Abstract
Background
Malaria cases persist in some remote areas in Sabah and Sarawak despite the ongoing and largely successful malaria control programme conducted by the Vector Borne Disease Control Programme, Ministry Of Health, Malaysia. Point mutations in the genes that encode the two enzymes involved in the folate biosynthesis pathway, dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS) enzymes confer resistance to pyrimethamine and sulfadoxine respectively, in both Plasmodium falciparum and P. vivax. The aim of the current study was to determine the mutation on both pvdhfr at codon 13, 33, 57, 58, 61, 117, and 173 and pvdhps genes at codon 383 and 553, which are potentially associated with resistance to pyrimethamine and sulfadoxine in P. vivax samples in Sabah.
Methods
Every individual was screened for presence of malaria infection using a commercial rapid dipstick assay, ParaMax-3™ (Zephyr Biomedical, India). Individuals tested positive for P. vivax had blood collected and parasite DNA extracted. The pvdhfr and pvdhps genes were amplified by nested-PCR. Restriction fragment length polymorphism (RFLP) was carried out for detection of specific mutations in pvdhfr at codons 13Leu, 33Leu, 57Ile/Leu, 58Arg, 61Met, 117Asn/Thr, and 173Leu and pvdhps at codons 383Gly and 553Gly. The PCR–RFLP products were analysed using the Agilent 2100 Bioanalyzer (Agilent Technology, AS).
Results
A total of 619 and 2119 individuals from Kalabakan and Kota Marudu, respectively participated in the study. In Kalabakan and Kota Marudu, 9.37 and 2.45 % were tested positive for malaria and the positivity for P. vivax infection was 4.2 and 0.52 %, respectively. No mutation was observed at codon 13, 33 and 173 on pvdhfr and at codon 553 on pvdhps gene on samples from Kalabakan and Kota Marudu. One-hundred per cent mutations on pvdhfr were at 57Leu and 117Thr. Mutation at 58Arg and 61Met was observed to be higher in Kota Marudu 72.73 %. Mutation at 383Gly on pvdhps was highest in Kalabakan with 80.77 %. There are four distinct haplotypes of pvdhfr/pvdhps combination.
Conclusions
The presence of triple and quintuple mutation combination suggest that the P. vivax isolates exhibit a high degree of resistant to sulfadoxine, pyrimethamine and sulfadoxine-pyrimethamine combination therapy.
Keywords: Plasmodium vivax, Molecular marker, Sulfadoxine-pyrimethamine, Dihydrofolate reductase (dhfr) gene, Dihydropteroate synthase (dhps) gen
Background
Malaysia is pursuing malaria elimination and has a national goal to eliminate malaria by 2020, and categorizes as in pre-elimination phase by the World Health Organization [1]. In Malaysia, malaria cases showed a decreased in 2010, 2011, 2012, and 2013, but slightly increased in 2014; incidents of malaria were 6650, 5306, 4725, 3850, and 3923, respectively [2]. Malaria is endemic in Sabah and most of the malaria cases occurs in remote areas of the interior region of Sabah or those areas along the borders of Malaysia-Indonesia, where mosquito vectors and infected individuals are present. Inadequate transport and communication facilities to these areas are a problem for access to diagnosis and treatment and pose challenges for control programmes. Sabah shares borders with North Kalimantan of Indonesia in the south, facing Sebatik Island, partly within Malaysia and partly within Indonesia. This geographic location, together with population movement, could facilitate malaria transmission in the country.
Since 2006, malaria cases in Malaysia were mostly attributed to Plasmodium vivax, which contributed to more than 50 % of malaria infection, followed by P. falciparum. However in 2014, the trend changed, when P. knowlesi infection was recorded in the highest number of cases, 65.9 % of all malaria cases in Malaysia, followed by P. vivax and P. falciparum, 18.6 and 9.2 %, respectively. Most human malaria and zoonotic malaria cases in Malaysia come from Sabah, followed by Sarawak [2].
Plasmodium vivax was considered to cause benign and uncomplicated cause of illness, but with new molecular tools, it is evident that P. vivax parasite infection could also be involved in multiple organ dysfunction and cause of severe malaria similar to P. falciparum in adults and children, including infants [3, 4].
According to the Malaysia National Antibiotic Guideline 2008, the treatment for vivax malaria is chloroquine and primaquine. However, for mixed infection cases (co-infection of P. falciparum and P. vivax), the treatment is similar to P. falciparum [5]. In the past, chloroquine (CQ) was the first-line anti-malarial drug used for the treatment of malaria. Sulfadoxine-pyrimethamine (SP), also known as Fansidar®, was used in areas with suspected CQ-resistance and for the treatment of mixed infection cases. Mixed infection of P. falciparum and P. vivax is frequent and thus the use of SP is likely to have exposed P. vivax to this drug. The use of SP in the treatment of uncomplicated falciparum malaria started in 1976. By 1996 resistance to SP was observed to be widely distributed in Peninsular Malaysia [6]. Additionally, SP combination is still occasionally used as presumptive treatment for individuals suspected of malaria in remote areas where diagnosis and treatment is not accessible. Therefore, P. vivax populations are often exposed to SP pressure and this might have caused the selection of the SP-resistant allele in P. vivax isolates [7].
Molecular epidemiology studies have shown that the point mutations in the genes that encode the two enzymes involved in the folate biosynthesis pathway, dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS) enzymes confer resistance to pyrimethamine and sulfadoxine, respectively, in both laboratory [8] and in natural parasite populations, in both P. falciparum and P. vivax parasites [9, 10]. Molecular studies have identified more than 20 different alleles for pvdhfr, of which mutations at codon 57Leu, 58Arg, 61Met, 117Thr/Asn, and 173Ile have been reported to be involved in clinical antifolate resistance. The presence of double mutation at codon 58Arg and 117Thr and triple mutation at codon 57Leu, 58Arg and 117Thr at pvdhfr gene are associated with delayed parasite clearance following SP treatment [11]. Quadruple mutations at 57Leu, 58Arg, 61Met, and 117Thr in this gene corresponded to therapeutic failure of SP treatment among P. vivax-infected patients [12, 13]. Five mutations have been identified on the pvdhps gene at codons 382, 383, 442, 512, 553, and 585. Mutation at codon 382Ala/Cys, 383Gly, 512Met/Thr/Glu, and 553Gly of pvdhps is associated with sulfadoxine resistance [13, 14].
