Abstract
Opioid use disorders are a significant public health problem, affecting over 2 million individuals in the US. Although opioid agonist treatment, predominantly offered in licensed methadone clinics, is both effective and cost-effective, many individuals do not receive it. Buprenorphine, approved in 2002 for prescription by waivered physicians, could improve opioid agonist treatment access for individuals unable or unwilling to receive methadone.
We examine the extent to which the geographic distribution of waivered physicians has enhanced potential opioid agonist treatment access, particularly in non-metropolitan areas with fewer methadone clinics. We found that while the approximately 90% of counties classified as methadone clinic shortage areas remained constant, buprenorphine shortage areas fell from 99% of counties in 2002 to 51% in 2011, lowering the US population percentage residing in opioid treatment shortage counties to approximately 10%. The increase in buprenorphine-waivered physicians has dramatically increased potential access to opioid agonist treatment, especially in non-metropolitan counties.
Introduction
Opioid use disorders (1) are a significant public health problem, affecting an estimated 2 million individuals in the United States in 2012.(2) Illicit opioid use contributes to medical morbidity, promotes risky behaviors, and complicates treatment for medical and mental health conditions.(3–5) In 2009, the annual societal costs of heroin and prescription opioid misuse, including overdose deaths, lost productivity, criminal justice costs, and individual health care costs, totaled an estimated $55.7 billion.(6, 7)
Opioid use disorders are chronic medical illnesses requiring treatment.(8) Opioid agonist therapy provided in structured and licensed addiction programs or in physician offices comprises evidence-based, cost-effective treatments that mitigate the negative health and societal effects of the disorders. Such therapies are more effective than counseling alone,(9–11) but historically the majority of individuals with opioid use disorders have not received opioid agonist treatment.(12, 13) One important treatment barrier is the geographic distribution of providers: opioid treatment programs, which treat patients with methadone and/or buprenorphineare, are located predominantly in urban areas.(14, 15) Opioid treatment programs commonly require patients to take daily medications administered on-site under direct observation, effectively limiting treatment access for rural patients.
The 2002 approval of buprenorphine opioid agonist therapy (hereafter defined as either buprenorphine or buprenorphine/naloxone formulations) was welcomed as an opportunity to increase access to treatment for many individuals.(13, 16) Through the Drug Addiction Treatment Act of 2000, physicians who completed an approved course (hereafter referred to as waivered physicians) or board certified in addiction medicine or psychiatry were waivered from the special registration requirements in the Controlled Substances Act and were permitted to prescibe medications such as buprenorphine outside of opioid treatment programs. Waivered physicians could expand treatment access to individuals who would not or could not attend opioid treatment programs for geographical, ideological, or practical considerations.(14, 17, 18)
The overall number of physicians waivered to prescribe buprenorphine has been increasing,(19, 20) but little is known about such physicians’ location and whether their distribution has increased potential access to opioid agonist treatment in non-metropolitan and smaller communities where opioid treatment programs are scarce.(14) Proximity to waivered physicians does not guarantee access to opioid agonist treatment, but it is a necessary condition.
Our goal is to describe the growth in the number of waivered physicians and the evolution of their geographic distribution over the period 2002–2011. We examine whether the increased number and distribution of waivered physicians has enhanced potential access to opioid agonist treatment, particularly in non-metropolitan counties with few or no opioid treatment programs. Because waivered physicians can prescribe burprenorphine outside of opioid treatment programs, and because there are relatively few opioid treatment programs in rural areas, we hypothesized that growth in the number of waivered physicians would have the greatest impact on potential access to treatment in less populated counties.
Methods
Building on the Health Resources and Services Administration (HRSA) methodology for identifying Health Professional Shortage Areas (HPSA), (21) we developed an approach to identify communities with opioid treatment provider shortages, including counties with shortages of waivered physicians, opioid treatment programs, and overall opioid agonist treatment.
