Abstract
Background:
Adenoid cystic carcinoma (ACC) accounts for 1% of all head and neck (HN) cancers.
Materials and Methods:
Demographic, clinical, treatment, and survival details of 66 patients were collected (1995-2011) and analyzed. Disease-free survival (DFS) was estimated by Kaplan-Meier method.
Results:
Primary disease sites were sinonasal (n = 27), salivary gland (n = 30), and others (n = 9). Median follow-up was 23 months (range: 12-211 months). Estimated DFS at 2- and 5-year were 75% and 67.2%, respectively. On univariate analysis, intra-cranial extension (ICE) (hazard ratio [HR]: 3.59, P = 0.0071), lymph node involvement (HR: 4.05, P = 0.0065), treatment modality (others vs. surgery plus adjuvant radiotherapy, HR: 2.39, P = 0.0286) and T stage (T3/4 vs. T1/2, HR: 3.27, P = 0.007) had significant impact on DFS. Lymph node involvement (P = 0.038) and ICE (P = 0.038) continued to have significant impact on DFS on multivariate analysis.
Conclusion:
Surgery followed by adjuvant radiotherapy remains the treatment of choice for HN ACC. Lymph node involvement and ICE confer poor prognosis.
Keywords: Adenoid cystic carcinoma, head and neck, radiotherapy, surgery
INTRODUCTION
Adenoid cystic carcinoma (ACC) accounts for 1% of all head and neck (HN) cancers and about 10-22% of all malignant tumors of the major and minor salivary glands.[1] Minor salivary glands (65%) are more frequently involved than major salivary glands (submandibular - 19%, parotid - 16%).[1] Occasionally, they arise from sites other than salivary glands, such as the lacrimal glands, the ceruminal glands of external auditory canal, nose, paranasal sinus, palate, nasophaynx, and larynx. ACC was initially described by Bilroth in 1856 and named as cylindroma for its classic histologic appearance.[2] Although these tumors are usually low-grade malignancies as per histologic differentiation, management of these tumors is a distinct therapeutic challenge because of the propensity for local invasion, perineural involvement, distant metastasis and ability to recur over a prolonged period.[3] Surgery is the cornerstone of management of ACC. Studies have demonstrated the utility of adjuvant radiation in terms of superior disease control over either modality alone.[4] However, the improvement in loco-regional control with combined modality treatment did not translate into a significant improvement in overall survival rate.[5] The role of chemotherapy is investigational and is often confined to recurrent, metastatic or advanced unresectable tumors. The response rate ranges from 10% to 25% depending on the choice of drugs.[6] Targeted therapy with biological agents has a response rate of <10% and has failed to improve treatment outcome.[6] Hence, no consensus exists on the appropriate modality for the management of these malignancies. Most studies concur that overall survival rate for ACC is favorable, with 5-year survival ranging between 64% and 89% and 10-year survival between 37% and 77%.[3,7,8] The current study aimed at reviewing the clinical experience of management of HN ACC and analyzing the prognostic factors in this malignancy at a tertiary care institute in North India.
MATERIALS AND METHODS
Medical records were reviewed and data collected on all HN ACC over a 16-year period (1995-2011) from the institutional archives. A total of 90 patients of HN ACC cases were identified. Fourteen cases of ACC of the lacrimal gland and 10 cases with missing data were excluded from analysis. This study thus reports on 66 patients of HN ACC. Collected data were analyzed for a demographic profile, clinical presentation, disease site, stage, treatment modalities, and survival outcome. Patients were retrospectively staged as per American Joint Committee on Cancer (2010) TNM classification. The philosophy of treatment over the study period has been to assess all the patients in a multi-disciplinary HN cancer clinic comprising of an otolaryngologist, radiation oncologist, and medical oncologist.
Baseline staging work-up consisted of contrast enhanced computed tomography or magnetic resonance imaging of HN and X-ray of the chest. Complete blood count, liver function test, and kidney function tests were carried out in all patients.
Surgery was done primarily for all medically operable patients. The type of procedure was dependent on the primary site, the extent of disease, cosmetic considerations, and discretion of the surgeon. In general, an attempt was made to maximize local control with preservation of cosmetic and functional outcomes. Unresectable tumors were treated with radiation alone, the intent being curative or palliative, depending on the performance status of patients and the loco-regional extent of disease.
