 | 36 | −3.00 | −32.00 | 36.00 | 3 | 2.24 |
| Sescousse | Middlee | 1.33 | −34.67 | 26.67 | 1.67 | −34.33 | 29.00 | 3 | 0.47 | |
| Meta-cluster | Middle | −0.67 | −33.17 | 32.50 | 3 | |||||
| Pre-SMA | Batra | Middle | −2 | 16 | 46 | −1.25 | 15.25 | 46.50 | 4 | 1.06 |
| Anterior Insula | Batra | Right | 32 | 20 | −6 | 32.00 | 20.50 | −6.00 | 2 | 0.50 |
| Sescousse | Right | 36 | 22 | −6 | 35.50 | 20.50 | −6.00 | 2 | 1.58 | |
| Meta-cluster | Right | 33.75 | 20.50 | −6.00 | 2 | |||||
| Anterior Insula | Batra | Left | −30 | 22 | −6 | −31.00 | 20.50 | −6.00 | 2 | 1.80 |
| Sescousse | Left | −28 | 16 | −8 | −27.50 | 17.00 | −9.50 | 2 | 1.12 | |
| Meta-cluster | Left | −29.25 | 18.75 | −7.75 | 3 | |||||
Coordinates reported in Bartra et al. [21] (Positive effects of subjective value on BOLD) and Sescousse et al. [22] (Monetary reward meta-analysis).
Mean coordinates of LORETA’s center of gravity of the cluster minimizing the distance (vectorc).
The distance (in mm) between the LORETA approximation and the fMRI coordinates
Averaged over two left regions
Averaged over right and left regions
fMRI=functional magnetic resonance imaging; BOLD=blood-oxygen-level dependent; LORETA=low-resolution electromagnetic tomography; OFC=orbitofrontal cortex; vmPFC=ventromedial prefrontal cortex; pgACC=pregenual anterior cingulate cortex; ACC=anterior cingulate cortex; PCC=posterior cingulate cortex; pre-SMA=pre-supplementary motor area.
Fig. 3.
Bartra et al. [21] whole-brain meta-analysis showing significant clustering of regions with greater blood-oxygen-level dependent (BOLD) activity in response to reward outcomes (A), the Sescousse et al. [22] activation likelihood estimation (ALE) map for monetary reward outcomes (B), and the low-resolution electromagnetic tomography (LORETA) clusters defined in the current study (C) with regions showing reduced activation in the remitted depressed (rMDD) group (anterior cingulate cortex and pregenual anterior cingulate cortex) circled in green.
Fig. 4.
Group differences in reward-related mean current density in the anterior cingulate cortex (ACC) meta-cluster (A) and the pregenual anterior cingulate cortex (pgACC) cluster (C), and correlations between mean current density in these regions and reward learning (=ΔRB) (B & D). In panel B and D, correlations remain significant after removing the control subject with the highest ΔRB. On the y-axis, numbers are negative due to the log10 transformation.
We also examined associations among the LORETA ROIs and the TF2/SF4 PCA factor. Consistent with this factor’s centro-parietal scalp topography, a significant positive correlation emerged between its amplitude and reward-related activity in pre-SMA (r = .32, p = .02, N = 51). Furthermore, activity within pre-SMA was associated with increased activity in both ACC clusters (ACC meta-cluster: r = .46, p = .001, N = 51; pgACC: r = .34, p = .01, N = 51), and a trend toward increased overall response bias on the PRT (r = .27, p = .056, N = 51). Additional correlations involving measures of depression chronicity are presented in the Supplement.
Discussion
The goal of this study was to examine the neural processes underpinning reward learning in individuals with rMDD relative to never-depressed controls. Although abnormalities in reward learning have been suggested as a trait-like characteristic of MDD [7], the neural mechanisms associated with reward learning deficits in remitted individuals remain unclear. Results showed that, unlike healthy controls, individuals with rMDD had a tendency toward poorer modulation of behavior based on prior reward contingencies. This behavioral impairment was mirrored by blunted neural responses to reward feedback (as indexed by scalp-recorded ERPs) and reduced reward-related activation in the ACC (estimated using LORETA). Collectively, these findings suggest that reward learning impairments observed in acute MDD persist into full remission, and that these impairments may be attributable to abnormalities in the neural substrates that support reward feedback monitoring.