Data
We used location and year of certification of waivered physicians from the Buprenorphine Waiver Notification System (2002–2011); yearly information about substance abuse treatment programs providing methadone and/or buprenorphine came from the National Survey of Substance Abuse Treatment Services (2002–2011).(2) We used the Area Resource File to obtain rural-urban continuum codes (2003), population, socio-economic, demographic, and health service supply data (2002–2011). To assess need for treatment, we obtained illicit opioid price information from the Drug Enforcement Agency’s System to Retrieve Information from Drug Evidence (2002–2011) and opioid-related overdose death counts from the National Vital Statistics System (2002–2011). We followed the CDCs approach and identified opioid-related deaths using ICD-10 underlying cause of death codes (X40–X44, X60–64, X85, or Y10–Y14) and injury and poisoning codes (T40.0–T40.4, T40.6).(22)
Defining Shortage Areas
We identified counties with a shortage of waivered physicians, opioid treatment programs, and overall opioid agonist treatment, drawing on definitions developed by HRSA.(21) HRSA’s HPSA defines shortage areas as areas in which the number of practitioners per capita is very low (below a specified value) or the number of practitioners per capita is low and there is high need in the area. We defined shortage area counties, separately for waivered physicians and opioid treatment programs, as those that 1) had no providers of that type; 2) fell in the lowest 10% of that type of provider per capita; or 3) fell in the lowest 20% of that type of provider per capita and were in a county with high treatment need. Need was a function of number of opioid-related overdose deaths, heroin prices, and socioeconomic and demographic characteristics (analytic approach details are available in the online Appendix, section A1).(23) We defined an opioid treatment shortage county (hereafter referred to as a treatment shortage county) as a county that had both a waivered physician shortage and an opioid treatment program shortage.
Analysis
For each year (2002–2011), we identified the number and proportion of each type of treatment-shortage county, and we estimated the number of individuals and fraction of the U.S. population living in each type. We categorized county urban-rural status as metropolitan (urban population >50,000), large non-metropolitan (population >20,000), medium non-metropolitan (population <20,000 and >2,500), and small non-metropolitan (population <2,500).(24) For each urban-rural category, we calculated the means of shortage indicators and the percent of the populations residing in shortage areas for each year. Using t and F-statistics we tested the hypotheses that (1) the fraction of shortage counties declined during the period across all counties and within each of the urban-rural population classifications, (2) the reduction in shortage counties differed across the urban-rural population classifications, and (3) the percent of population living in shortage counties declined the least in metropolitan counties and the most in rural counties with relatively large populations. These reductions were associated with commensurate increases in potential access for county residents.
To examine the robustness of our results, we repeated the analyses using two alternative definitions of shortage areas: a) a narrow metric limiting shortage to counties with no providers and b) a broad metric expanding shortage by relaxing the classification criteria to increase the number of high need counties (for details see Appendix Section A1).(23) We found no substantive differences in any result; therefore we present only the results using the shortage area definition most consistent with HRSA methodology. The Institutional Review Boards of the RAND Corporation, the University of Pittsburgh, and Pennsylvania State University approved the study.
Limitations
Study limitations include the following. Not all waivered physicians actively treat patients with buprenorphine,(18, 25, 26) and we do not know which waivered physicians are actively treating patients, or the extent to which the geographical distribution of active waivered physicians differs from non-active physicians. As a result, our approach characterizes potential access to treatment, not actual use. However, presence of waivered physicians in non-metropolitan counties is significantly associated with greater use of both buprenorphine (27) and opioid agonist therapy among Medicaid-enrollees.(28) Our data are only available through 2011; we do not know if more recent data would change our findings. Office-based buprenorphine prescribers are restricted to prescribing for either 30 or 100 patients. This restriction may limit the ability of waivered physicians to address an unmet need for opioid agonist treatment; thus we may be underestimating the number of counties with a true shortage of opioid agonist treatment. Not all individuals living in a shortage county may have substantial difficulties accessing opioid agonist treatment, since some can access treatment in another county. Our data contain no information about the appropriateness or quality of opioid agonist treatment provided, or the use of non-pharmacologic interventions, both of which may influence clinical outcomes.
