Abstract
Background
Healthcare-associated infection (HAI) rates have fallen with development of multifaceted infection prevention programs. However, these programs require ongoing investments. Our objective was to examine the cost effectiveness of hospitals’ ongoing investments in HAI prevention in intensive care units (ICUs).
Methods
Five years of Medicare data were combined with HAI rates, cost and quality of life estimates drawn from literature. Life years (LYs), quality adjusted life years (QALYs), and health care expenditures with and without central line associated bloodstream infection (CLABSI) and/or ventilator associated pneumonia (VAP) as well as incremental cost-effectiveness ratios (ICERs) of multifaceted HAI prevention programs were modeled.
Results
Total LYs and QALYs gained per ICU due to infection prevention programs were 15.55 LY and 9.61 QALY for CLABSI and 10.84 LY and 6.55 QALY for VAP. Reductions in index admission ICU costs were $174,713.09 for CLABSI and $163,090.54 for VAP. The ICERs were $14,250.74 per LY gained and $23,277.86 per QALY gained.
Conclusions
Multifaceted HAI prevention programs are cost-effective. Our results are a reminder of the importance of maintaining the ongoing investments in HAI prevention. The welfare benefits implied by the advantageous incremental cost-effectiveness ratios would be lost if the investments were suspended.
Annually, approximately 1.7 million patients suffer a health care-associated infection (HAI) in the United States and nearly 100,000 are estimated to die.1 The overall direct annual cost of these infections to our nation has been estimated to range from $28 to $45 billion dollars; and health care-associated sepsis and pneumonia are among the most costly in terms of mortality as well as financially.2 In patients with invasive surgery, the attributable mean length of stay has been reported to be 10.9 days, costs were $32,900 and mortality was 19.5% for each case of hospital-acquired sepsis; the corresponding values for hospital-acquired pneumonia were 14.0 days, $46,400 and 11.4%.3 Furthermore, the majority of these infections are associated with external devices inserted in intensive care units (ICU), namely central line catheters and ventilators.4
In an effort to decrease HAIs in the ICU, a number of evidence-based clinical interventions such as bundles and guidelines have been published.5-7 Effective implementation of these interventions is crucial to support clinician adherence at the bedside and reduce HAI rates.8-10 Indeed, focusing on improving the organizational culture by promoting standardized evidence based practice protocols, providing clinician compliance audit and feedback loops, expert-led educational sessions, and forums for dissemination are often needed to improve clinician adherence and patient quality. Such interventions are most often accomplished in hospitals by investing in multifaceted infection prevention programs.7,11
As a result of past investments, the United States has seen vast improvements in many HAI rates with impressive progress to the 5-year targets set out in the HAI Action Plan.12 However, focusing on infection prevention uses limited and competing resources and requires an ongoing financial commitment by the institution. Additionally, the cost and resource utilization estimates described above are limited to those directly incurred by the institution during the hospitalization in which the infection occurred. The societal welfare benefits of improved HAI prevention in the hospital includes not only the immediate health benefits and cost reductions of infection prevention, but also the long-term benefits of improved survival and the value of future health care expenditures. Furthermore, these long-term post-hospitalization costs are important to those needing a societal perspective, such as the Centers for Medicaid and Medicare Services (CMS), other insures and/or to those implementing accountable care organizations.
Cost-effectiveness modeling can guide public policy and institutional investment decisions by quantifying the long-term health and economic consequences attributable to different strategies, and aid in the understanding of the full impact of the infections, as well as the value of past and future investments in reducing infections. Using a societal perspective, the objective of our study was to examine the cost-effectiveness of a hospitals’ ongoing investment in preventing HAIs in an ICU. To do so, we developed a model that estimates the attributable long-term patient outcomes and health care expenditures associated with a multifaceted infection prevention program designed to decrease central line-associated bloodstream infection (CLABSI) and ventilator-associated pneumonia (VAP). The comparator was usual care without an ongoing investment in infection prevention programs.