Pvdhfr mutant alleles of 58Arg and 117Thr/Asn were found in the southeast Asian countries of East Timor [15], Thailand [11, 16], Myanmar [16], Vietnam and Philippines [17], and India [18]. Mutation in pvdhps at amino acids 383Gly and 553Gly were highly prevalent in Thailand [19, 20]. In Malaysia, P. falciparumdhfr and dhps genes have been studied extensively in endemic areas of Sabah, the villages in the districts of Kalabakan, Tawau bordering to North Kalimantan [21], the interior part of Sabah, Keningau, and Nabawan [22]. Both studies have indicated the high prevalence of mutation in pfdhfr and pfdhps conferring resistance to SP, which could predict treatment failure.
The aim of the current study was to investigate the frequency of mutation on both pvdhfr and pvdhps genes potentially associated with resistance to pyrimethamine and sulfadoxine in P. vivax field isolates in malaria-endemic areas of Sabah. This study will establish an epidemiological map of the distribution of SP-resistant vivax malaria in Sabah. The information obtained could contribute towards achieving the goal of the malaria elimination programme.
Methods
Study area
Plasmodium vivax samples were collected from 23 villages in Kota Marudu, and 26 villages, road construction and loggers’ camps in Kalabakan, Sabah. Kalabakan is located 100 km from Tawau, towards the south of Sabah bordering Kalimantan, Indonesia while Kota Marudu is located in Kudat Division in the northern region of Sabah with 1083 km from Philippine (Fig. 1). The samples from Kalabakan were collected in 2008 and 2009 by active case detection. During those years, Kalabakan contributed to most of the malaria cases with 21.54 and 22.79 %, respectively, of the total cases in Sabah (Vector Borne Disease Control, 2008, 2009 unpublished data). Sample collections in Kota Marudu in 2011 and 2012 were by both active case detection and passive case detection. At the time the study was conducted, the number of malaria cases in Kota Marudu contributed 10 % of total cases in Sabah in 2011 (Vector Borne Disease Control, 2011 unpublished data).
Fig. 1.

Map of Sabah showing the geographical sites where the 37 P. vivax isolates were collected: Kota Marudu (n = 11), Kalabakan (n = 26). In this study, samples were collected from two of Sabah divisions (illustrated in the square in top left corner), from Kota Marudu district in the Kudat Division and Kalabakan district in the Tawau Division. This map were generated by OpenStreetMap: http://www.openstreetmap.org/ and USGS National Map Viewer (public domain): http://viewer.nationalmap.gov/viewer/
Sample collection
The study protocol was reviewed and approved by the Research Review Committee of the Institute for Medical Research (IMR) and Medical Research Ethics Committee (MREC), Ministry of Health Malaysia. For active case detection, the team visited the villages and the study was announced and briefed to all the population. Study information sheets were given to all who came to the briefing. All the population received the study information sheet, informed consent form and they were assisted whenever required in understanding the study. Individuals who consented to participate in the study were screened for malaria infection by finger-prick blood diagnosis in the field using rapid diagnosis test (RDT) kit (Paramax-3™, Zephyr Biomedicals, India). Some finger-prick blood was also used to prepare blood film for malaria parasite (BFMP) for determination of parasite density.
Individuals diagnosed positive for malaria infection by RDT had approximately 500 µl of blood collected by venipuncture and transferred to EDTA tubes. BFMP and blood in anticoagulant EDTA tubes were brought to the field station to be processed. The EDTA blood was spotted onto filter paper 3MM® Whatman (Brentford, UK). The filter paper was allowed to dry completely by hanging on a wire line in the field station. After drying, they were packed into a small plastic zipper bags containing silica gels, labelled appropriately and transported to Institute for Medical Research for further molecular study. The BFMP were stained with Giemsa and examined for presence of malaria parasites by microscopy to determine the density of the infection. Individuals diagnosed positive with malaria were treated with a standard anti-malaria treatment by health clinic staff. The study managed to collect 58 samples from Kalabakan and 52 samples from Kota Marudu.
DNA extraction and molecular diagnosis for confirmation of malaria species
Parasite DNA was extracted from blood spot filter paper by using QIAamp® DNA Mini Kit (QIAmp; QIAGEN, Hilden, Germany) following the manufacturer’s instruction (Dried Blood Spot Protocol). The DNA was eluted with 150 µl AE Buffer (10 mM Tris–HCl pH 9.0 and 0.5 mM EDTA). The parasite DNA was used for molecular diagnosis of the malaria species and for genotyping studies. All samples underwent molecular speciation using Fuehrer et al. [23] for P. falciparum, P. vivax and Plasmodium malariae. All samples also underwent speciation for P. knowlesi using protocol from Imwong et al. [24]. Samples confirmed positive for P. vivax infection by the molecular diagnosis were included in the study.
Amplification of pvdhfr and pvdhps gene
The pvdhfr and pvdhps genes were amplified from genomic DNA by nested-PCR. The amplifications of the pvdhfr was carried out as described previously [11, 25] with some modification on the final volume of the PCR reaction mix. In the amplification of P. vivaxdhfr-ts gene, size of 1876 bp, excluding the stop codon, the PCR reaction was amplified by using the oligonucleotide pair VDT OF and VDT OR. The final volume of the reaction mix was 50 µl, consisted of 50 ng of template DNA, 1X Buffer I-Taq (BioRad Laboratories Inc), 125 µM dNTPs (BioRad Laboratories Inc), 2.0 mM of Magnesium (Mg) (BioRad Laboratories Inc), 200 nM of each primer, and 2.5 U i-Taq DNA polymerase (BioRad Laboratories Inc). The cycling parameters for the amplification reactions were as follow: an initial denaturation step at 95 °C for 5 min was followed by 25 cycles of denaturation steps at 94 °C for 1 min, annealing steps at 68 °C for 2 min and extension at 72 °C for 2 min. PCR product were hold at 16 °C after a final extension at 72 °C for 5 min.
Two microlitre of the PCR reaction was then used to amplify the P. vivaxdhfr (pvdhfr) domain of size 711 bp by using oligonucleotide pair VDT OF and VDF OR (first PCR). The PCR reaction mixtures were the same as used in the first PCR reaction except for the Mg concentration and the primer concentration. The cycling temperature was also similar to the first PCR amplification except for the annealing temperature and the number of cycles (see Table 1).
Table 1.