Results
Counties with a shortage of opioid treatment programs, waivered physicians, and overall opioid agonist treatment
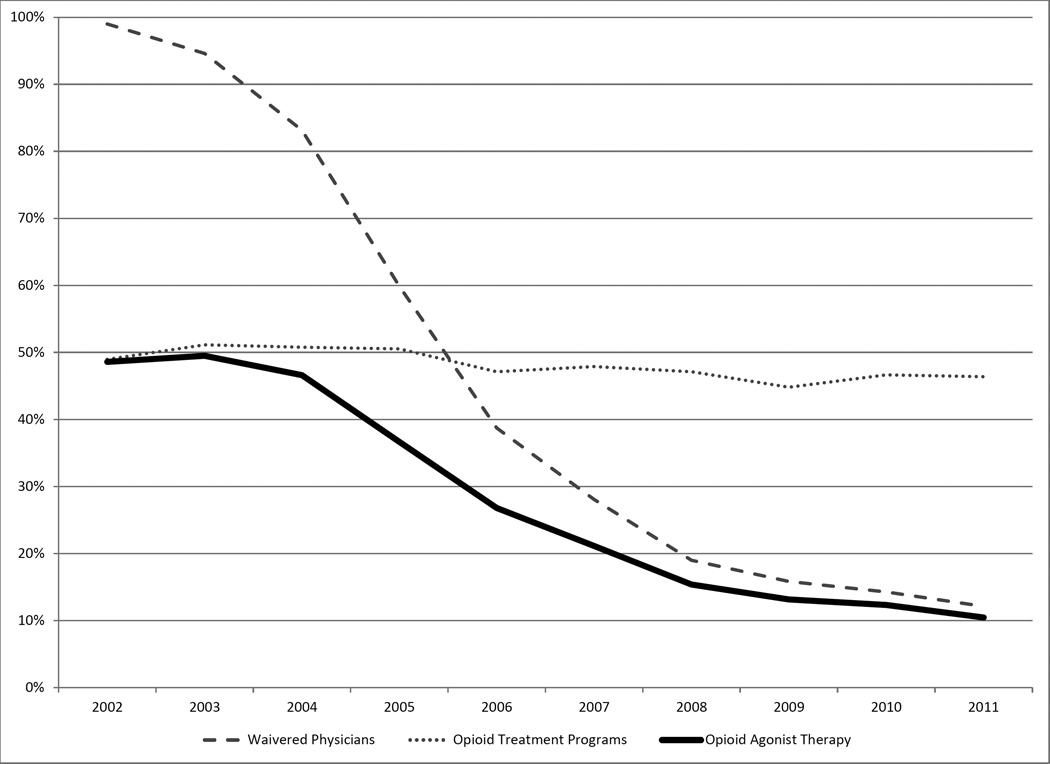
The percentage of counties with a shortage of opioid treatment programs changed only slightly over time (P<0.001), trending from 90% in 2002 to approximately 87% in 2011. (Exhibit 1; online Appendix Figure A4)(23) In contrast, the percentage of counties with a shortage of waivered physicians fell sharply from 99% in 2002 to 47% in 2011 (P<0.001), substantially increasing the number of counties that were no longer treatment shortage counties and in which individuals had potential access to opioid agonist treatment.
Exhibit 1.
The Change in Opioid Agonist Treatment Shortage Counties between 2002 and 2011 Source: Authors’ analysis
| 2002 | 2011 | ||
|---|---|---|---|
| % of shortage counties |
% of shortage counties |
||
| Overall | |||
| Opioid Treatment Program | 90.3 | 87.0**** | |
| Waivered Physicians | 98.9 | 46.8**** | |
| Overall Opioid Agonist Therapy | 86.9 | 45.8**** | |
| Metro | |||
| Opioid Treatment Program | 74.6 | 68.6*** | |
| Waivered Physicians | 98.6 | 26.1**** | |
| Overall Opioid Agonist Therapy | 73.3 | 24.1**** | |
| Large non -Metro | |||
| Opioid Treatment Program | 95.0 | 88.6*** | |
| Waivered Physicians | 99.1 | 31.3**** | |
| Overall Opioid Agonist Therapy | 94.4 | 29.7**** | |
| Medium non -Metro | |||
| Opioid Treatment Program | 99.0 | 97.7** | |
| Waivered Physicians | 98.6 | 54.6**** | |
| Overall Opioid Agonist Therapy | 97.5 | 54.1**** | |
| Small non -Metro | |||
| Opioid Treatment Program | 100 | 99.4 | |
| Waivered Physicians | 99.6 | 75.9**** | |
| Overall Opioid Agonist Therapy | 99.6 | 75.7**** | |
Difference between 2002–2011: * p< 0.1;
p< 0.05;
p< 0. 01;
p < 0.001
In 2002, slightly less than half of the U.S. population resided in treatment shortage counties (Exhibit 2), and the percentage of the population living in counties with a shortage of opioid treatment programs fell only modestly between 2002 and 2011. However, the substantial decrease in counties with a shortage of waivered physicians meant that by 2011, the percentage of the population residing in a treatment shortage county had declined from 49% to 10%, providing an estimated 74% increase in the fraction of the U.S. population with potential access to treatment.