Methods
The Long-term Cost Effectiveness HAI Prevention Policy Model
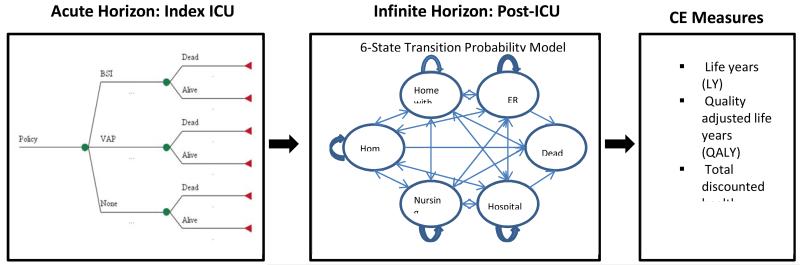
We developed a long-term HAI cost-effectiveness policy model that starts with an index hospitalization during which an elderly subject has an ICU stay. Each subject’s experience during the index ICU stay was characterized, including the probability of developing a CLABSI or VAP, the conditional probability of inpatient deaths due to the specific HAI type, and the incremental hospital costs associated with each infection. We modeled 5-year post-discharge experiences using a 6-state (living in community, using home health care, living in a nursing home, inpatient hospital, emergency room visit and dead) discrete-time dynamic model to estimate post-discharge survival, quality of life, and health care costs, each of which were conditional upon having or not having experienced an HAI during the index ICU stay (see Figure 1). We examined alternative assumptions about the lasting consequences of infections. The time horizon for the model was lifetime, and we calculated incremental cost-effectiveness ratios (ICERs) both as costs per life year (LY) and as costs per quality-adjusted life year (QALYs). Because the intervention is applied at the unit level we therefore present ICERs at the unit level.
Figure 1.
A long-term HAI cost-effectiveness policy model featuring a decision model for the index ICU stay and a 6-state transition probability model for ICU stay.
The ICU was the chosen setting because critically ill patients have the highest risk of infection, surveillance data regarding infections are readily available from ICUs, evidence of efficacy of infection prevention programs exist and are the focus of national targets. The choice of the elderly is relevant because the majority of ICU patients are elderly and also pragmatic because it allowed us to use Medicare data for long-term follow up. The six health states were chosen because they reflect meaningful changes in quality of life and have associated health care utilization.
Data
Base case model parameters were drawn from the literature (see Table 1) or estimated from two unique data sets compiled by the research team. The first data set was a cohort of 17,537 elderly Medicare patients admitted to 31 hospitals during 2002 with clinical data about HAI outcomes from infection surveillance that was conducted by infection preventionists and submitted to the Centers for Disease Control and Prevention’s (CDC) National Nosocomial Infection Surveillance (NNIS) system; these data were augmented with five years of Medicare claims data that allowed us to assess the long-term health outcomes and health care utilization attributable to HAIs.13 The second data set was from a recent National Healthcare Safety Network (NHSN) research group in which 701 hospitals provided ICU-specific device utilization and HAI rates for 2011.14 Other parameters required to determine the health benefits and economic costs included estimates of the deaths attributable to HAI; estimates of the incremental index hospital costs due to HAI, which were derived from the literature3 and life-tables.15
Table 1. Overview of Variables and Values.
