Abstract
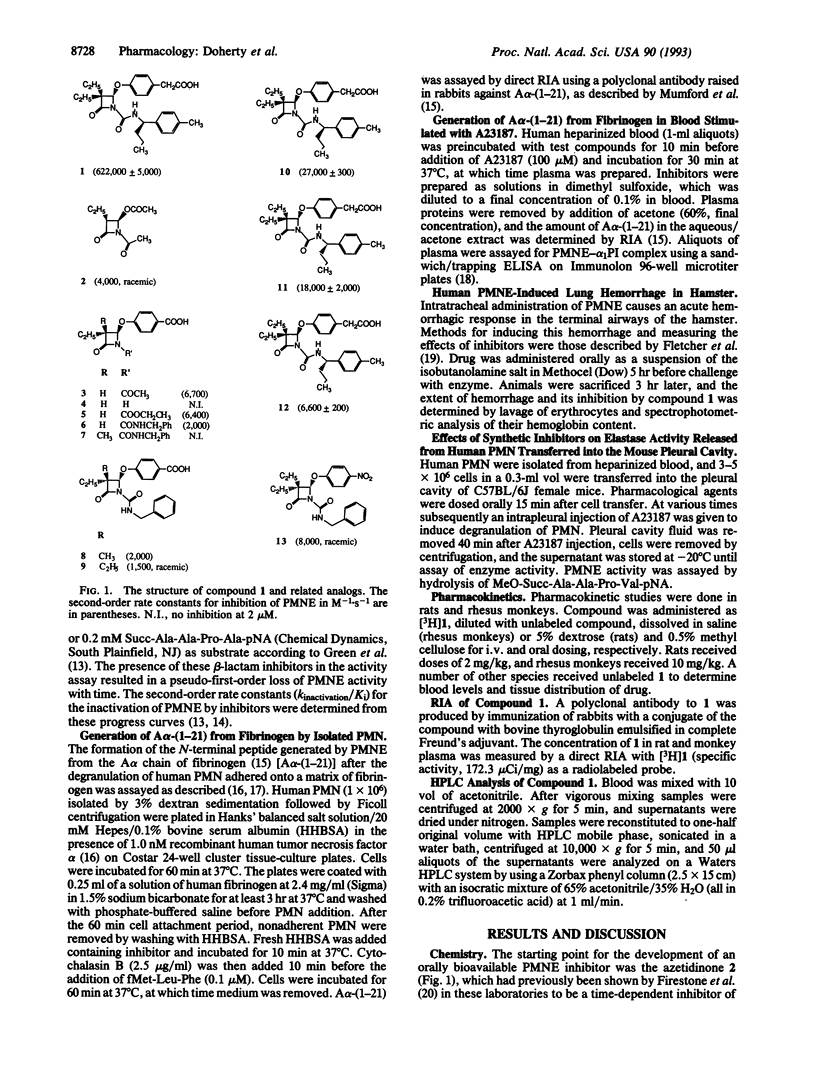
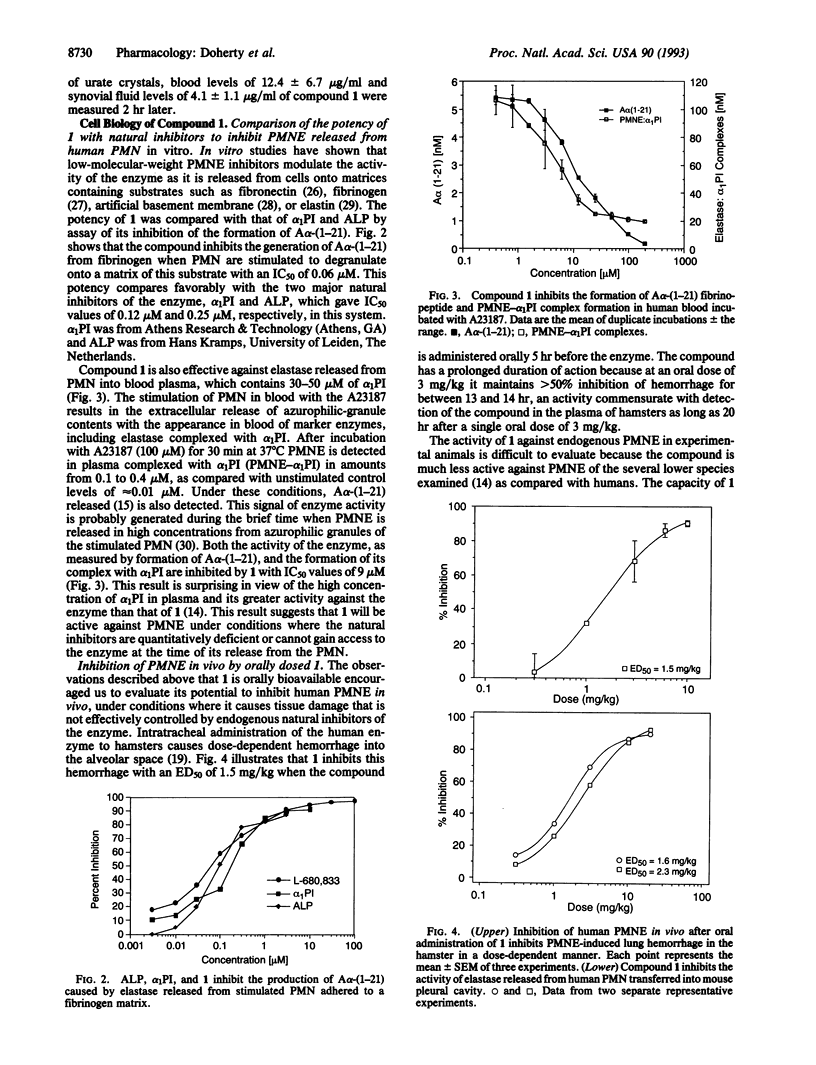
A series of potent and highly selective time-dependent monocyclic beta-lactam inhibitors of human polymorphonuclear leukocyte elastase (PMNE, EC 3.4.21.37) is described. The intrinsic potency of these compounds, as exemplified by L-680,833 (k(inactivation)/K(i) of 622,000 M-1.s-1), is reflected at the cellular level where it inhibits generation of the specific N-terminal cleavage product A alpha-(1-21) from the A alpha chain of fibrinogen by enzyme released from isolated polymorphonuclear leukocytes stimulated with fMet-Leu-Phe with an IC50 of 0.06 microM. The inhibitory activity of L-680,833 is also apparent in whole blood stimulated with A23187, where it inhibits formation of A alpha-(1-21) and PMNE-alpha 1-proteinase inhibitor complex formation with IC50 values of 9 microM. Pharmacokinetic studies indicate that after oral dosing L-680,833 is bioavailable in rats and rhesus monkeys. This oral bioavailability is reflected by the inhibition (i) of tissue damage elicited in hamster lungs by intratracheal instillation of human PMNE and (ii) enzyme released from human PMN stimulated after their transfer into the pleural cavity of mice. The properties of L-680,833 allow it to effectively supplement the activity of natural inhibitors of PMNE in vivo, suggesting that this type of low-molecular-weight synthetic inhibitor could have therapeutic value in diseases where PMNE damages tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode W., Meyer E., Jr, Powers J. C. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry. 1989 Mar 7;28(5):1951–1963. doi: 10.1021/bi00431a001. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G. Alpha 1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J Clin Invest. 1990 May;85(5):1343–1352. doi: 10.1172/JCI114578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Ashe B. M., Bonney R. J., Dorn C., Finke P., Fletcher D., Hanlon W. A., Humes J. L., Maycock A., Mumford R. A. The discovery and biologic properties of cephalosporin-based inhibitors of PMN elastase. Ann N Y Acad Sci. 1991;624:219–229. doi: 10.1111/j.1749-6632.1991.tb17021.x. [DOI] [PubMed] [Google Scholar]