Primers and profiles used for amplification of pvdhfr gene
| Primer pairs (5′–3′) | Concentration of Mg2 (mM) | Concentration of primer (nM) | Annealing Temperature (°C/Min) | Cycle | Size (bp) |
|---|---|---|---|---|---|
| VDT OF: ATGGAGGACCTTTCAGATGTATTTGACATT VDT OR: GGCGGCCATCTCCATGGTTATTTTATCGTG |
2.0 | 200 | 68/2 | 25 | 1876 |
| VDT OF: ATGGAGGACCTTTCAGATGTATTTGACATT VDF OR: CTTGCTGTAAACCAAAAAGTCCA |
2.0 | 400 | 66/2 | 30 | 711 |
| VDF 13NF: GACCTTTCAGATGTATTTGACATTTACGGC VDF NR58: GGTACCTCTCCCTCTTCCACTTTAGCTTCT |
1.5 | 300 | 62/2 | 30 | 232 |
| VDNF57: CATGGAAATGCAACTCCGTCGATATGATGT VDF NR: TCACACGGGTAGGCGCCGTTGATCCTCGTG |
1.5 | 400 | 50/2 | 30 | 472 |
| VDT OF: ATGGAGGACCTTTCAGATGTATTTGACATT VDF NR: TCACACGGGTAGGCGCCGTTGATCCTCGTG |
1.0 | 300 | 60/2 | 30 | 608 |
Nested PCR were carried out separately to amplify three regions on the pvdhfr genes. The PCR reaction used primer pairs VDF13NF and VDFNR58 amplifying 232 base pair (bp) fragment containing Ile13Leu, Pro33Leu, Ser58Arg, and Thr61Met, primer pair using VDNF58and VDF NR amplifying 472 bp fragment containing Phe58Ile/Leu and Ser117Thr/Asn, and primer pair VDTOF and VDFNR amplifying 608 bp fragment containing Phe58Ile/Leu and Ile173Leu. The PCR reaction were similar to the PCR reaction of the first PCR, except for the concentration of Mg and primer concentration. The cycling temperature was also similar to that of the first PCR except for the annealing temperature and the cycles (see Table 1).
Amplification of the P. vivax dhps (pvdhps) gene was carried out as described previously [19]. The first round PCR was performed by using the primer sets VDHPS OF and VDHPS OR, followed by two sets of second round PCR using primer sets VDHPS NF and VDHPS NR amplifying 703 bp fragment containing codon Ala383Gly, and primer sets VDHPS-553OF and VDHPS NR amplifying 170 bp fragment containing codon 553. The first and second rounds PCR were carried out in a final volume of 50 µl with similar reaction as carried out for pvdhfr, except for the primers and Mg concentration and the cycling temperature (see Table 2).
Table 2.
Primers and profiles used for amplification of pvdhps gene
| Primer pairs (5′–3′) | Concentration of Mg2 (mM) | Concentration of primer (nM) | Annealing Temperature (°C/Min) | Cycle | Size (bp) |
|---|---|---|---|---|---|
| VDHPS OF: ATTCCAGAGTATAAGCACAGCACATTTGAG VDHPS OR: CTAAGGTTGATGTATCCTTGTGAGCACATC |
2.0 | 250 | 69/2 | 21 | 1354 |
| VDHPS NF: AATGGCAAGTGATGGGGCGAGCGTGATTGA VDHPS NR: CAGTCTGCACTCCCCGATGGCCGCGCCACC |
2.0 | 250 | 70.5/2 | 25 | 703 |
| VDHPS 553OF: TTCTCTTTGATGTCGGCCTGGGGTTGGCCA VDHPS NR: CAGTCTGCACTCCCCGATGGCCGCGCCACC |
2.0 | 250 | 69.5/1 | 30 | 170 |
The PCR products were electrophoresed on 2 % agarose gel (Biorad Laboratories Inc) with GelTed™ Nucleic Acid Gel Stain and visualized on an ultraviolet transilluminator. The lack of cross-contamination was monitored by the inclusion of negative control samples (water was used to replace the DNA template) in each of amplifications carried out.
RFLP (Restriction fragment length polymorphism) for detection of specific mutations in pvdhfr and pvdhps
The presence of mutations on pvdhfr at codons 13Leu, 33Leu, 57Ile/Leu, 58Arg, 61Met, 117Asn/Thr, and 173Leu and mutation on pvdhps at codons 383Gly and 553Gly were analysed by RFLP following protocol as described previously [7]. The enzyme digestions were conducted according to manufacturer’s instruction (New England Biolabs, Beverly, MA, USA). Details of the restriction enzymes used and the digestion product sizes indicating wild type and mutant are described in Table 3. The detection of mutation at 13Leu, 33Leu, 58Arg, 61Met, and 173Leu was determined by HaeII, SacII, AluI, Tsp451, and StyI, respectively. The detection of mutation at 57Ile/Leu was determined by reaction of PCR (VDT-OF/VDFNR) with Xmn1, which did not cleave the 608 bp product. To confirm the mutation, either 57Ile or 57Leu, the PCR (VDNF57/VDF NR) was digested with BsrGI to digest the 472 bp products to 444 and 28 bp (mutation 57Ile) and no digestion (mutation 57Leu). The detection of mutation 117Thr/Asn was determined by reaction of PCR (VDNF57/VDF NR) with PvuII, which did not digest the 472 bp product. The codon Ser117Thr was determined by reaction with BsrI, which did not digest the 472 bp. The mutation at 117Thr was determined by reaction with BstNI, which cleaved the 472 bp product to 258 and 215 bp. The mutation at 117Asn was determined by reaction with BsrI which cleaved the 472 bp to 253 and 219 bp. The mutation at 117Asn was confirmed by reaction with BstNI, which did not cleave the 472 bp product. Amplification of pvdhps gene, region VDHPS NF/VDHPS NR, detected mutation at 383Gly. The mutation was detected by reaction of the PCR product with MspI, which cleaved the 703 bp product to 655 and 48 bp. The mutation at 553Gly was detected by reaction of PCR VDHPS553OF/VDHPSNR with MscI, which did not digest the 170 bp.
Table 3.