| Variable | Base Case | High | Low | Reference | |||
|---|---|---|---|---|---|---|---|
| Intervention Costs (per year): | $145,000.00 | $217,500.00 | $72,500.00 | Waters16 | |||
|
| |||||||
| CLABSI | VAP | CLABSI | VAP | CLABSI | VAP | ||
| Base Case | Base Case | Reference | |||||
|
| |||||||
| Index ICU Stay: | |||||||
|
| |||||||
| Device Days | |||||||
| Usual care comparator | 3324 | 2918 | - | - | - | - | NNIS 200417 |
| Multifaceted infection prevention program | 2846 | 2028 | 4269 | 3042 | 1423 | 1014 | NHSN 201118 |
|
| |||||||
| Incidence (per 1000 device days) | |||||||
| Usual care comparator | 5.0 | 4.9 | - | - | - | - | NNIS 200417 |
| Multifaceted infection prevention program | 1.2 | 1.1 | 4.8 | 4.7 | 0.0 | 0.0 | NHSN 201118 |
|
| |||||||
| Attributable Death Rate | 0.160 | 0.103 | 0.250 | 0.161 | 0.120 | 0.077 | Eber3 |
|
| |||||||
| Costs | |||||||
| Inpatient $ / HAI case | $16,155.00 | $21,163.00 | $24,232.50 | $31,744.50 | $8,077.50 | $10,581.50 | CDC2 |
|
| |||||||
| Base Case | High | Low | Reference | ||||
|
| |||||||
| Post-index ICU stay experiences: | |||||||
|
| |||||||
| Discount rate | 3% | 5% | 0% | ||||
|
| |||||||
| Quality of life decrements | |||||||
| Hospital admission* | 0.121 | 0.182 | 0.061 | Scherbaum30 | |||
| Home care day** | 0.570 | 0.855 | 0.285 | ||||
| Long-term care day** | 0.570 | 0.855 | 0.285 | ||||
| Emergency room visit day** | 0.400 | 0.600 | 0.200 | ||||
| Age 65*** | 0.200 | ||||||
| Age > 65 / per year*** | 0.005 | Wagner31 | |||||
|
| |||||||
| Costs | |||||||
| Hospital day | $2,048.36 | $3,072.54 | $1,024.18 | ||||
| Home care day | $143.83 | $215.75 | $71.92 | ||||
| Emergency room visit | $5,655.57 | $8,483.36 | $2,827.79 | ||||
| Long-term care day | $99.54 | $149.30 | $49.77 | ||||
The hospital admission utility decrement is an annual decrement
The home care, long-term care, and emergency room decrements are per day
We apply an age-based decrement to utility, setting QALY to 0.80 at age 65 and decreasing by 0.005 per year thereafter (See Wagner31).
CLABSI: Central Line-Associated Bloodstream Infections, HAI: Healthcare-Associated Infections, ICU: Intensive Care Unit, QALY: Quality-Adjusted Life Years, VAP: Ventilator-Associated Pneumonia
Infection Prevention Costs
The cost of a multifaceted infection prevention program was based on published estimates from the Michigan Keystone ICU Patient Safety Program.16 The in-depth costing analysis was based on a stratified random sample of six Michigan hospitals participating in the Program. We chose these cost estimates because the infection prevention program focused on decreasing CLABSI and VAP in ICUs. Using an in-depth activity based costing approach in the six hospitals, the average annualized infection prevention program was estimated to cost $161,000 per year per hospital, which included both start up ($16,000) and ongoing implementation ($145,000) costs. Since we were interested in the ongoing investment, we used the $145,000 ongoing implementation costs in our base case analysis.
The Comparator and Post-Intervention HAI Rates
The usual care comparator associated HAI rates and device days for CLABSI and VAP came from 2004 published NNIS data;17 the post-intervention ICU HAI rates were drawn from 2011 NHSN published data.18 These HAI rates represent a 76% reduction CLABSI and 77.6% reduction in VAP, which is similar to rates found in clinical trials assessing the effectiveness of multifaceted infection prevention programs.7,19
Post-Discharge Health States, Quality of Life and Costs
Medicare data were used to estimate the monthly conditional probabilities of the health states for those that had or had not experienced a HAI. These five-year (60 month) post-index ICU stay estimates were developed to characterize health status and associated resource utilization as a function of HAI while controlling for demographic characteristics (i.e., age, gender and race), chronic comorbidities (for which we used 30 aggregated condition codes and 184 hierarchical condition codes using the DxCG software)20-22 and dual eligibility to Medicare and Medicaid.13 We extended the survival functions to end of life by applying age specific mortality rates using life tables.15 Because impending death has substantial effects on health status, we enhanced the models by conditioning on the time until death and used alternative assumptions about the lasting consequences of infections to extend the utilization predictions through end-of-life.
Quality of life adjustments were based on similar health states described in the literature. To find the quality of life adjustments, we searched the Cost-effectiveness Analysis Registry database.23 From these quality of life adjustments, dis-utilities were calculated based on the estimated number of days in each health state. To account for the effects of aging on quality of life, we included an age-specific QALY assuming 0.80 at age 65 and included an annual decrement of 0.005 for each year thereafter. We also present the results in life years (LY) gained without the quality adjustment.