EXHIBIT 2.
Percent of Population Living in Counties with Opioid Treatment Program, Waivered Physician, and Opioid Agonist Treatment Shortages Source: Authors’ analysis
Shortage Counties by Metropolitan Status and Size of County Population
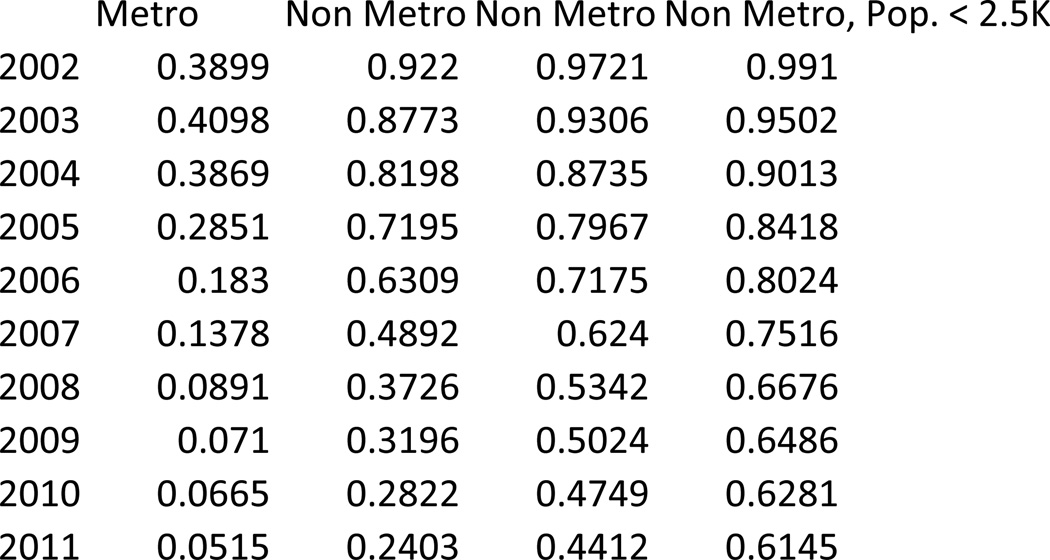
From 2002 to 2011, the percentage of treatment shortage counties declined substantially among both metropolitan and non-metropolitan counties, resulting in increased access to potential treatment in every urban-rural classification (P<0.001). (Exhibit 1, Appendix Exhibit A5)(23) Among the urban-rural classifications, the decline (94% to 30%, P<0.001), and associated increase in potential access, was greatest among large non-metropolitan counties; the next greatest decline (73% to 24%, P<0.001) and associated increased potential access occurred in metropolitan counties. The declines in these two types of counties were significantly different from one another (P<0.001) and significantly greater (P<0.001 in each case) than the decline in either medium non-metropolitan counties (98% to 54%, P<0.001) or small non-metropolitan counties (99% to 76%, P<0.001). Thus potential access increased less in these latter types of counties.
Counties of each urban-rural classification had statistically significant and substantial declines from 2002 to 2011 in the percentage of population living in treatment shortage counties (P<0.001 for each urban-rural classification) (Exhibit 3), with smaller but substantial declines in the most densely and least densely populated counties. By 2011, only 5% of metropolitan residents lived in a treatment shortage county (down from nearly 39% in 2002) and just over 60% of small non-metropolitan county residents lived in a treatment shortage county (down from nearly 100% in 2002).
EXHIBIT 3.
Percent of Population Living in Opioid Agonist Treatment Shortage Counties by Population and Urban Status Source: Authors’ analysis
Online Appendix Exhibit A5 (23), a map of the United States, illustrates that although potential access to opioid agonist therapy improved substantially from 2002 and 2011, particularly on the East and West coasts, large areas in the Midwest were still designated as shortages areas in 2011.