Postoperative radiotherapy was offered to almost all of these patients. Target volume included the postoperative bed along with any residual disease. Elective nodal irradiation was not done. The base of the skull was routinely included in the radiation portal for all tumors with named cranial nerve, invasion of the base of the skull or intra-cranial extension (ICE).
The dose of adjuvant radiation and addition of concurrent chemotherapy were decided based on the presence of high-risk factors in the postoperative histopathology report e.g., presence of positive or close margin, perinodal spread or more than one lymph node involvement. The dose of postoperative radiation (PORT) was 60-64 gray at 2 gray per fraction over 6-6.5 weeks depending on the presence of the aforementioned high-risk features. Patients treated with reirradiation on recurrence received radiation dose of 45 gray at 1.8 gray per fraction over 5 weeks and for metastatic bone disease, either 8 gray in single fraction or 20 gray in 5 fractions over 5 days were used. Extrapolating from the data of use of postoperative concurrent chemoradiation in high-risk squamous cell carcinoma of HN, cisplatin (40 mg/m2 body surface area intravenous weekly) was added to PORT in patients with positive margin and extracapsular extension in patients considered eligible for this intensified approach, else a higher dose of radiation was used in these cases.
Weekly toxicity assessment was done during the course of radiotherapy according to Radiation Therapy and Oncology Group acute radiation morbidity scoring criteria.[9] Radiation was withheld in the emergence of Grade 3/4 toxicity.
Disease-free survival (DFS) was defined as the interval between the date of diagnosis and the date of recurrence and was estimated by Kaplan-Meier product-limit method. Log-rank test was used for univariate analysis of factors impacting DFS. Cox proportional hazard regression model was used for multivariate analysis. SPSS version 12.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. P ≤ 0.05 was considered to be statistically significant for all analysis.
RESULTS
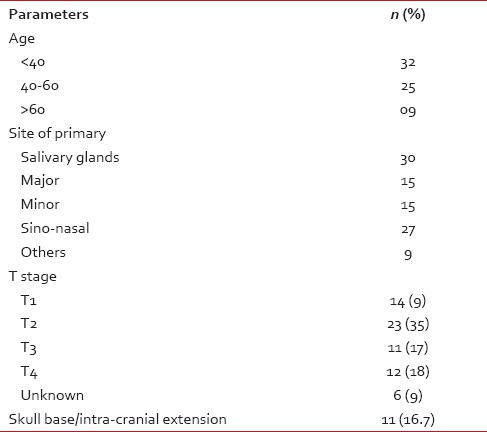
A total of 66 patients met the study criteria. The male:female ratio was 34:32. Median age at presentation was 40 years (range: 17-73 years). Ten patients had clinical node-positive disease and of 11 necks sampled, 8 patients had pathological node positivity. The rest of the patient and tumor characteristics have been summarized in Table 1.
Table 1.
Patient and tumor characteristics (n = 66)
Fifty-seven patients underwent surgery. On postoperative histopathology, positive margin and perineural invasion were noted in 18 and 10 patients, respectively. Postoperative radiotherapy was administered in 54 (81.8%) patients. Three patients underwent surgery alone (one was early stage oral cavity, and two were salivary gland carcinoma). Six patients with advanced disease received palliative radiotherapy (20-30 Gray in 5-10 fractions over 1-2 weeks). Three patients underwent radical radiotherapy.
Patients were treated with megavoltage photons and/or electron beams after ensuring proper immobilization using customized thermoplastic cast. Treatment planning had evolved with time from two-dimensional fluoroscopy based radiation therapy to three-dimensional conformal radiation therapy (3D-CRT) to intensity modulated radiotherapy (IMRT) and use of image-guidance (IG). Two-dimensional radiation techniques were used in 26 patients, 3D-CRT was used in 25 patients, and IMRT was used in 4 patients, whereas IG-IMRT was used in 6 patients. Mixed beam therapy (photon:electron combination ratio = 1:4) was used in 2 patients. Median radiotherapy dose was 60 gray in 30 fractions over 6 weeks. Of the 18 patients with margin positive disease, concomitant chemotherapy (cisplatin 40 mg/m2 weekly) along with adjuvant radiation was used in 8 of these patients, seven patients received higher dose of radiation - 64 gray in 32 fractions over 6.5 weeks and 3 patients had re-surgical excision with negative margins and received conventional radiation doses only. In addition, 5 patients received 64 gray in 32 fractions over 6.5 weeks due to the presence of other high-risk factors.