Average cost estimates obtained from published literature were assigned for the utilization of the following resources: hospital day, homecare day, emergency room day and long-term care day. We accumulated the costs of utilization and generated the net present value of post-discharge health care costs at the time of the index ICU admission. A 3% discount rate was applied to costs and health benefits.24 All survival models were analyzed using STATA (StataCorp LP, College Station, TX). The final cost-effectiveness models were developed in EXCEL. All cost data are presented in 2013 US dollars. To determine the robustness of the results a number of one-way sensitivity analyses were conducted testing the assumptions of the intervention effectiveness, underlying device days in the ICU, attributable mortality, intervention costs, downstream utilization costs, discount rates and utilities used in the QALYs. We also examined the robustness of the results in a less heterogeneous population and varied the age cohorts limiting the analysis to those 65 to 69 years and those 75 to 79 years of age. The consolidated health economic evaluation reporting standards (CHEERS) were followed in developing this report.25 Institutional Review Board approvals were obtained from Columbia University and RAND.
Results
Table 2 provides sample characteristics for the sample of elderly ICU that form the basis of our model estimates, by sample cohorts including all elderly in the sample, those that experienced a CLABSI, those that experienced a VAP, and two age-specific cohorts that were used in sensitivity analysis. The mean age of the sample was 77.1 years old and 49% were female. The majority was White (91%), only 13% of the sample was dual eligible for Medicare and Medicaid and the average number of aggregated condition codes was 4.83. Over half of the sample (57%) died during the five year follow-up period.
Table 2. Sample Characteristics, Elderly Index-ICU Residents Discharged Alive.
| Infection Cohorts | Age Specific Cohorts | ||||
|---|---|---|---|---|---|
| All Elderly | CLABSI | VAP | Ages 65-69 | Ages 75-79 | |
| N=15311 | N=915 | N=1512 | N=2581 | N=3741 | |
| Demographics and index ICU comorbid conditions | |||||
| Age | 77.12 | 77.75 | 78.05 | 67.21 | 76.97 |
| Female | 0.49 | 0.51 | 0.51 | 0.44 | 0.48 |
|
| |||||
| Race | |||||
| White | 0.91 | 0.89 | 0.90 | 0.87 | 0.91 |
| Black | 0.07 | 0.08 | 0.07 | 0.09 | 0.06 |
| Other | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 |
|
| |||||
| Dual Eligible | 0.13 | 0.16 | 0.17 | 0.17 | 0.12 |
| Number of ACC | 4.83 | 5.73 | 5.08 | 4.70 | 4.89 |
|
| |||||
| Post-Discharge Mortality | |||||
| Died during follow-up | 0.57 | 0.75 | 0.77 | 0.42 | 0.56 |
| Days alive (mean) | 1252.58 | 898.84 | 876.45 | 1457.86 | 1289.67 |
|
| |||||
| Post-Discharge Health Care Utilization | |||||
| Hospital Admissions (mean)* | |||||
| Year 1 post-discharge | 2.13 | 2.37 | 2.28 | 2.13 | 2.16 |
| Year 2 | 1.95 | 2.19 | 2.08 | 2.11 | 1.87 |
| Year 3 | 1.87 | 1.90 | 1.95 | 1.99 | 1.82 |
| Year 4 | 1.88 | 1.93 | 1.99 | 1.93 | 1.89 |
| Year 5 | 1.87 | 1.72 | 1.94 | 1.94 | 1.77 |
| ED Visits (mean)* | |||||
| Year 1 post-discharge | 2.13 | 2.17 | 2.09 | 2.32 | 2.10 |
| Year 2 | 2.21 | 2.26 | 2.31 | 2.40 | 2.10 |
| Year 3 | 2.22 | 2.39 | 2.27 | 2.29 | 2.04 |
| Year 4 | 2.18 | 2.32 | 2.20 | 2.18 | 2.14 |
| Year 5 | 2.12 | 1.96 | 2.15 | 2.25 | 2.00 |
|
| |||||
| HHC Visits (mean)* | |||||
| Year 1 post-discharge | 3.16 | 3.48 | 2.64 | 2.41 | 3.32 |
| Year 2 | 4.66 | 7.12 | 4.62 | 2.79 | 4.60 |
| Year 3 | 4.28 | 7.24 | 5.20 | 2.35 | 3.54 |
| Year 4 | 3.50 | 6.10 | 4.15 | 2.48 | 2.75 |
| Year 5 | 3.38 | 5.81 | 2.80 | 2.80 | 3.17 |
|
| |||||
| LTC Days (mean)* | |||||
| Year 1 | 7.34 | 8.50 | 7.33 | 6.71 | 7.57 |