- Doherty J. B., Ashe B. M., Argenbright L. W., Barker P. L., Bonney R. J., Chandler G. O., Dahlgren M. E., Dorn C. P., Jr, Finke P. E., Firestone R. A. Cephalosporin antibiotics can be modified to inhibit human leukocyte elastase. Nature. 1986 Jul 10;322(6075):192–194. doi: 10.1038/322192a0. [DOI] [PubMed] [Google Scholar]
- Doherty J. B., Ashe B. M., Barker P. L., Blacklock T. J., Butcher J. W., Chandler G. O., Dahlgren M. E., Davies P., Dorn C. P., Jr, Finke P. E. Inhibition of human leukocyte elastase. 1. Inhibition by C-7-substituted cephalosporin tert-butyl esters. J Med Chem. 1990 Sep;33(9):2513–2521. doi: 10.1021/jm00171a028. [DOI] [PubMed] [Google Scholar]
- Finke P. E., Ashe B. M., Knight W. B., Maycock A. L., Navia M. A., Shah S. K., Thompson K. R., Underwood D. J., Weston H., Zimmerman M. Inhibition of human leukocyte elastase. 2. Inhibition by substituted cephalosporin esters and amides. J Med Chem. 1990 Sep;33(9):2522–2528. doi: 10.1021/jm00171a029. [DOI] [PubMed] [Google Scholar]
- Finke P. E., Shah S. K., Ashe B. M., Ball R. G., Blacklock T. J., Bonney R. J., Brause K. A., Chandler G. O., Cotton M., Davies P. Inhibition of human leukocyte elastase. 4. Selection of a substituted cephalosporin (L-658,758) as a topical aerosol. J Med Chem. 1992 Oct 16;35(21):3731–3744. doi: 10.1021/jm00099a002. [DOI] [PubMed] [Google Scholar]
- Fletcher D. S., Osinga D. G., Hand K. M., Dellea P. S., Ashe B. M., Mumford R. A., Davies P., Hagmann W., Finke P. E., Doherty J. B. A comparison of alpha 1-proteinase inhibitor methoxysuccinyl-Ala-Ala-Pro-Val-chloromethylketone and specific beta-lactam inhibitors in an acute model of human polymorphonuclear leukocyte elastase-induced lung hemorrhage in the hamster. Am Rev Respir Dis. 1990 Mar;141(3):672–677. doi: 10.1164/ajrccm/141.3.672. [DOI] [PubMed] [Google Scholar]
- Green B. G., Weston H., Ashe B. M., Doherty J., Finke P., Hagmann W., Lark M., Mao J., Maycock A., Moore V. PMN elastases: a comparison of the specificity of human isozymes and the enzyme from other species toward substrates and inhibitors. Arch Biochem Biophys. 1991 Apr;286(1):284–292. doi: 10.1016/0003-9861(91)90042-h. [DOI] [PubMed] [Google Scholar]