The overall rate of Grade 2 or higher acute nonhematological toxicity was 8% among those treated with combined modality approach. Only one patient developed Grade 4 toxicity (dermatitis and mucositis). The most common acute morbidities were dermatitis, conjunctivitis, and mucositis. Only 3 patients developed Grade 2 or higher hematological toxicity.
Median follow-up duration was noted to be 23 months (range: 12-211 months). Nineteen patients had a recurrence at the time of last follow-up. Thirteen patients failed locally, 4 patients had distant metastasis (3 had lung metastasis, and 1 had bone metastasis), and 2 patients had both local recurrence and distant metastasis (lung metastasis). At failure, surgical excision followed by reirradiation (3 patients), only reirradiation (2 patients), palliative chemotherapy (3 patients), palliative radiotherapy alone to involved metastatic sites of bone (1 patient) and rest received only best supportive care.
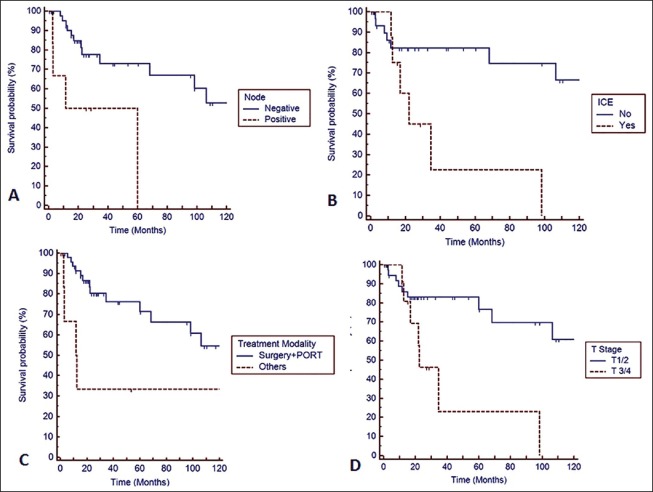
Two years and 4 years DFS rate [Figure 1] were 75% and 71%, respectively. Univariate analysis indicated increased predisposition [Table 2 and Figure 2] toward failure with skull base erosion/ICE at presentation (HR: 3.59, 95% confidence interval [CI]: 1.89-14.46; P = 0.007), lymph node involvement (HR: 4.06, 95% CI: 1.63-26.17; P = 0.006), use of treatment modality other than surgery and postoperative radiotherapy (HR: 2.39, 95% CI: 1.63-9.12; P = 0.01) and higher T stage (T3/4) (HR: 3.27, 95% CI: 1.36-11.43; P = 0.007). Other prognostic factors [Table 2] viz. age (P = 0.196), margin positivity (P = 0.605) and extent of surgery (P = 0.573) did not impact DFS significantly. Lymph node involvement (P = 0.038) and skull base invasion/ICE (P = 0.038) continued to have significant impact on DFS even on multivariate analysis.
Figure 1.
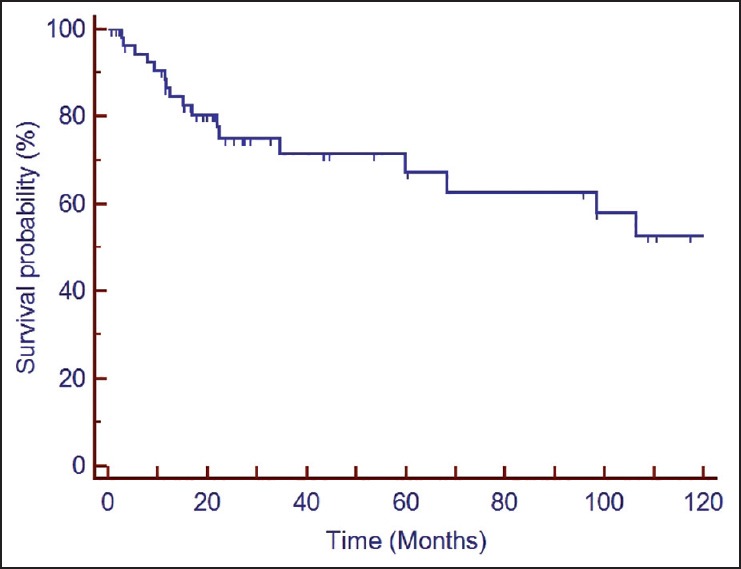
Kaplan-Meier survival curve depicting disease free survival of the entire cohort
Table 2.