| Year 2 | 10.14 | 12.03 | 11.17 | 11.76 | 10.79 |
| Year 3 | 10.50 | 16.27 | 12.22 | 11.35 | 10.86 |
| Year 4 | 11.10 | 14.96 | 11.94 | 13.24 | 10.30 |
| Year 5 | 11.03 | 13.02 | 11.24 | 12.95 | 11.14 |
Utilization figures are conditional on being alive during the given year post-discharge from the index hospitalization. ACC: Aggregate Condition Codes, CLABSI: Central Line-Associated Bloodstream Infections, ED: Emergency Department, HHC: Home Health Care, ICU: Intensive Care Unit, LTC: Long-Term Care, VAP: Ventilator-Associated Pneumonia
Table 3 presents the model base case results for the average ICU. Starting with the estimated number of infections and deaths averted for both CLABSI and VAP per ICU, we estimated the total number of life years gained (i.e., increased LY of 15.55 and 10.84 from reduced risk of CLABSI and VAP, respectively) and the total number of QALYs gained (i.e., 9.61 and 6.55 from the reduced risk of CLABSI and VAP, respectively). We estimated the total reduction in ICU costs due to the reduction in the number of cases of CLABSI and VAP to be $174,713.09 and $163,090.54, respectively. Because of the increased post-discharge LY for those for whom infection was averted, the total net present value of post-discharge health care costs increase substantially (i.e., $366,901.52 for CLABSI and $201,965.92 for VAP). By combining the ICU-level health benefits and cost differences (both acute and post-discharge) for the CLABSI and the VAP cohorts, and including the ICU-level intervention costs, we estimate that the ICER is $14,250.74 per LY gained and $23,277.86 per QALY gained.
Table 3. Base Case Results.
| CLABSI | VAP | |
|---|---|---|
| Number of cases averted (index ICU stay) | 10.81 | 7.71 |
| Infections averted among attributable deaths | 1.73 | 0.79 |
| Infections averted among discharged alive | 9.08 | 6.91 |
|
| ||
| Health effects (post-discharge from index ICU) | ||
| Life expectancy / case | ||
| No infection | 4.44 | 4.59 |
| Infection | discharged alive | 3.57 | 3.55 |
| Infection | died | ||
|
| ||
| QALY / case | ||
| No infection | 2.16 | 2.26 |
| Infection | discharged alive | 1.67 | 1.68 |
| Infection | died | ||
|
| ||
| Health benefits from infections averted | ||
| LY gained | 15.55 | 10.84 |
| QALY gained | 8.12 | 5.81 |
|
| ||
| Costs | ||
| Index ICU savings / case | $16,155.00 | $21,163.00 |
| Total index ICU savings | $174,713.09 | $163,090.54 |
|
| ||
| Post-discharge from index ICU | ||
| No infection / case | $124,737.28 | $124,581.68 |
| Infection | survived / case | $108,108.82 | $109,670.15 |
| Infection | died / case | ||
| Total Cost Savings | ($366,901.52) | ($201,965.92) |
|
| ||
| Combining BSI and VAP | ||
| Total ICU Gain in LY | 26.39 | |
| Total ICU Gain in QALY | 13.93 | |
|
| ||
| Total ICU Inpatient Cost Increase | ($337,803.64) | |
| Total ICU downstream Cost Increase | $568,867.44 | |
|
| ||
| Intervention Cost / case (CLABSI + VAP) | $7,828.87 | |
| Total Intervention Cost / ICU | $145,000.00 | |
| Total Cost Increase | $376,063.80 | |
|
| ||
| ICER (LE) | $14,250.74 | |
| ICER (QALY) | $26,996.33 | |
CLABSI: Central Line-Associated Bloodstream Infection, ICER: Incremental Cost-Effectiveness Ratios, ICU: Intensive Care Unit, LY: Life Years, QALY: Quality-Adjusted Life Years, VAP: Ventilator-Associated Pneumonia
Table 4 displays the results of the sensitivity analyses. In all cases the ICERS are less than $85,000 per outcome. If the intervention is only effective in reducing 10% of the infections, the ICER is the highest at just over $83,000 per QALY and $50,000 per LY. When the intervention is only applied to the eldest cohort, those 75 to 79 years of age, the resulting ICER is slightly higher than when applied to the younger ICU patients.