The primer pairs, PCR product sizes, restriction enzymes used and the digestion product size in detection of gene polymorphism on pvdhfr and pvdhps gene
| Gene/primer pair | PCR product size (bp) | To detectmutation at codon | Restriction enzyme used | Digestion product size (bp) | |
|---|---|---|---|---|---|
| Wild type | Mutation | ||||
| DHFR Forward: VDF 13NF Reserve: VDF NR58 |
232 | Ile13Leu Pro33Leu Ser58Arg Thr61Met |
HaeII SacII AluI Tsp451 |
Ile: 232 Pro: 138 and 94 Ser: 167, 40 and 25 Thr: 200 and 32 |
Leu: 200 and 32 Leu: 232 Arg: 207 and 25 Met: 232 |
| DHFR/ Forward: VDNF57 Reserve: VDF NR |
472 | Phe57Ile/Leu |
BsrGI PvuII BstNI BsrI |
Phe: 472 Ser: 258 and 214 Ser: 472 Ser: 472 |
Ile: 444 and 28 Leu: 472 Asn/Thr: 472 Thr: 257 and 215 Asn: 472 Asn: 253 and 219 Thr: 472 |
| DHFR/ Forward: VDT OF Reverse: VDF NR |
608 | Phe57Ile/Leu Ile173Leu |
XmnI StyI |
Phe: 442 and 166 Ile: 472 and 136 |
Ile/Leu: 608 Leu: 438, 97 and 73 |
| DHPS/ Forward: VDHPS NF Reverse: VDHPS NR |
703 | Ala383Gly | MspI | Ala: 703 | Gly: 655 and 48 |
| DHPS/ Forward: VDHPS553OF Reverse: VDHPSNR |
170 | Ala553Gly | MscI | Ala: 143 and 27 | Gly: 170 |
Analysis of PCR–RFLP products using the Agilent 2100 Bioanalyzer
The PCR–RFLP products were analysed using the Agilent 2100 Bioanalyzer and the Agilent DNA 1000 Kit (Agilent Technologies, Molecular Probes Inc, USA). The procedures were conducted according to manufacturer’s instruction. The result was then viewed and analysed using Agilent 2100 software.
Results
Sample collection
A total of 619 and 2119 individuals from 23 and 26 sites (villagers, logger camps, road construction workers) in Kalabakan and Kota Marudu, respectively, participated in the study. In Kalabakan, 58 individuals (9.37 %) (95 % [CI] 7.07–11.67) tested positive for malaria of which 26 (4.2 %) (95 % [CI] 2.6–5.8 %) were positive for P. vivax. While in Kota Marudu, 52 individuals (2.45 %) (95 % [CI]1.8–3.1 %) tested positive for malaria of which 11 (0.52 %) (95 % [CI] 0.2–0.8 %) were positive for P. vivax. Total number of P. vivax samples used in the study was 37. The pvdhfr and pvdhps genes were successfully amplified and RFLP analysis for detection of mutation on the genes was conducted.
Analysis of mutation on the pvdhfr and pvdhps genes
Findings of the pvdhfr gene showed presence of mutations at 58Arg and 61Met (Fig. 2), at 57Leu and 117Thr (Fig. 3) and 57Leu (Fig. 4). Findings of pvdhps gene showed mutation at 383Gly (Fig. 5). Wild type was observed at Ile13, Pro33, Ile173 on pvdhfr gene and Ala553 on pvdhps gene.The prevalent of wild type and mutant on pvdhfr and pvdhps genes is summarized in Table 4. No mutation was observed at codon 13, 33 and 173 on pvdhfr and at codon 553 on pvdhps gene on samples from Kalabakan and Kota Marudu. Common mutations (100 %) on pvdhfr were at 57Leu and 117Thr (Table 4). Mutations at 58Arg and 61Met were observed to be higher in Kota Marudu, 72.73 % (95 % [CI] 43.44–90.26). Mutation at 383Gly on pvdhps was highest in Kalabakan with 80.77 % (95 % [CI] 62.12–91.49) (Table 4).
Fig. 2.
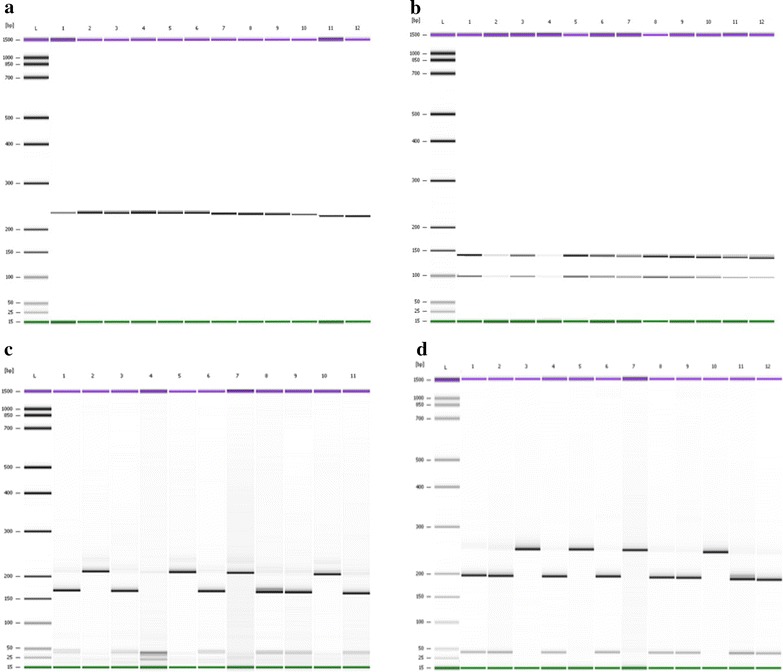
PCR–RFLP of the pvdhfr gene. PCR–RFLP products of VDF13NF/VDFNR58 with HaeIII showed no cleavage of the 232 bp fragment indicating of wild type Ile13 (a). Reaction with SacII cleaved the PCR products to 138 and 94 bp indicating wild type Pro33 (b). Reaction with AluI cleaved the PCR products to 208 and 25 bp fragments, indicating a mutation 58Arg, while cleavage to 167, 40 and 25 bp indicated a wild type Ser58 (c). Reaction with Tsp451 showed no cleavage (232 bp) indicating a mutation 61Met, while wild type Thr61 showed a cleavage to 200 and 32 bp fragment (d). a, b and d: Lane L DNA ladder of Agilent DNA 1000 Kit (Agilent Technologies, Molecular Probes Inc, USA), Lanes 1–12 PCR–RFLP products of samples. c: Lanes L DNA ladder of Agilent DNA 1000 Kit (Agilent Technologies, Molecular Probes Inc, USA), Lane 1–11 PCR–RFLP products of samples
Fig. 3.