Consistent with predictions and with our prior work [7], individuals with rMDD showed poorer ability to modulate behavior as a function of reinforcement, as evidenced by a reduction in reward learning on the PRT. Although the Group × Block interaction for response bias fell just below significance in the current study, this is possibly due to the smaller sample size, as our prior significant effects were observed in a larger sample of 37 controls and 47 remitted individuals [7].
Prior work using the PRT has shown evidence of similar impairments in response bias development in individuals with acute MDD [3] and individuals high on depressive symptoms [25], and further studies point to disruptions in reward-related phasic DA signaling as a potential source of this impairment. Specifically, blunted response bias has been observed following administration of a low dose of the D2/D3 agonist pramipexole (which is assumed to reduce phasic DA signaling via DA autoreceptor stimulation [34]) and following a lab-based stressor hypothesized to interfere with phasic DA signaling [35]. Deficits on the PRT have also been found to improve in acutely depressed [36] as well as remitted depressed [37] individuals who smoke, putatively because nicotine increases phasic DA firing [38, 39]. These separate lines of evidence provide support for the hypothesis that the blunted response bias observed in our rMDD sample may reflect a trait-like dysfunction in phasic DA signaling that persists beyond depression remission.
In line with blunted response bias, individuals with rMDD evidenced reduced modulation in the ERP waveform at PCA factor TF2/SF4, which was a positive-going component that peaked at 294 ms following receipt of reward feedback. The polarity and timing of this factor suggests that it most likely captured variance attributable to the RewP. Prior research indicates that the RewP varies in accordance with mesolimbic reward circuit activity elicited during early evaluation of outcomes against predictions [40]. Consistent with the hypothesized role of mesolimbic deficits in depression [41], previous studies have found reductions in RewP amplitude (as measured by FRN amplitude changes under win versus loss conditions) in individuals with acute MDD [17, 18]. Our findings extend this work by showing that RewP amplitude is also present in asymptomatic individuals who are in complete remission from depression. Importantly, the RewP factor could be distinguished from another positive-going, slower wave component (TF2/SF2) that peaked 426 ms, which likely captured variance associated with P300. Taken together, the findings indicate that reward learning deficits in individuals with rMDD may be linked to deficits in neural responses to reward feedback occurring at the point in neural processing where the value of reward feedback is evaluated against internal predictions.
Our LORETA analyses clarified that the rMDD group had reduced reward-related neural activation in the ACC. Specifically, we found reduced activity to reward feedback in the ACC and the pgACC clusters [21, 22], which was associated with poorer behavioral reward learning. These findings align with prior evidence of reduced ACC activity during reward anticipation in individuals with MDD [42], and the role of these regions in reward learning [43], particularly in experimental settings requiring integration of reinforcement history over time [20]. These findings extend prior work by showing that reward processing deficits in individuals with rMDD a) arise very early in the course of information processing, and b) involve disruption in an area (the ACC) that forms action-outcome associations for the purpose of guiding goal-directed behavior.
In addition to the involvement of the ACC, we found that reduced RewP amplitude and poorer overall response bias were both linked to lower reward-related activity in pre-SMA. Reduced activity in pre-SMA was also associated with blunted activity in the two ACC clusters. Although directionality cannot be inferred from these correlations, one possible explanation for this relationship is that it captures the interactive effects of disruption in reward processing originating in the striatum and ACC. Specifically, during reward learning, striatal reward signals project to the ACC and pyramidal neurons in the ACC then project to the primary and supplementary motor areas [44], putatively transforming motivation and cognitive inputs into preparatory actions. Therefore, it is possible that in individuals with reduced reward-related ACC activity (e.g., those with rMDD), there may be a disruption in the reward-outcome representations necessary for the development of preparatory motor responses in pre-SMA, which then causes reward learning deficits.