- Hanlon W. A., Stolk J., Davies P., Humes J. L., Mumford R., Bonney R. J. rTNF alpha facilitates human polymorphonuclear leukocyte adherence to fibrinogen matrices with mobilization of specific and tertiary but not azurophilic granule markers. J Leukoc Biol. 1991 Jul;50(1):43–48. doi: 10.1002/jlb.50.1.43. [DOI] [PubMed] [Google Scholar]
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985 Aug;132(2):417–433. doi: 10.1164/arrd.1985.132.2.417. [DOI] [PubMed] [Google Scholar]
- Knight W. B., Chabin R., Green B. Inhibition of human serine proteases by substituted 2-azetidinones. Arch Biochem Biophys. 1992 Aug 1;296(2):704–708. doi: 10.1016/0003-9861(92)90630-f. [DOI] [PubMed] [Google Scholar]
- Knight W. B., Green B. G., Chabin R. M., Gale P., Maycock A. L., Weston H., Kuo D. W., Westler W. M., Dorn C. P., Finke P. E. Specificity, stability, and potency of monocyclic beta-lactam inhibitors of human leucocyte elastase. Biochemistry. 1992 Sep 8;31(35):8160–8170. doi: 10.1021/bi00150a007. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., Rudolphus A., Stolk J., Willems L. N., Dijkman J. H. Role of antileukoprotease in the human lung. Ann N Y Acad Sci. 1991;624:97–108. doi: 10.1111/j.1749-6632.1991.tb17010.x. [DOI] [PubMed] [Google Scholar]
- Mumford R. A., Williams H., Mao J., Dahlgren M. E., Frankenfield D., Nolan T., Schaffer L., Doherty J. B., Fletcher D., Hand K. Direct assay of A alpha(1-21), a PMN elastase-specific cleavage product of fibrinogen, in the chimpanzee. Ann N Y Acad Sci. 1991;624:167–178. doi: 10.1111/j.1749-6632.1991.tb17016.x. [DOI] [PubMed] [Google Scholar]
- Navia M. A., McKeever B. M., Springer J. P., Lin T. Y., Williams H. R., Fluder E. M., Dorn C. P., Hoogsteen K. Structure of human neutrophil elastase in complex with a peptide chloromethyl ketone inhibitor at 1.84-A resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(1):7–11. doi: 10.1073/pnas.86.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia M. A., Springer J. P., Lin T. Y., Williams H. R., Firestone R. A., Pisano J. M., Doherty J. B., Finke P. E., Hoogsteen K. Crystallographic study of a beta-lactam inhibitor complex with elastase at 1.84 A resolution. Nature. 1987 May 7;327(6117):79–82. doi: 10.1038/327079a0. [DOI] [PubMed] [Google Scholar]