Table 4. Model Results, Sensitivity Analyses.
| Alternative Values for Acute Stage | Low | High |
|---|---|---|
| Intervention effectiveness (% reduction; baseline: %76) | 10% | 90% |
| ICER (LY) | $50,904.58 | $13,477.29 |
| ICER (QALY) | $83,140.71 | $22,012.00 |
|
| ||
| Device days (% of baseline) | 50% | 150% |
| ICER (LY) | $19,745.43 | $12,419.17 |
| ICER (QALY) | $32,253.18 | $20,286.09 |
|
| ||
| Inpatient mortality attributable to infection (% of baseline) | 50% | 150% |
| ICER (LY) | $10,919.14 | $16,611.52 |
| ICER (QALY) | $17,736.25 | $27,242.51 |
|
| ||
| Inpatient costs attributable to infection (% of baseline) | 50% | 150% |
| ICER (LY) | $20,651.18 | $7,850.29 |
| ICER (QALY) | $33,732.67 | $12,823.06 |
|
| ||
| Intervention Cost (% of baseline) | 50% | 150% |
| ICER (LY) | $11,503.39 | $16,998.08 |
| ICER (QALY) | $18,790.21 | $27,765.52 |
| Alternative Values for Post-Discharge Stage | Low | High |
|---|---|---|
| Discount Rate | 0% | 5% |
| ICER (LY) | $17,255.08 | $12,609.61 |
| ICER(QALY) | $28,908.21 | $25,731.16 |
|
| ||
| Prices | 50% | 150% |
| ICER (LY) | $3,472.27 | $25,029.20 |
| ICER (QALY) | $6,577.81 | $47,414.85 |
|
| ||
| Utilities | 50% | 150% |
| ICER (LY) | $14,250.74 | $14,250.74 |
| ICER (QALY) | $25,028.05 | $29,302.43 |
| Alternative Population Scenarios | ||
|---|---|---|
| Age Cohort | 65 to 69 | 75 to 79 |
| ICER (LY) | $13,512.33 | $14,996.95 |
| ICER (QALY) | $21,048.68 | $24,384.17 |
ICER: Incremental Cost-Effectiveness Ratios, LY: Life Years, QALY: Quality-Adjusted Life Ye ars
Discussion
This policy model assessed the long-term cost-effectiveness of a multifaceted infection prevention program in the ICU setting from the societal perspective. We found ongoing investment in infection prevention programs designed to decrease CLABSI and VAP to be a cost-effective strategy; and these findings were robust to a number of assumptions. While many hospitals in the U.S. have already invested in these types of programs, hence the improvement toward national targets, our results serve as a reminder to health administrators and policy makers of the importance of ongoing investment to keep HAI rates down. That is, the welfare improvement implied by the advantageous incremental cost-effectiveness ratio results would be lost if the ongoing investments in prevention policies were suspended.
Other cost-effectiveness analysis models have been developed for specific infection prevention interventions. For example, a decision analytic cost-effectiveness analysis model comparing antiseptic impregnated catheters to standard catheters was published.26 Additionally, the use of antimicrobial catheters has been compared to investment in multifaceted infection prevention programs in Australia.27 However, neither of these analyses took the societal perspective or included long-term outcomes and costs attributable to HAIs. Unlike these studies, we have developed a long-term cost-effectiveness model that evaluates a composite policy, which includes a variety of elements as represented by the Michigan Keystone ICU Patient Safety Program, and uses 5 years of follow-up data to develop long-term projections of health and cost outcomes.