Additional studies are needed to confirm this speculation and address the limitations of the current work. First, the predictive validity of reward feedback monitoring deficits in identifying risk for relapse in rMDD needs to be examined. Although blunted RewP has been found to predict MDE onset in adolescent girls [10], it remains unclear whether it has sensitivity and specificity in identifying individuals who will go on to develop recurrent depression and who may warrant targeted preventive intervention. Second, LORETA analyses did not correct for multiple comparisons (as the ROIs were defined a priori), and thus these findings await independent replications. Third, the design of the reward task used in the current study precludes the assessment of ERP difference waves that capture processing of reward versus non-reward (e.g., penalty) feedback. Finally, we only examined responses elicited during reward receipt and not during reward anticipation. This is an important area for future research as evidence indicates that reward anticipation may be blunted in acute MDD [45] but intact in rMDD [46, 47]. Specifically, although Dichter and colleagues found blunted neural responses to reward outcomes in rMDD, reward-related hyperactivation within the pgACC emerged during reward anticipation [46]. This was interpreted as evidence of relatively greater neural resources needed to represent the value of anticipated rewards in rMDD individuals. Similarly, although individuals with rMDD have been shown to have reduced ventral striatal responses to pleasant stimuli (e.g., the smell of chocolate), differences in the subjective wanting of these stimuli were not found [47]. Taken together, findings of normative reward anticipation but blunted responses during reward outcome in rMDD indicate that vulnerability to depression may be characterized by reductions in hedonic capacity, whereas acute MDD may be associated with deficits that extend to the ‘wanting’ of rewards. Future longitudinal studies are needed to confirm this conjecture.
To conclude, the current findings extend our understanding of depression by showing that reward learning impairments observed in acute MDD persist into remission, and that these impairments are accompanied by abnormalities in the neural processes that support reward feedback monitoring, particularly those involving the ACC. These findings provide a basis for future longitudinal research to assess whether abnormalities in these neural systems can identify rMDD individuals at risk for relapse who may benefit from targeted preventive intervention.
Supplementary Material
Acknowledgements
The authors wish to acknowledge Eva Woodward and Nancy Hall-Brooks for their contribution to diagnostic screening of participants in the current study.
Over the past three years, Dr. Pizzagalli has received honoraria/consulting fees from Otsuka America Pharmaceutical and Pfizer for activities unrelated to this project.
Role of Funding Source: This research was supported by National Institute of Mental Health grant R01 MH068376 awarded to Dr. Pizzagalli and The William Rosenberg Family Foundation (Carol Silverstein and Jill Gotlieb). Dr. Whitton was partially supported by Fellowships from the Andrew P. Merrill Memorial Fund and the American Australian Association. No funding sources played a role in the study design, collection, analyses and interpretation of the data, in the writing of the report or the decision to submit the paper for publication
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: All other authors declare no biomedical financial interests or potential conflicts of interest.
When comorbidities in the rMDD group were taken into account, we found that both rMDD subsamples failed to show a significant increase in response bias from block 1 to block 2 of the PRT (p = .88 for those with past depression only; p = .34 for those with a comorbidity), indicating that effects did not differ according to comorbidity status.