Univariate and multivariate analysis of factors predictive of disease free survival
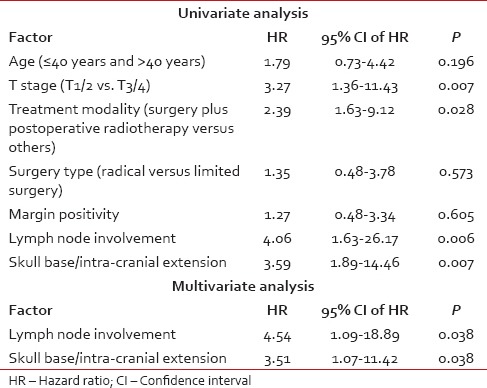
Figure 2.
Kaplan-Meier survival curve depicting impact of prognostic factors on disease free survival. (a) Lymph node positivity (b) intra-cranial extension (ICE) (c) treatment modality (PORT – Postoperative radiotherapy) (d) T stage of the disease
DISCUSSION
In this current single institutional analysis of 66 patients of HN ACC, predominantly treated with the multi-modality approach, we observed 2- and 4-year DFS rate of 75% and 71% respectively. Surgery along with PORT emerged to be a better treatment modality than the rest. Skull base invasion/ICE, lymph node involvement and higher T stage were found to be poor prognostic factors with respect to DFS. Finally, lymph node involvement and presence of skull base invasion or ICE continued to retain their prognostic significance on multivariate analysis.
The results of this study are in accordance with those of other contemporary series of HN ACC [Table 3].
Table 3.
Comparative analysis of prognostic factors and survival outcomes in contemporary series
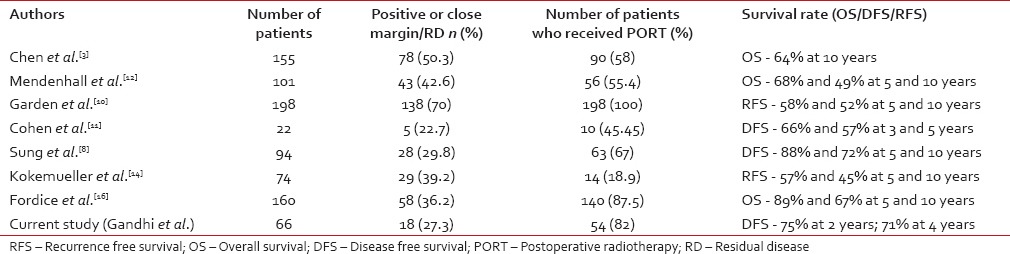
One of the largest series on HN ACC (n = 155) has been reported by Chen et al.[3] from University of California, San-Francisco (UCSF). The 10-year overall survival and distant metastasis-free survival were 64% and 66%, respectively. The authors concluded that T4 disease (P = 0.0001), perineural invasion (P = 0.008), omission of PORT (P = 0.007), and major nerve involvement (P = 0.02) were independent predictors of local recurrence. Study by Simpson et al.[9] from Mallinckrodt Institute of Radiology also established the superiority of surgery, followed by radiation over surgery alone. Ten years local control was 83% for patients treated with surgery, followed by adjuvant radiation compared with only 25% for those treated with surgery alone. According to single institute data from M.D. Anderson Cancer Centre,[10] local control rates were 95% and 86% at 5- and 10-year, respectively in patients of HN ACC (n = 198) treated with combination of surgery and PORT. In another series reported from University of California, Los Angeles by Cohen et al.,[11] local control rates were 82% and 70% for patients treated with or without adjuvant radiation after surgery, respectively. Mendenhall et al. reported 5- and 10-year local control rates of 94% and 91% in a cohort of 56 patients treated at the University of Florida with multi-modality approach and identified T stage as an independent predictor of local failure.[12] However, studies by Khan et al.[13] and Kokemueller et al.[14] failed to show any benefit of PORT. On the contrary, Silverman et al.[15] showed the utility of PORT in patients of HN ACC with T4 tumors and positive margins. However, the benefit of adjuvant radiation was not observed in other subsets of patients of HN ACC. Some of the other contemporary series also had comparatively higher rate of positive margins,[3,10,12] which might be attributed as a potential reason for the superiority of surgery and PORT over surgery alone. In the current study, we used PORT in all patients with positive margin, thus nullifying the negative prognostic impact of margin positivity on local control.