This analysis has a number of strengths and limitations. The strengths include the use of long-term societal data, providing a minimum of five years of detailed follow-up data on health care outcomes, utilization, and costs. However, by necessity the data were only available on the elderly. Other researchers from a large accountable care organization may be able to use a similar model with data from patients of all ages. The probability of HAI came from data submitted to the CDC. Strengths of these data include the consistent definitions that are used across multiple hospitals; however, even with the definitions there continues to be heterogeneity in the application of the definitions.28 Nonetheless, the NHSN data are the best multi-site HAI data available. The cost of the intervention was based on implementing a multifaceted infection prevention program nearly a decade ago. While all cost data were inflated to 2013 dollars, contextual changes such as the development of new technologies or mandatory reporting of HAIs may impact workflow and subsequent costs. However, our results were not sensitive to the cost of the intervention. Last, another important limitation of our work is that it is based on estimates from retrospective data, and although we included detailed health status controls in our analyses, our estimates of the long-term effects of infection on health outcomes and health care costs could be biased.
We examined the cost-effectiveness of ongoing investments in multifaceted HAI prevention programs in hospital ICUs that have been initiated over the last decade. We believe this cost-effectiveness model, which integrates parameter estimates from the literature with empirical estimates of the long-term consequences of HAI, provides a more complete story of the costs and benefits of HAI than has previously been published, and reveals the value of continued efforts to prevent HAI. Our findings show that the incremental cost effectiveness ratios are small and that they are robust to alternative assumptions indicating that ongoing investments in HAI prevention should be continued. There is no implicit cost-effectiveness threshold; however, it is generally assumed that an ICER less than $50,000 is cost-effective and one more than $100,000 is not.29 The improvements in infection rates brought about by these policies have been substantial, and our long-term modeling of the health and cost-consequences of these infections highlights the value of the interventions and the potential consequences of discontinuing them.
Although HAI rates have been dramatically reduced over the last decade, infections are an ongoing problem, particularly emerging and resistant infections such as those caused by multiple drug resistant organisms including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci as well as Clostridium difficile diarrhea, which is associated with antibiotic use. The social cost of these infections is large, as revealed by our model, and as a consequence, effort to develop new prevention strategies is warranted.
Acknowledgments
This study was funded by the National Institute of Nursing Research (R01NR010107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew Dick, RAND Corporation.
Eli N. Perencevich, University of Iowa, Carver College of Medicine, Iowa City VA.
Monika Pogorzelska-Maziarz, Thomas Jefferson University, School of Nursing.
Jack Zwanziger, University of Illinois, Chicago.
Elaine L. Larson, Columbia University, Mailman School of Public Health, School of Nursing, Center for Health Policy.
Patricia W. Stone, Columbia University, School of Nursing, Center for Health Policy.
References
- 1.Klevens RM, Edwards JR, Richards CL, Jr., Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott R. The Direct Medical Costs of Health care-Associated Infections in U.S. Hospitals and the Benefits of Prevention. Centers for Disease Control and Prevention; Atlanta, GA: [Accessed on November 21, 2013]. 2009. Available at http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. [Google Scholar]
- 3.Eber MR, Laxminarayan R, Perencevich EN, Malani A. Clinical and economic outcomes attributable to health care-associated sepsis and pneumonia. Arch Intern Med. 2010;170:347–353. doi: 10.1001/archinternmed.2009.509. [DOI] [PubMed] [Google Scholar]
- 4.Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Pollock DA, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control. 2011;39:798–816. doi: 10.1016/j.ajic.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Yokoe DS, Mermel LA, Anderson DJ, Arias KM, Burstin H, Calfee DP, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:S12–21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 6.Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD. The 100,000 lives campaign: setting a goal and a deadline for improving health care quality. JAMA. 2006;295:324–327. doi: 10.1001/jama.295.3.324. [DOI] [PubMed] [Google Scholar]
- 7.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 8.Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One. 2011;6:e15452. doi: 10.1371/journal.pone.0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pogorzelska M, Stone PW, Furuya EY, Perencevich EN, Larson EL, Goldmann D, et al. Impact of the ventilator bundle on ventilator-associated pneumonia in intensive care unit. Int J Qual Health Care. 2011;23:538–544. doi: 10.1093/intqhc/mzr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. Am J Infect Control. 2011;39:640–646. doi: 10.1016/j.ajic.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Scales DC, Dainty K, Hales B, Pinto R, Fowler RA, Adhikari NK, et al. A multifaceted intervention for quality improvement in a network of intensive care units: a cluster randomized trial. JAMA. 2011;305:363–372. doi: 10.1001/jama.2010.2000. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Health & Human Services [Accessed on September 9, 2013];National Action Plan to Prevent Healthcare-Associated Infections: National Targets and Metrics. Available at http://www.hhs.gov/ash/initiatives/hai/nationaltargets/index.html.