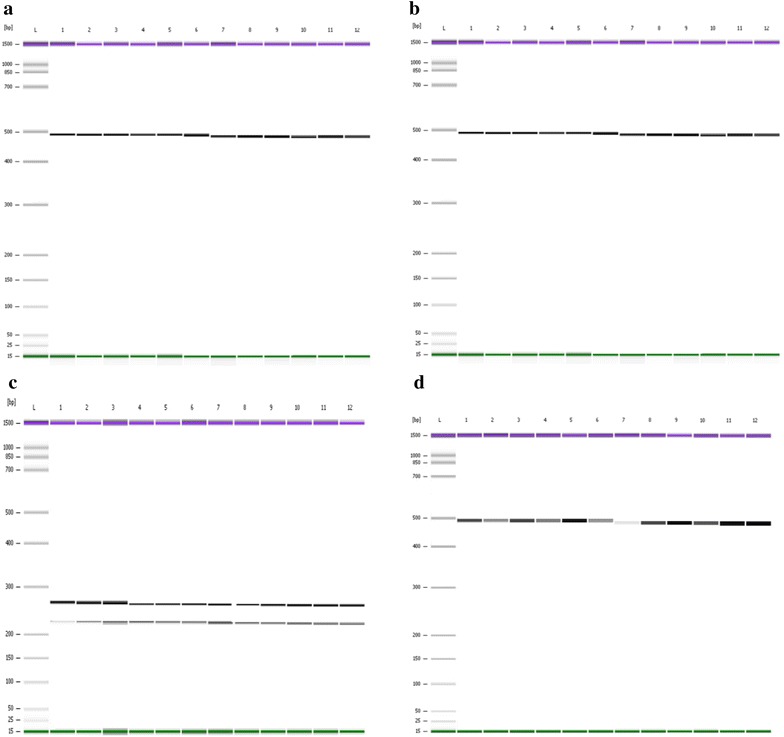
PCR–RFLP of the pvdhfr gene. PCR–RFLP product of VDNF 57/VDF NR with BsrGI showed no cleavage of the 472 bp indicating wild type Phe58 and mutation 57Leu (a). Reaction with PvuII showed no cleavage (472 bp) indicating mutation 117Thr/Asn (b). Reaction with BstN1 cleaved the 472 bp to 258 and 215 bp indicating mutation 117Thr (c). Reaction with Bsr1 showed no cleavage indicating wild type Ser118 and mutation 117Thr (d). a, b, c and d: Lane L DNA ladder of Agilent DNA 1000 Kit (Agilent Technologies, Molecular Probes Inc, USA), Lanes 1–12 PCR–RFLP products of samples
Fig. 4.
PCR–RFLP of the pvdhfr gene. PCR–RFLP of VDTOF/VDFNR with StyI cleaved the 608 bp fragment into 472 and 136 bp indicating of wild type Ile173 (a). Reaction with XmnI showed no cleavage (608 bp) indicating mutation 57Ile/Leu (b). a and b: Lane L DNA ladder of Agilent DNA 1000 Kit (Agilent Technologies, Molecular Probes Inc, USA). Lanes 1–12 PCR-RFLP products of samples
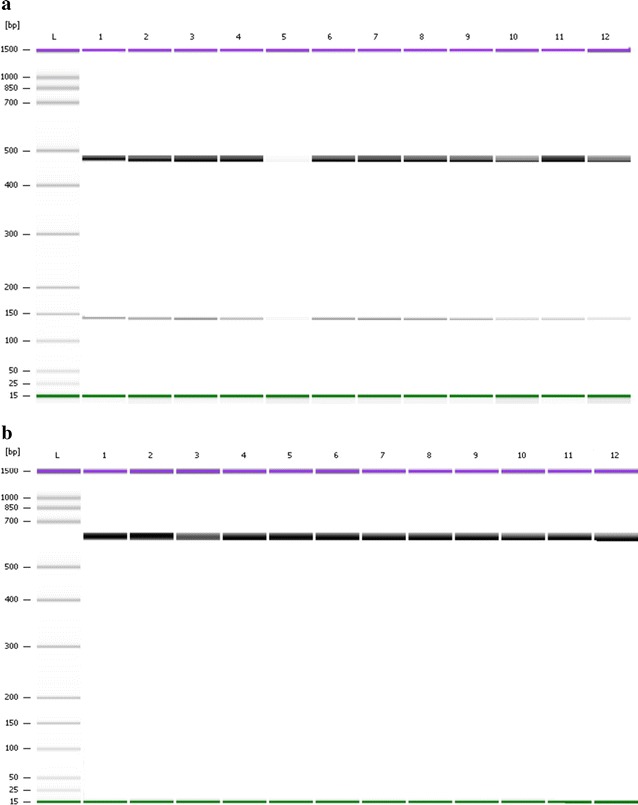
Fig. 5.

PCR-RFLP of the pvdhps gene. PCR-RFLP of VDHPSNF/VDHPSNR with MspI cleaved the 703 bp fragment into 655 and 48 bp indicating mutation 383Gly (a). PCR–RFLP of VDHPS553OF/VDHPSNR with MscI digest the 170 bp to 143 and 28 bp indicating wild type Ala553 (b). a and b: Lane L DNA ladder of Agilent DNA 1000 Kit (Agilent Technologies, Molecular Probes Inc, USA). a: Lanes 1, 3, 5, 7, 9, and 11 PCR products of VDHPSNF/VDHPSNR, Lanes 2, 4, 6, 8, 10, and 12 digestion reaction with MspI. b: Lanes 1, 3, 5, 7, 9, and 11 PCR products of VDHPS553OF/VDHPSNR, Lanes 2, 4, 6, 8, 10, and 12 digestion reaction with MscI
Table 4.
Frequency of wild type and mutation on the dhfr and dhps gene from 37 P. vivax samples collected in Kalabakan and Kota Marudu, Sabah
| Genes | Codon | Kalabakan (n = 26) | Kota Marudu (n = 11) | ||
|---|---|---|---|---|---|
| Wild type (%) | Mutant (%) | Wild type (%) | Mutant (%) | ||
| Pvdhfr | 13 | 100 | 0 | 100 | 0 |
| 33 | 100 | 0 | 100 | 0 | |
| 57 | 0 | 100 | 0 | 100 | |
| 58 | 69.23 | 30.77 | 27.27 | 72.73 | |
| 61 | 69.23 | 30.77 | 27.27 | 72.73 | |
| 117 | 0 | 100 | 0 | 100 | |
| 173 | 100 | 0 | 100 | 0 | |
| pvdhps | 383 | 19.23 | 80.77 | 27.27 | 72.73 |
| 553 | 100 | 0 | 100 | 0 | |
Distribution of pvdhfr and pvdhps combination alleles
The findings showed that there are four distinct haplotypes of pvdhfr/pvdhps combination among the 37 samples (Table 5). The four haplotypes are:
13Ile, 33Pro, 57Leu, 58Arg, 61Met, 117Thr, 173Ile, 383Gly, 553Ala,
13Ile, 33Pro, 57Leu, 58Ser, 61Thr, 117Thr, 173Ile, 383Gly, 553Ala,
13Ile, 33Pro, 57Leu, 58Arg, 61Met, 117Thr, 173Ile, 383Ala, 553Ala and
13Ile, 33Pro, 57Leu, 58Ser, 61Thr, 117Thr, 173Ile, 383Ala, 553Ala
Table 5.