The difference in the results regarding the impact of PORT and the variation in survival rates across the studies may be attributed to patient selection criteria and different therapeutic approach like the extent of surgery and radiotherapy target volume. Existing literature has heterogeneity regarding elective nodal irradiation of neck[3,17,18] due to individualization of treatment decisions. The coverage of skull base has also differed from series to series. We have routinely included the base of the skull in the postoperative treatment volume in case of perineural invasion of any major named nerve, the base of skull invasion or ICE. The discrepancies can also be partially due to the difference in the demographic and clinicopathologic factors across these studies done from different parts of the world. Results of our series are in concordance with other reported studies in the literature [Table 3].
Lymph node involvement and base of skull invasion or ICE were noted to be risk factors for poor DFS on univariate analysis in our series. Study from Memorial Sloan-Kettering Cancer Centre showed that T4 stage and gross or clinical nerve involvement (P = 0.002) were associated with decreased progression-free survival, whereas T4 stage and lymph node involvement were associated with decreased overall survival.[19] The association of lymph node involvement with poorer outcome has also been demonstrated by Fordice et al.[16] Multiple studies have pointed out the adverse impact of perineural invasion or nerve involvement on local control and survival. However in our study, perineural involvement did not have any significant impact on DFS. This might be because of our cautious approach in the inclusion of base of the skull in the radiation target volume in all such patients, which nullified the risk of recurrence.
In our series, local failure was observed to be the predominant pattern of failure that is in accordance with the results obtained by Khan et al.,[13] Kokenmueller et al.[14] and other studies.[20,21] However, in the UCSF series reported by Chen et al.,[3] distant metastasis was the most common pattern of failure. Sites of distant failure in this study were as follows: 25 lung (71%), 5 bone (14%), 3 liver (9%), and 2 brain (6%) metastasis. A longer follow-up in our study might unveil further distant metastasis.
The current study, in accordance with literature from different parts of the world, shows excellent tolerance to PORT with favorable acute toxicity profile. However, lack of attention to late toxicity, which might be significant in long-term survivors, is a limitation of our study.
Although subject to inherent limitations of any retrospective study, the current study highlights the superiority of surgery, followed by adjuvant radiation in managing patients of HN ACC. Invasion of skull base or ICE was found to be an independent prognostic factor in our study. However, the adverse prognostic impact of positive margin was negated by the routine use of high dose PORT. The median follow-up duration in the present study is comparatively shorter than other contemporary series, which is essentially due to poor compliance to long-term follow-up policy, mandatory for HN ACC. Last but not the least; more attention needs to be focused on late toxicity, functional outcome, and quality of life in long-term survivors.
CONCLUSION
The current single institutional analysis clearly demonstrates the superiority of multi-modality management in the form of surgery and adjuvant radiation in the management of HN ACC. Invasion of skull base or ICE confers poor prognosis. However, the prognostic impact of positive margin on local control and survival can be nullified by the routine use of high dose radiation therapy in such cases. Special attention needs to be given to late morbidities and quality of life issues in long-term survivors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128:512–20. doi: 10.1016/0002-9610(74)90265-7. [DOI] [PubMed] [Google Scholar]
- 2.Billroth T. Observations on tumors of the salivary glands. Virchows Arch Pathol Anat. 1859;17:357–75. [Google Scholar]