Discussion
We found that buprenorphine treatment has significantly decreased the number of counties and the percent of the U.S. population living in counties that have potential shortages of access to opioid agonist therapy, substantially increasing potential access to opioid agonist therapy for millions of Americans. Improved access to such treatment may be particularly important and timely: rising rates of heroin use and a surge in prescription opioid misuse have intensified the need for treatment of opioid dependence.(6, 29–31)
Consistent with studies of treatment access in other areas of healthcare,(32) we find that shortages of qualified opioid agonist treatment providers remain more problematic in less populated counties than in metropolitan counties, despite the fact that the less populated counties had the greatest increase in opioid agonist treatment providers between 2002 and 2011. The potential impact of buprenorphine waivered physicians in non-metropolitan counties may be large relative to that of new opioid treatment programs in these areas because the additional clinical infrastructure required by a physician to provide office-based buprenorphine is minimal compared to that required to establish a new opioid treatment program.
Policy Implications
With fewer than 3.5% of office-based U.S. physicians waivered to prescribe buprenorphine, potential access to buprenorphine has not diffused as widely as hoped when it was first introduced,(16, 25, 26, 33), and obtaining opioid agonist treatment remains challenging in large swathes of the country. Many of these areas are in the Midwest, a region of the country in which Medicaid policies facilitating access to opioid agonist therapy have historically been less generous than in other regions of the country, and where there have been fewer policy changes in recent years to enhance such access.(34)
It also remains to be seen to what extent Medicaid expansion and other changes following passage of the Affordable Care Act (ACA) influences the availability of opioid agonist therapy for Medicaid enrollees in the future.(35) The government is already the largest payer for substance abuse treatment services, (36, 37) and given the increased number of Medicaid enrollees in many states and Medicaid enrollees’ higher rates of opioid use disorders,(38) the potential for increased demand for opioid use disorder treatment is quite high. Parity of behavioral health services under the ACA may also affect the availability of opioid agonist treatment, given that prior Federal parity legislation (39) was associated with an increase in substance abuse treatment facilities use of buprenorphine (40) but not broader substance use disorder treatment expansion.(41)
It is unlikely that the number of opioid treatment programs dispensing methadone will substantially increase, given the regulatory burden involved in opening and running such programs,(18, 42–44) and many communities’ unwillingness to accept a nearby opioid treatment program.(18) It is therefore likely that improved access will occur primarily through office-based physician treatment.(14, 45) To increase the number of buprenorphine waivered physicians, federal agencies and professional organizations are increasing the availability of trainings to certify physicians (46–48) and mentorship and support for waivered physicians.(49) In addition, states can implement policies that appear to increase the number of waivered physicians.(20) In addition to these activities, policymakers seeking to increase access should consider other strategies, with an emphasis on increasing the number of waivered physicians in non-metropolitan counties. These could include increasing the number of and support for physicians in shortage areas (49) and further relaxing limits on the number of individuals each waivered physician can treat.(50) Such policies could potentially substantially increase the use of buprenorphine, particularly in rural communities,(27) which may be particularly relevant and timely, given buprenorphine’s effectiveness in treating dependence on prescription opioids,(51) a growing problem in many smaller counties.(6)
However, the lack of supportive infrastructure may also present challenges to office-based waivered physicians (52) with respect to adequately monitoring appropriate buprenorphine use, as well as being challenged to meet the waiver’s requirement of being able to refer buprenorphine patients for appropriate counseling and other non-pharmacologic therapies.(53) Further research is needed to better understand to what extent quality of care and clinical outcomes vary across different settings in which individuals with opioid use disorders receive opioid agonist treatment.
Conclusion
Expansion of buprenorphine-waivered physicians throughout the country, particularly in rural areas, has dramatically expanded the geographic distribution of qualified providers, increasing potential access to opioid agonist treatment. Given our findings, policies that increase the number of waivered physicians and expand the number of patients who can be treated by each waivered physician, may be the most rapid approach to enhancing capacity for opioid agonist treatment, particularly outside of metropolitan counties.
However, increasing the supply of providers able to treat patients addresses only one of the challenges to expanding potential access to effective treatment for opioid use disorders. A range of other important barriers exist for many individuals, including stigma,(54, 55) inadequate insurance coverage,(56) onerous treatment requirements,(57, 58) and inadequate training for waivered physicians regarding the mental and physical health disorders commonly comorbid with opioid misuse.(59, 60) Future efforts are needed to better understand and address these additional barriers, as reducing the number of areas that have a shortage of providers able to treat with opioid agonist therapy is an essential step in addressing this substantial public health problem.