- 13.Dick A, Liu H, Zwanziger J, Perencevich EN, Furuya EY, Larson EL, et al. Long-term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432. doi: 10.1186/1472-6963-12-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone PW, Pogorzelska-Maziarz M, Herzig CTA, Wiener LM, Furuya EY, Dick A, et al. State of Infection Prevention in U.S. Hospitals Enrolled in the National Health and Safety Network. Am J Infect Control. 2014;42:94–9. doi: 10.1016/j.ajic.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention NCfHS [Accessed on September 10, 2013];United States Life Tables. Available at http://www.cdc.gov/nchs/products/life_tables.htm#life.
- 16.Walters HR, Korn R, Jr, Colantuoni E, Berenholtz SM, Goeschel CA, Needham DM, et al. The business case for quality: economic analysis of the Michigan Keystone Patient Safety Program in ICUs. Am J Med Qual. 2011;26:333–339. doi: 10.1177/1062860611410685. [DOI] [PubMed] [Google Scholar]
- 17.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 18.Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Antilla A, et al. National Healthcare Safety Network report, data summary for 2011, device-associated module. Am J Infect Control. 2013;41:286–300. doi: 10.1016/j.ajic.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reduction in central line-associated bloodstream infections among patients in intensive care units--Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1013–1016. [PubMed] [Google Scholar]
- 20.Ash AS, Posner MA, Speckman J, Franco S, Yacht AC, Bramwell L. Using claims data to examine mortality trends following hospitalization for heart attack in Medicare. Health Serv Res. 2003;38:1253–1262. doi: 10.1111/1475-6773.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis RP, Ash A. Refinements to the Diagnostic Cost Group (DCG) model. Inquiry. 1995;32:418–429. [PubMed] [Google Scholar]
- 22.Petersen LA, Pietz K, Woodard LD, Byrne M. Comparison of the predictive validity of diagnosis-based risk adjusters for clinical outcomes. Med Care. 2005;43:61–67. [PubMed] [Google Scholar]
- 23.Institute for Clinical Research and Health Policy Studies, Tufts Medical Center [Accessed on September 10, 2013];Cost-Effectiveness Analysis Registry. Available at https://research.tufts-nemc.org/cear4.
- 24.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectivenss in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 25.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)--explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Veenstra DL, Saint S, Sullivan SD. Cost-effectiveness of antiseptic-impregnated central venous catheters for the prevention of catheter-related bloodstream infection. JAMA. 1999;282:554–560. doi: 10.1001/jama.282.6.554. [DOI] [PubMed] [Google Scholar]
- 27.Halton KA, Cook D, Paterson DL, Safdar N, Graves N. Cost-effectiveness of a central venous catheter care bundle. PLoS One. 2010:5e12815. doi: 10.1371/journal.pone.0012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller SC, Linkin DR, Fishman NO, Lautenbach E. Variations in identification of healthcare-associated infections. Infect Control Hosp Epidemiol. 2013;34:678–686. doi: 10.1086/670999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers JD, Neumann PJ, Buxton Does Medicare Have an Implicit Cost-Effectiveness Threshold? Med Decis Making. 2010;30:E14–E27. doi: 10.1177/0272989X10371134. [DOI] [PubMed] [Google Scholar]
- 30.Scherbaum WA, Goodall G, Erny-Albrecht KM, Massi-Benedetti M, Erdmann E, Valentine WJ. Cost-effectiveness of pioglitazone in type 2 diabetes patients with a history of macrovascular disease: a German perspective. Cost Eff Resour Alloc. 2009;7:9. doi: 10.1186/1478-7547-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner M, Lindgren P, Merikle E, Goetghebeur M, Jonsson B. Economic evaluation of high-dose (80 mg/day) atorvastatin treatment compared with standard-dose (20 mg/day to 40 mg/day) simvastatin treatment in Canada based on the Incremental Decrease in End-Points Through Aggressive Lipid-Lowering (IDEAL) trial. Can J Cardiol. 2009;25:e362–369. doi: 10.1016/s0828-282x(09)70159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]