References
- 1.Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 3.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cogn Emot. 2000;14:711–724. [Google Scholar]
- 5.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland BR, Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety. 2009;26:117–122. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- 7.Pechtel P, Dutra SJ, Goetz EL, Pizzagalli DA. Blunted reward responsiveness in remitted depression. J Psychiatr Res. 2013;47:1864–1869. doi: 10.1016/j.jpsychires.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry. 2010;67:380–387. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J Abnorm Psychol. 2014;123:287–297. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 2013;50:74–81. doi: 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- 11.Walsh MM, Anderson JR. Learning from experience: Event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neurosci Biobehav Rev. 2012;36:1870–1884. doi: 10.1016/j.neubiorev.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45:688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 13.Baker TE, Holroyd CB. Dissociated roles of the anterior cingulate cortex in reward and conflict processing as revealed by the feedback error-related negativity and N200. Biol Psychol. 2011;87:25–34. doi: 10.1016/j.biopsycho.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. NeuroImage. 2011;57:1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Becker MP, Nitsch AM, Miltner WH, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J Neurosci. 2014;34:3005–3012. doi: 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santesso DL, Evins AE, Frank MJ, Schetter EC, Bogdan R, Pizzagalli DA. Single dose of a dopamine agonist impairs reinforcement learning in humans: Evidence from event-related potentials and computational modeling of striatal-cortical function. Hum Brain Mapp. 2009;30:1963–1976. doi: 10.1002/hbm.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W-h, Wang L-z, Shang H-r, Shen Y, Li Z, Cheung EFC, Chan RCK. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Foti D, Carlson JM, Sauder CL, Proudfit GH. Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp. 2011;32:2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 21.Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sescousse G, Caldú X, Segura B, Dreher J-C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- 24.First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV-TR Axis I disorders research version non-patient edition (SCID-I/NP) New York: Biometric Research New York State Psychiatric Institute; 2002. [Google Scholar]
- 25.Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacMillan NA, Creelman DC. Detection theory: a user’s guide. New York: Cambridge University Press; 1991. [Google Scholar]
- 27.Hautus MJ. Corrections for extreme proportions and their biasing effects on estimated values of d′. Behav Res Meth Instr. 1995;27:46–51. [Google Scholar]
- 28.Makeig S, Jung T-P, Ghahremani D, Sejnowski TJ. Independent component analysis of simulated ERP data. Institute for Neural Computation, University of California; 1996. technical report INC-9606. [Google Scholar]
- 29.Perrin F, Pernier J, Bertnard O, Giard M, Echallier J. Mapping of scalp potentials by surface spline interpolation. Electroencephalogr Clin Neurophysiol. 1987;66:75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- 30.Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods. 2010;187:138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Dien J. Evaluating two step PCA of ERP data with geomin, infomax, oblimin, promax, and varimax rotations. Psychophysiology. 2010;47:170–183. doi: 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- 32.Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- 33.Pizzagalli DA, Lehmann D, Hendrick AM, Regard M, Pascual-Marqui RD, Davidson RJ. Affective judgments of faces modulate early activity (~ 160 ms) within the fusiform gyri. NeuroImage. 2002;16:663–677. doi: 10.1006/nimg.2002.1126. [DOI] [PubMed] [Google Scholar]
- 34.Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology. 2008;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogdan R, Santesso DL, Fagerness J, Perlis RH, Pizzagalli DA. Corticotropin-releasing hormone receptor type 1 (CRHR1) genetic variation and stress interact to influence reward learning. J Neurosci. 2011;31:13246–13254. doi: 10.1523/JNEUROSCI.2661-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liverant GI, Sloan DM, Pizzagalli DA, Harte CB, Kamholz BW, Rosebrock LE, et al. Associations among smoking, anhedonia, and reward learning in depression. Behav Ther. 2014;45:651–663. doi: 10.1016/j.beth.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janes AC, Pedrelli P, Whitton AE, Pechtel P, Douglas S, Martinson MA, et al. Reward responsiveness varies by smoking status in women with a history of major depressive disorder. Neuropsychopharmacology. 2015;40:1940–1946. doi: 10.1038/npp.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice ME & Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H & Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 40.Proudfit GH. The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- 41.Pizzagalli DA. Depression, stress, and anhedonia: Toward a synthesis and integrated model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ubl B, Kuehner C, Kirsch P, Ruttorf M, Diener C, Flor H. Altered neural reward and loss processing and prediction error signalling in depression. Soc Cogn Affect Neurosci. 2015 doi: 10.1093/scan/nsu158. nsu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Van Hoesen GW, Morecraft RJ, Vogt BA. Neurobiology of cingulate cortex and limbic thalamus. Springer; 1993. Connections of the monkey cingulate cortex, in; pp. 249–284. [Google Scholar]
- 45.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136:1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.