Supplementary Material
Acknowledgements
The authors are indebted to Steve Mason of SAMHSA for assistance with the Buprenorphine Waiver Notification System data, Mary Vaiana, PhD, Sebastian Bauhoff PhD, Carrie Farmer PhD, and Todd Mandell MD for review and feedback on prior manuscript versions, and Gina Boyd, MLIS, for research assistance.
Funding Source: The National Institute on Drug Abuse of the NIH supported (1R01DA032881-01A1) this study.
Footnotes
Conflict of Interest Statement: Dr. Stein has served on an Advisory Board for Otsuka Pharmaceuticals. Dr. Gordon receives royalties from Cambridge University Press and UptoDate for work un-related to this topic. None of the other authors have conflicts of interest to disclose.
Author Contributions: See accompanying forms
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Office of Applied Studies SAMHSA. The DASIS Report. [cited 2013 June 23];The national survey of substance abuse treatment services (N-SSATS): Substance Abuse Mental Health Services Administration. 2012 Available from: systemhttp://www.oas.samhsa.gov/2k3/NSSATS/NSSATS.pdf.
- 3.Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008 Apr 1;94(1–3):38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Becker WC, Fiellin DA, Desai RA. Non-medical use, abuse and dependence on sedatives and tranquilizers among U.S. adults: psychiatric and socio-demographic correlates. Drug Alcohol Depend. 2007 Oct 8;90(2–3):280–287. doi: 10.1016/j.drugalcdep.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson KM, Fibbi M, Langer D, Silva K, Lankenau SE. Prescription drug misuse and risk behaviors among young injection drug users. J Psychoactive Drugs. 2013 Apr-Jun;45(2):112–121. doi: 10.1080/02791072.2013.785811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulozzi LJ. Prescription drug overdoses: a review. J Safety Res. 2012 Sep;43(4):283–289. doi: 10.1016/j.jsr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011 Apr;12(4):657–667. doi: 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- 8.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 9.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Fudala PJ, Bridge TP, Herbert S, Williford WO, Chiang CN, Jones K, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349(10):949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 12.American Methadone Treatment Association. Methadone Maintenance Program and Patient Census in the US. New York: American Methadone Treatment Association; 1998. [Google Scholar]
- 13.O'Brien CP. A 50-year-old woman addicted to heroin: review of treatment of heroin addiction. JAMA. 2008;300(3):314–321. doi: 10.1001/jama.300.1.jrr80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark RE, Samnaliev M, Baxter JD, Leung GY. The evidence doesn't justify steps by state Medicaid programs to restrict opioid addiction treatment with buprenorphine. Health Aff (Millwood) 2011;30(8):1425–1433. doi: 10.1377/hlthaff.2010.0532. [DOI] [PubMed] [Google Scholar]
- 15.Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services. The determinations report: A report on the physician waiver program established by the Drug Addiction Treatment Act of 2000 ("DATA") Rockville, MD: 2006. [Google Scholar]
- 16.Ducharme L, Abraham A. State policy influence on the early diffusion of buprenorphine in community treatment programs. Subst Abuse Treat Prev Policy. 2008;3(1):17–27. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiellin DA, O'Connor PG. Clinical practice. Office-based treatment of opioid-dependent patients. N Engl J Med. 2002;347(11):817–823. doi: 10.1056/NEJMcp013579. [DOI] [PubMed] [Google Scholar]
- 18.Oliva EM, Maisel NC, Gordon AJ, Harris AH. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr Psychiatry Rep. 2011 Oct;13(5):374–381. doi: 10.1007/s11920-011-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham C, Kunins H, Roose R, Elam R, Sohler N. Barriers to obtaining waivers to prescribe buprenorphine for opioid addiction treatment among HIV physicians. J Gen Intern Med. 2007;22(9):1325. doi: 10.1007/s11606-007-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein BD, Gordon AJ, Dick AW, Burns RM, Pacula RL, Farmer CM, et al. Supply of buprenorphine waivered physicians: the influence of state policies. J Subst Abuse Treat. 2015 Jan;48(1):104–111. doi: 10.1016/j.jsat.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health Resources and Services Administration. HPSA Designation Criteria: U.S Department of Health and Human Services. [cited 2013 November 4]; Available from: http://bhpr.hrsa.gov/shortage/hpsas/designationcriteria/designationcriteria.html.