Frequency distribution of the combination pvdhfr and pvdhps from 37 P. vivax samples collected in Kalabakan and Kota Marudu, Sabah demonstrated four distinct haplotypes
| Pvdhfr alleles | Pvdhps alleles | No of isolates (%) | No of dhfr/dhps combination mutant genotype | |
|---|---|---|---|---|
| Kalabakan (n = 26) | Kota Marudu (n = 11) | |||
| 13Ile/33Pro/57Leu/58Arg/61Met/117Thr/173Ile | 383Gly553Ala | 7 (26.92) | 6 (54.55) | 4 dhfr, 1 dhps |
| 13Ile/33Pro/57Leu/58Ser/61Thr/117Thr/173Ile | 383Gly/553Ala | 14 (53.85) | 2 (18.18) | 2 dhfr, 1 dhps |
| 13Ile/33Pro/57Leu/58Arg/61Met/117Thr/173Ile | 383Ala/553Ala | 1 (3.85) | 2 (18.18) | 4 dhfr |
| 13Ile/33Pro/57Leu/58Ser/61Thr/117Thr/173Ile | 383Ala/553Ala | 4 (15.38) | 1 (9.09) | 2 dhfr |
Ile isoleucine, Leu leucine, Ser serine, Thr threonine, Pro proline, Arg arginine, Met methionine, Ala alanine, Gly glycine
Discussion
From 2006 to 2012, more than 50 % of malaria cases in Malaysia were attributed by P. vivax species, although recently P. knowlesi cases has been reported to be prevalent at Lipis Pahang in the Peninsular Malaysia and Sarawak, in Malaysia Borneo [26]. The malaria cases are decreasing; however, cases still occur in confined, remote, interior, hilly or forested areas of endemic regions. Malaria control and elimination strategy plays an important role in decreasing the disease burden in the country [27]. The present study is undertaken to investigate the prevalence of mutation associated with the pvdhfr and pvdhps in P. vivax isolates in the endemic areas of Kalabakan and Kota Marudu in Sabah. In Kalabakan, of the 619 individuals screened for malaria, 9.37 % were positive, of which 4.2 % were P. vivax infection, while in Kota Marudu, of the 2119 population screened, 2.45 % positive for malaria, of which 0.52 % were positive for P. vivax.
Genotype analysis of the P. vivax samples identified that the most prevalent mutations of pvdhfr gene from both Kalabakan and Kota Marudu are single mutations at codon 57Leu (100 %) and 117Thr (100 %). Findings from Kuesap et al. [28] in Mae Sot area in Thailand showed a similar observation, where mutation at codon 117Thr was prevalent. Unlike this study, where mutation at 57Leu (100 %) was observed, the finding in Mae Sot, Thailand was found to be the less [28]. Recent studies from Philippine, showed that there are no mutation at acid amino 57 meanwhile all their samples were mutant 117Asn [29].Meanwhile, study from Indonesia showed the allele with the highest prevalence was 117Asn (27 %), followed by 117Thr (18 %) [30]. However,all samples from Kalabakan and Kota Marudu did not contain any 117Asn mutation. Mutation at 57Leu and 117Thr, on its own, confers resistance to pyrimethamine [11, 31] and likely to be precursory to further dhfr mutations.
The present study also showed mutation at codon 58Arg (Kalabakan 30.77 % and Kota Marudu 72.73 %) and at codon 61Met (Kalabakan 30.77 % and Kota Marudu 72.73 %). Mutations at 57Leu, 58Arg, 61Met, and 117Thr have been reported to be involved in clinical antifolate resistance [11, 32]. In Kota Marudu, mutation at 58Arg and 61Met was found to be high compared to Kalabakan. However in Kalabakan, a higher proportion of the P. vivax populations are still wild type at codon 58Arg and 61Met. Kota Marudu region is nearly with Philippines meanwhile Kalabakan is more nearly to Indonesia. Recent study by from Philippine showed that all their sample represent the mutation at 58 Arg [29] compare with study from Indonesia showed that their sample still have wild type at codon 58 (58Ser) [30]. Studies conducted in the region indicated that mutation at 58Arg is common, similar to other regions, such as East Timor [15], Thailand [25], Philippines [17], and India [31].
Study of the pvdhps gene on samples collected from Kalabakan and Kota Marudu showed that mutation at codon 383Gly (80.77 and 72.73 %, respectively) is common. Mutation at codon 383Gly indicated a reduced sensitivity to sulfa drugs and sulfones [14, 19]. Unlike this study, the prevalence of 383Gly mutation is low in other parts of the region, East Timor [33], Iran [34], Pakistan [7], and Korea [16]. Similar with recent study from neighbouring region, Indonesia showed 50 % sample carried mutant allele, 383Gly meanwhile mutant allele 553Gly was not observed in any of the isolates examined [30].
No mutations were detected at codon 13Leu, 33Leu and 173Leu of pvdhfr gene and codon 553Gly on the pvdhps. Mutation at codon 173Leu was found to be involved in clinical anti-folate resistance [25]. With the presence of 383Gly mutation along with double mutant of pvdhfr, which observed in the present study, implicated the use of either pyrimethamine or sulfadoxine (with other anti-malarial drugs) or a combination (SP), in post treatment failure.
In the present findings, there are four distinct haplotypes of pvdhfr/pvdhps. The most common combination is Ile13, Pro33, 57Leu, Ser58, Thr61, 117Thr, Ile173, 383Gly, Ala553, (two dhfr, one dhps) (53.85 %), and was found to be prevalent in Kalabakan. Haplotype Ile13, Pro33, 57Leu, 58Arg, 61Met, 117Thr, Ile173, 383Gly, Ala553, (four dhfr, one dhps) was observed in both Kalabakan and Kota Marudu. Clinical studies have showed that quadruple pvdhfr mutant parasite (57Leu, 58Arg, 61Met, 117Thr), had been associated with SP treatment failure [9, 11, 32]. The mutant parasite in this study, strongly suggested that P. vivax isolates from Kalabakan and Kota Marudu carry the resistant allele to antifolate drugs, SP. This observation is also seen in neighbouring countries, Indonesia, Papua New Guinea and India [9, 35].