- 3.Chen AM, Bucci MK, Weinberg V, Garcia J, Quivey JM, Schechter NR, et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: Prognostic features of recurrence. Int J Radiat Oncol Biol Phys. 2006;66:152–9. doi: 10.1016/j.ijrobp.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 4.King JJ, Fletcher GH. Malignant tumors of the major salivary glands. Radiology. 1971;100:381–4. doi: 10.1148/100.2.381. [DOI] [PubMed] [Google Scholar]
- 5.Miglianico L, Eschwege F, Marandas P, Wibault P. Cervico-facial adenoid cystic carcinoma: Study of 102 cases. Influence of radiation therapy. Int J Radiat Oncol Biol Phys. 1987;13:673–8. doi: 10.1016/0360-3016(87)90284-7. [DOI] [PubMed] [Google Scholar]
- 6.Papaspyrou G, Hoch S, Rinaldo A, Rodrigo JP, Takes RP, van Herpen C, et al. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: A review. Head Neck. 2011;33:905–11. doi: 10.1002/hed.21458. [DOI] [PubMed] [Google Scholar]
- 7.Rapidis AD, Givalos N, Gakiopoulou H, Faratzis G, Stavrianos SD, Vilos GA, et al. Adenoid cystic carcinoma of the head and neck. Clinicopathological analysis of 23 patients and review of the literature. Oral Oncol. 2005;41:328–35. doi: 10.1016/j.oraloncology.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Sung MW, Kim KH, Kim JW, Min YG, Seong WJ, Roh JL, et al. Clinicopathologic predictors and impact of distant metastasis from adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2003;129:1193–7. doi: 10.1001/archotol.129.11.1193. [DOI] [PubMed] [Google Scholar]
- 9.Simpson JR, Thawley SE, Matsuba HM. Adenoid cystic salivary gland carcinoma: Treatment with irradiation and surgery. Radiology. 1984;151:509–12. doi: 10.1148/radiology.151.2.6324280. [DOI] [PubMed] [Google Scholar]
- 10.Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–26. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 11.Cohen AN, Damrose EJ, Huang RY, Nelson SD, Blackwell KE, Calcaterra TC. Adenoid cystic carcinoma of the submandibular gland: A 35-year review. Otolaryngol Head Neck Surg. 2004;131:994–1000. doi: 10.1016/j.otohns.2004.06.705. [DOI] [PubMed] [Google Scholar]
- 12.Mendenhall WM, Morris CG, Amdur RJ, Werning JW, Hinerman RW, Villaret DB. Radiotherapy alone or combined with surgery for adenoid cystic carcinoma of the head and neck. Head Neck. 2004;26:154–62. doi: 10.1002/hed.10380. [DOI] [PubMed] [Google Scholar]
- 13.Khan AJ, DiGiovanna MP, Ross DA, Sasaki CT, Carter D, Son YH, et al. Adenoid cystic carcinoma: A retrospective clinical review. Int J Cancer. 2001;96:149–58. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 14.Kokemueller H, Eckardt A, Brachvogel P, Hausamen JE. Adenoid cystic carcinoma of the head and neck — a 20 years experience. Int J Oral Maxillofac Surg. 2004;33:25–31. doi: 10.1054/ijom.2003.0448. [DOI] [PubMed] [Google Scholar]
- 15.Silverman DA, Carlson TP, Khuntia D, Bergstrom RT, Saxton J, Esclamado RM. Role for postoperative radiation therapy in adenoid cystic carcinoma of the head and neck. Laryngoscope. 2004;114:1194–9. doi: 10.1097/00005537-200407000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: Predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149–52. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 17.Balamucki CJ, Amdur RJ, Werning JW, Vaysberg M, Morris CG, Kirwan JM, et al. Adenoid cystic carcinoma of the head and neck. Am J Otolaryngol. 2012;33:510–8. doi: 10.1016/j.amjoto.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Lupinetti AD, Roberts DB, Williams MD, Kupferman ME, Rosenthal DI, Demonte F, et al. Sinonasal adenoid cystic carcinoma: The M.D. Anderson Cancer Center experience. Cancer. 2007;110:2726–31. doi: 10.1002/cncr.23096. [DOI] [PubMed] [Google Scholar]
- 19.Gomez DR, Hoppe BS, Wolden SL, Zhung JE, Patel SG, Kraus DH, et al. Outcomes and prognostic variables in adenoid cystic carcinoma of the head and neck: A recent experience. Int J Radiat Oncol Biol Phys. 2008;70:1365–72. doi: 10.1016/j.ijrobp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Prokopakis EP, Snyderman CH, Hanna EY, Carrau RL, Johnson JT, D’Amico F. Risk factors for local recurrence of adenoid cystic carcinoma: The role of postoperative radiation therapy. Am J Otolaryngol. 1999;20:281–6. doi: 10.1016/s0196-0709(99)90028-5. [DOI] [PubMed] [Google Scholar]
- 21.Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992;164:623–8. doi: 10.1016/s0002-9610(05)80721-4. [DOI] [PubMed] [Google Scholar]