- 22.Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug poisoning deaths in the United States, 1980–2008. NCHS data brief. 2011 Dec;(81):1–8. [PubMed] [Google Scholar]
- 23.To access the Health Affairs Appendix, click on the Appendix link in the box to the right of the article online.
- 24.United States Department of Agriculture Economic Research Service. 2013 rural-urban continuum codes 2013. [cited 2014 September 3]; updated 05/10/2013; Available from: http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx - .VAcgRcVdXOE.
- 25.Gordon AJ, Liberto J, Granda S, Salmon-Cox S, Andrée T, McNicholas L. Outcomes of DATA 2000 certification trainings for the provision of buprenorphine treatment in the Veterans Health Administration. Am J Addict. 2008;17(6):459–462. doi: 10.1080/10550490802408613. [DOI] [PubMed] [Google Scholar]
- 26.Gordon AJ, Trafton JA, Saxon AJ, Gifford AL, Goodman F, Calabrese VS, et al. Implementation of buprenorphine in the Veterans Health Administration: Results of the first 3 years. Drug Alcohol Depend. 2007;90(2–3):292–296. doi: 10.1016/j.drugalcdep.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Stein BD, Burns RM, Pacula RL, Dick AW, Bauhoff S, Sorbero M, et al. Where is buprenorphine dispensed? The Role of Private Offices, OTP’s and Substance Abuse Treatment Facilities in Urban and Rural Areas. doi: 10.1111/1468-0009.12137. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein BD, Dick AW, Sorbero M, Burns RM, Pacula RL, Gordon AJ, et al., editors. Patterns of buprenorphine use Among Medicaid-enrolled individuals with opioid Use disorders. 2014 Addiction Health Services Research (AHSR) Conference; 2014 October 15; Boston, MA. 2014. [Google Scholar]
- 29.Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013;132(1–2):95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Oliva EM, Trafton JA, Harris AH, Gordon AJ. Trends in opioid agonist therapy in the Veterans Health Administration: is supply keeping up with demand? Am J Drug Alcohol Abuse. 2013;39(2):103–107. doi: 10.3109/00952990.2012.741167. [DOI] [PubMed] [Google Scholar]
- 31.Substance Abuse and Mental Health Services Administration. Results from the 2012 national survey on drug use and health: Summary of national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 32.Ku L, Jones K, Shin P, Bruen B, Hayes K. The states' next challenge--securing primary care for expanded Medicaid populations. N Engl J Med. 2011;364(6):493–495. doi: 10.1056/NEJMp1011623. [DOI] [PubMed] [Google Scholar]
- 33.Roman PM, Abraham AJ, Knudsen HK. Using medication-assisted treatment for substance use disorders: evidence of barriers and facilitators of implementation. Addict Behav. 2011;36(6):584–589. doi: 10.1016/j.addbeh.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns RM, Pacula RL, Bauhoff S, Gordon AJ, Hendrikson H, Leslie DL, et al. Policies supporting opioid agonist therapy of opioid use disorders: The evolution of state policies from 2004 to 2013. doi: 10.1080/08897077.2015.1080208. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barry CL, Huskamp HA. Moving beyond parity--mental health and addiction care under the ACA. N Engl J Med. 2011 Sep 15;365(11):973–975. doi: 10.1056/NEJMp1108649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levit KR, Mark TL, Coffey RM, Frankel S, Santora P, Vandivort-Warren R, et al. Federal spending on behavioral health accelerated during recession as individuals lost employer insurance. Health Aff (Millwood) 2013 May;32(5):952–962. doi: 10.1377/hlthaff.2012.1065. [DOI] [PubMed] [Google Scholar]
- 37.Levit KR, Stranges E, Coffey RM, Kassed C, Mark TL, Buck JA, et al. Current and future funding sources for specialty mental health and substance abuse treatment providers. Psychiatr Serv. 2013 Jun;64(6):512–519. doi: 10.1176/appi.ps.201200298. [DOI] [PubMed] [Google Scholar]
- 38.Becker WC, Fiellin DA, Merrill JO, Schulman B, Finkelstein R, Olsen Y, et al. Opioid use disorder in the United States: Insurance status and treatment access. Drug Alcohol Depend. 2008;94(1–3):207–213. doi: 10.1016/j.drugalcdep.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 39.Paul Wellstone and Pete Domenici Mental Health Parity and Addiction Equity Act of 2008. 2008 [PubMed] [Google Scholar]