Mutation combinations at 57Leu, 58Arg with 383Gly and 58Arg, 117Thr with 383Gly, have been implicated in reducing sensitivity to SP drugs in P. vivax population [36], and mutation at 58Arg and 117Thr has been implicated in in vivo pyrimethamine resistance as it could cause structural changes in pvdhfr and lead to decrease binding to the drug [37], and appeared first under drug pressure [34]. One in vitro study at Indonesia, showed that most of samples that carried the quadruple mutant allele (57Arg/58Leu/61Met/117Thr) were 23 times more likely to experience therapeutic failure, compare with subjects infected by parasites that carried only wild type, single, double or triple mutant allele of dhfr [13].
Conclusion
The study suggests the presence of sulfadoxine, pyrimethamine and SP pressure on P. vivax isolates in Kalabakan and Kota Marudu, Sabah. The presence of triple and quintuple mutation combination suggests that P. vivax isolates exhibit a high degree of resistance to sulfadoxine and pyrimethamine and SP. The use of parasite molecular markers as tools for surveillance for drug resistance provides support to malaria control programmes and malaria elimination strategies, so that resistance status can be assessed and the most effective treatment can be selected and deployed.
This study served as scientific evidence that there is resistance to sulfadoxine, pyrimethamine and SP in the study area. Any malaria drug combination, such as artesunate plus SP and chloroquine plus SP needs to be reconsidered for the treatment of P. vivax infection, especially in the area of chloroquine and sulfadoxine-pyrimethamine-resistant P. vivax.
Authors’ contributions
NRA prepared the study proposal, study design, led the study in Kalabakan and Kota Marudu, Sabah, data analysis and interpretation and preparation of manuscript. URS conducted the molecular genetic studies, analysis of data, data statistics and partly drafted the manuscript. NAN, MNFS and PKM contributed reagents/materials/analysis tools. JJ, MT and NSY participated in the study design in the field. NRA and HMS edited and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank the Director General of Health Malaysia for the permission to publish this paper. We thank the Director of the Institute for Medical Research, Kuala Lumpur, for her critical review and support in publishing this paper. Thanks also to all the patients who participated in the study, and the clinical staff who supported the sample collections and assisted in the clinical and laboratory procedures.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Umi Rubiah Sastu, Email: umie_1902@yahoo.com.my.
Noor Rain Abdullah, Email: noorrain@imr.gov.my.
Nor Azrina Norahmad, Email: azrina@imr.gov.my.
Muhammad Nor Farhan Saat, Email: mnfarhan@imr.gov.my.
Prem Kumar Muniandy, Email: prem_khs@hotmail.com.
Jenarun Jelip, Email: drjenarun@gmail.com.
Moizin Tikuson, Email: tmoizin@yahoo.com.
Norsalleh Yusof, Email: hazimnsy@yahoo.com.
Hasidah Mohd Sidek, Email: hasidahmohdsidek@yahoo.com.
References
- 1.WHO: World Malaria Report 2012. Geneva: World Health Organization; 2012.
- 2.Ministry of Health Malaysia: Vector Borne Disease Control Program: vector control in Malaysia. Ministry of Health Malaysia; 2014.
- 3.Poespoprodjo JR, Fobia W, Kenangalem E, Lampah DA, Hasanuddin A, Warikar N, et al. Vivax malaria: a major cause of morbidity in early infancy. Clin Infect Dis. 2009;48:1704–1712. doi: 10.1086/599041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandre M, Ferreira CO, Siqueira AM, Magalhaes BL, Mourao MP, Lacerda MV, et al. Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611–1614. doi: 10.3201/eid1610.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health Malaysia:Vector Borne Disease Control Program: Vector Control in Malaysia. Ministry of Health Malaysia; 2008.
- 6.Lokman Hakim S, Sharifah Roohi SW, Zurkunai Y, Noor Rain A, Mansor SM, Palmer K, et al. Plasmodium falciparum: increased propotion of severe resistance (RII and RIII) to chloroquine and high rate of resistance to sulfadoxine-pyrimethamine in Peninsular Malaysia after two decades. Trans R Soc Trop Med Hyg. 1996;90:294–297. doi: 10.1016/S0035-9203(96)90258-8. [DOI] [PubMed] [Google Scholar]
- 7.Zakeri S, Afsharpad M, Ghasemi F, Raeisi A, Kakar Q, Atta H, et al. Plasmodium vivax: prevalence of mutations associated with sulfadoxine-pyrimethamine resistance in Plasmodium vivax clinical isolates from Pakistan. Exp Parasitol. 2011;127:167–172. doi: 10.1016/j.exppara.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Gregson A, Plowe C. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol Rev. 2005;57:117–145. doi: 10.1124/pr.57.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Hastings MD, Maguire JD, Bangs MJ, Zimmerman PA, Reeder JC, Baird JK, et al. Novel Plasmodium vivax dhfr alleles from Indonesia Archipelago and Papua New Guinea: association with pyrimethamine resistance determined by a Saccharomyces cerevisiae expression system. Antimicrob Agents Chemother. 2005;49:733–740. doi: 10.1128/AAC.49.2.733-740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leartsakulpanich U, Imwong M, Pukrittakayamee N, White J, Snounou G, Sirawaraporn S, et al. Molecular characterization of dihydrofolate reductase in relation to antifolate resistance in Plasmodium vivax. Mol Biochem Parasitol. 2002;119:63–73. doi: 10.1016/S0166-6851(01)00402-9. [DOI] [PubMed] [Google Scholar]
- 11.Imwong M, Pukrittayakamee S, Looareesuwan S, Pasvol G, Poirreiz J, White NJ, Snounou G. Association of genetic mutations in Plasmodium vivax DHFR with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob Agents Chemother. 2001;45:3122–3127. doi: 10.1128/AAC.45.11.3122-3127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tjitra E, Baker J, Suprianto S, Cheng Q. Abstey. N: therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob Agents Chemother. 2002;46:3947–3953. doi: 10.1128/AAC.46.12.3947-3953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastings MD, Porter KM, Maguire JD, Susanti I, Kania W, Bangs MJ, et al. Dihydrofolate reductase mutations in Plasmodium vivax from Indonesia and therapeutic response to sulfadoxine plus pyrimethamine. J Infect Dis. 2004;189:744–750. doi: 10.1086/381397. [DOI] [PubMed] [Google Scholar]