- Rice W. G., Weiss S. J. Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science. 1990 Jul 13;249(4965):178–181. doi: 10.1126/science.2371565. [DOI] [PubMed] [Google Scholar]
- Shah S. K., Brause K. A., Chandler G. O., Finke P. E., Ashe B. M., Weston H., Knight W. B., Maycock A. L., Doherty J. B. Inhibition of human leukocyte elastase. 3. Synthesis and activity of 3'-substituted cephalosporins. J Med Chem. 1990 Sep;33(9):2529–2535. doi: 10.1021/jm00171a030. [DOI] [PubMed] [Google Scholar]
- Shah S. K., Dorn C. P., Jr, Finke P. E., Hale J. J., Hagmann W. K., Brause K. A., Chandler G. O., Kissinger A. L., Ashe B. M., Weston H. Orally active beta-lactam inhibitors of human leukocyte elastase-1. Activity of 3,3-diethyl-2-azetidinones. J Med Chem. 1992 Oct 16;35(21):3745–3754. doi: 10.1021/jm00099a003. [DOI] [PubMed] [Google Scholar]
- Stolk J., Davies P., Kramps J. A., Dijkman J. H., Humes J. J., Knight W. B., Green B. G., Mumford R., Bonney R. J., Hanlon W. A. Potency of antileukoprotease and alpha 1-antitrypsin to inhibit degradation of fibrinogen by adherent polymorphonuclear leukocytes from normal subjects and patients with chronic granulomatous disease. Am J Respir Cell Mol Biol. 1992 May;6(5):521–526. doi: 10.1165/ajrcmb/6.5.521. [DOI] [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Curnutte J. T., Regiani S. Neutrophil-mediated solubilization of the subendothelial matrix: oxidative and nonoxidative mechanisms of proteolysis used by normal and chronic granulomatous disease phagocytes. J Immunol. 1986 Jan;136(2):636–641. [PubMed] [Google Scholar]
- Weitz J. I., Huang A. J., Landman S. L., Nicholson S. C., Silverstein S. C. Elastase-mediated fibrinogenolysis by chemoattractant-stimulated neutrophils occurs in the presence of physiologic concentrations of antiproteinases. J Exp Med. 1987 Dec 1;166(6):1836–1850. doi: 10.1084/jem.166.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]