- 40.Bauhoff S, Stein BD, Pacula RL, Dick AW, Gordon AJ, Burns RM, et al., editors. Do substance abuse policies influence opioid agonist therapies in substance abuse treatment facilities?. Presented at the Addiction Health Services Research Conference; 2014; Boston, MA. [Google Scholar]
- 41.Busch SH, Epstein AJ, Harhay MO, Fiellin DA, Un H, Leader D, Jr, et al. The effects of federal parity on substance use disorder treatment. Am J Manag Care. 2014;20(1):76–82. [PMC free article] [PubMed] [Google Scholar]
- 42.Barnett PG, Zaric GS, Brandeau ML. The cost-effectiveness of buprenorphine maintenance therapy for opiate addiction in the United States. Addiction. 2001 Sep;96(9):1267–1278. doi: 10.1046/j.1360-0443.2001.96912676.x. [DOI] [PubMed] [Google Scholar]
- 43.Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- 44.Doran CM, Shanahan M, Mattick RP, Ali R, White J, Bell J. Buprenorphine versus methadone maintenance: a cost-effectiveness analysis. Drug Alcohol Depend. 2003;71(3):295–302. doi: 10.1016/s0376-8716(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 45.Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J, Farmer C. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: Recent service utilization trends in the use of buprenorphine and methadone. Drug Alcohol Depend. 2012 Jun 1;123(1–3):72–78. doi: 10.1016/j.drugalcdep.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 46.SAMHSA. Buprenorphine training events. [cited 2014 September 22]; Available from: http://buprenorphine.samhsa.gov/training_main.html.
- 47.American Osteopathic Academy of Addiction Medicine. PCSS-MAT self study. [cited 2014 September 22]; Available from: http://www.aoaam.org/content.php?pg=2. [Google Scholar]
- 48.American Academy of Addiction Psychiatry. Buprenorphine waiver training. [cited 2014 September 22]; Available from: http://www.aaap.org/education-training/buprenorphine/. [Google Scholar]
- 49.Egan JE, Casadonte P, Gartenmann T, Martin J, McCance-Katz EF, Netherland J, et al. The Physician Clinical Support System-Buprenorphine (PCSS-B): a novel project to expand/improve buprenorphine treatment. J Gen Intern Med. 2010 Sep;25(9):936–941. doi: 10.1007/s11606-010-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Recovery Enhancement for Addiction Treatment Act, Pub. L. No. S.2645. 2014 Jul 23; [Google Scholar]
- 51.Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O'Connor PG, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, et al. Office-based management of opioid dependence with buprenorphine: clinical practices and barriers. J Gen Intern Med. 2008 Sep;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Substance Abuse Mental Health Services Administration. Buprenorphine: Physician Waiver Qualifications. [cited 2014 December 9]; Available from: http://buprenorphine.samhsa.gov/waiver_qualifications.html.
- 54.Link BG, Struening EL, Rahav M, Phelan JC, Nuttbrock L. On stigma and its consequences: evidence from a longitudinal study of men with dual diagnoses of mental illness and substance abuse. J Health Soc Behav. 1997 Jun;38(2):177–190. [PubMed] [Google Scholar]
- 55.Luoma JB, Twohig MP, Waltz T, Hayes SC, Roget N, Padilla M, et al. An investigation of stigma in individuals receiving treatment for substance abuse. Addict Behav. 2007 Jul;32(7):1331–1346. doi: 10.1016/j.addbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Kissin W, McLeod C, Sonnefeld J, Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J Addict Dis. 2006;25(4):91. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- 57.Galanter M, Keller DS, Dermatis H, Egelko S. The impact of managed care on substance abuse treatment: a report of the American Society of Addiction Medicine. J Addict Dis. 2000;19(3):13–34. doi: 10.1300/J069v19n03_02. [DOI] [PubMed] [Google Scholar]
- 58.D'Aunno T. The role of organization and management in substance abuse treatment: Review and roadmap. J Subst Abuse Treat. 2006 Oct;31(3):221–233. doi: 10.1016/j.jsat.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 59.Hutchinson E, Catlin M, Andrilla CH, Baldwin LM, Rosenblatt RA. Barriers to primary care physicians prescribing buprenorphine. Annals of family medicine. 2014 Mar-Apr;12(2):128–133. doi: 10.1370/afm.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011 Mar;5(1):21–27. doi: 10.1097/ADM.0b013e3181d41ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.