- 14.Korsinczky M, Fischer K, Chen N, Baker J, Rickmann K, Cheng Q. Sulfadoxine resistance in Plasmodium vivax is associated with a specific amino acid in dihydropteroate synthase at the putative sulfadoxine-binding site. Antimicrob Agents Chemother. 2004;48:2214–2222. doi: 10.1128/AAC.48.6.2214-2222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Almeida A, Rosário VE, Henriques G, Arez AO, Cravo P. Plasmodium vivax in the Democratic republic of East Timor: parasite prevelance and antifolate resistance-associated mutations. Acta Trop. 2010;115:288–292. doi: 10.1016/j.actatropica.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Lu F, Lim CS, Nam DH, Kim K, Kim TS, Lee HW, et al. Mutations in the antifolate-resistance-associated genes dihydrofolate reductase and dihydropteroate synthase in Plasmodium vivax isolates from malaria-endemic countries. Am J Trop Med Hyg. 2010;83:474–479. doi: 10.4269/ajtmh.2010.10-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auliff A, Wilson DW, Russell B, Gao Q, Chen N, le Anh N, et al. Amino acid mutations in Plasmodium vivax dhfr and dhps from several geographical regions and susceptibility to antifolate drugs. Am J Trop Med Hyg. 2006;75:617–621. [PubMed] [Google Scholar]
- 18.Alam MT, Bora H, Bharti PK, Saifi MA, Das MK, Dev V, et al. Similar trends of pyrimethamine resistance-associated mutations in Plasmodium vivax and P. falciparum. Antimicrob Agents Chemother. 2007;51:857–863. doi: 10.1128/AAC.01200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imwong M, Pukrittayakamee S, Cheng Q, Moore C, Looareesuwan S, Snounou G, et al. Limited polymorphism in the dihydropteroate synthase gene (dhps) of Plasmodium vivax isolates from Thailand. Antimicrob Agents Chemother. 2005;49:4393–4395. doi: 10.1128/AAC.49.10.4393-4395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rungsihirunrat K, Na-Bangchang K, Hawkins VN, Mungthin M, Sibley CH. Sensitivity to antifolates and genetic analysis of Plasmodium vivax isolates from Thailand. Am J Trop Med Hyg. 2007;76:1057–1065. [PubMed] [Google Scholar]
- 21.Noor Rain A, Nor Azrina N, Jenarun J, Lokman Hakim S, Hasidah MS, Zakiah I, et al. High prevalence of mutation in the Plasmodium falciparum dhfr and dhps genes in field isolates from Sabah, Northern Borneo. Malar J. 2013;12:198–205. doi: 10.1186/1475-2875-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau TY. M S, Timothy W: mutational analysis of Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase genes in the interior division of Sabah, Malaysia. Malar J. 2013;12:445. doi: 10.1186/1475-2875-12-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuehrer H-P, Fally MA, Habler VE, Starzengruber P, Swoboda P, Noedl H. Novel nested direct PCR technique for malaria diagnosis using filter paper samples. J Clin Microbiol. 2011;49:1628–1630. doi: 10.1128/JCM.01792-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imwong M, Tanomsing N, Pukrittayakamee S, Day NPJ, White NJ, Snounou G. Spurious amplification of a Plasmodium vivax Small-subunit rna gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol. 2009;47:4173–4175. doi: 10.1128/JCM.00811-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imwong M, Pukrittayakamee S, Rénia L, Letourneur F, Charlieu JP, Leartsakulpanich U, et al. Novel mutations in the dihydrofolate reductase gene of Plasmodium vivax: evidence for sequential selection by drug pressure. Antimicrob Agents Chemother. 2003;47:1514–1521. doi: 10.1128/AAC.47.5.1514-1521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life-threatening. Clin Infect Dis. 2008;46:165–171. doi: 10.1086/524888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO . Guidelines for the treatment of malaria. 2. Geneva: World Health Organization; 2010. pp. 1–194. [PubMed] [Google Scholar]
- 28.Kuesap J, Rungsrihirunrat K, Thongdee P, Ruangweerayut R, Na-Bangchang K. Change in mutation patterns of Plasmodium vivax dihydrofolate reductase (pvdhfr) and dihydropteroate synthase (pvdhps) in P. vivax isolates from malaria endemic areas of Thailand. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2011;106:130–133. doi: 10.1590/S0074-02762011000900017. [DOI] [PubMed] [Google Scholar]
- 29.Thongdee P, Kuesap J, Rungsihirunrat K, Dumre SP, Espino E, Noedl H, et al. Genetic polymorphisms in Plasmodium vivax Dihydrofolate Reductase and Dihydropteroate Synthase in isolates from the Philippines, Bangladesh, and Nepal. Korean J Parasitol. 2015;53:227–232. doi: 10.3347/kjp.2015.53.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asih PBS, Marantina SS, Nababan R, Lobo NF, Rozi IE, Sumarto W, et al. Distribution of Plasmodium vivax pvdhfr and pvdhps alleles and their association with sulfadoxine-pyrimethamine treatment outcomes in Indonesia. Malar J. 2015;14:36. doi: 10.1186/s12936-015-0903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Pécoulas PE, Tahar R, Ouatas T, Mazabraud A, Basco L. Sequences variations in the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene and their relationship with pyrimethamine resistance. Mol Biochem Parasitol. 1998;92:265–273. doi: 10.1016/S0166-6851(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins VN, Joshi H, Rungsihirunrat K, Na-Bangchang K, Sibley CH. Antifolates can have a role in the treatment of Plasmodium vivax. Trends Parasitol. 2007;23:213–222. doi: 10.1016/j.pt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Zakeri S, Motmean SR, Afsharpad M, Djadid ND. Molecular characterization of antifolates resistance-associated genes, (dhfr and dhps) in Plasmodium vivax isolates from the Middle East. Malar J. 2009;8:20. doi: 10.1186/1475-2875-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson DS, Walliker D, Wellems TE. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci USA. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suminder K, Prajapati SK, Kalyanaraman K, Mohmmed A, Joshi H, Chauhan VS. Plasmodium vivax dihydrofolate reductase point mutations from the Indian subcontinent. Acta Trop. 2006;97:174–180. doi: 10.1016/j.actatropica.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Kongsaeree P, Khongsuk P, Leartsakulpanich U, Chitnumsub P, Tarnchompoo B, Walkinshaw MD, et al. Crystal structure of dihydrofolate reductase from Plasmodium vivax: pyrimethamine displacement linked with mutation-induced resistance. Proc Natl Acad Sci USA. 2005;102:13046–13051. doi: 10.1073/pnas.0501747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zakeri S, Kakar Q, Ghasemi F, Raeisi A, Butt W, Safi N, et al. Detection of mixed Plasmodium falciparum and P. vivax infections by nested-PCR in Pakistan, Iran & Afghanistan. Indian J Med Res. 2010;132:31–35. [PubMed] [Google Scholar]


