Abstract
The t(5;17) variant of acute promeylocytic leukemia (APL) expresses a fusion of nucleophosmin (NPM) with the retinoic acid receptor alpha (RARA). We have previously shown that NPM-RAR is a binding partner of the tumor necrosis factor receptor type-I –associated DEATH domain protein TRADD. Binding of TNF to its receptor, TNFR1, induces recruitment of TRADD, and subsequent recruitment of a cascade of proteins that ultimate activate caspase 3, NFκB , and JNK. We have previously shown that NPM-RAR interaction with TRADD blocks TNF activation of caspase 3, caspase 8, PARP cleavage, and ultimately, apoptosis. We now report that NPM-RAR expression is permissive for TNF activation of NFκB and JNK. We propose that inhibition of TNF activation of apoptosis, while preserving TNF activation of NFκB and JNK pathways that stimulate cell growth and survival, represents a novel mechanism through which NPM-RAR contributes to development of the leukemic phenotype.
Keywords: NPM-RAR, acute promyelocytic leukemia, TNF, TRADD, NFκB, JNK
INTRODUCTION
Acute promyelocytic leukemia (APL) is caused by the fusion of the tumor suppressor gene PML at 15q22 with the retinoic acid receptor alpha (RAR) locus at 17q21[1]. The resulting PML-RAR fusion protein perturbs myeloid maturation through a number of mechanisms, serving as a dominant-negative for the transcriptional activation properties of RARA, as a dominant negative for PML (a regulator of senescence and apoptotic pathways), and potentially as a rogue transcriptional activator[1].
In an effort to understand the contribution of these different mechanisms towards development of the APL phenotype, we have been studying the variant translocations in APL. Though rare, these “experiments of nature” have similar phenotype, though different genotype, with PML-RAR APL, and serve as important tools with which to dissect the pathways underlying leukemogenesis. To date nine variant translocations have been characterized, all of which share the same C-terminal domains of RARA as PML-RAR. The N-terminal sequences of the fusions involve PLZF in t(11;17)q(23;q21) [2]; NPM in t(5;17)(q35;q21) [3]; NUMA in t(11;17)(q13;q21) [4]; STAT5b in der17[5]; PRKAR1A in t(17;17)(q24;q24) [6]; BCOR in t(X;17)(p11;q21) [7]; FIP1L1 in t(4;17)(q12;q21) [8]; OBFC2A in der (2)t(2;17)(q32;q21) [9]; and TBLR1 in t(3;17)(q26;q21) [10]. We have focused our studies on t(5;17), which stands out as the second most common variant after t(11;17)q(23;q21) and which shares a similar phenotype with t(15;17), including ATRA responsiveness[11].
t(5;17) fuses the N-terminal 117 amino acids of the chaperonin nucleophosmin (NPM) to the C-terminal 402 amino acids of RARA[3]. Like PML-RAR, ectopic expression of NPM-RAR produces an APL-like phenotype in vitro and in vivo[12,13]. NPM-RAR is primarily nuclear[14], and interacts with co-activator and co-repressor complexes in a ligand-dependent fashion[15]. It binds to DNA both as homodimers and heterodimers with RXR, and similar to PML-RAR, its activity as a transcriptional regulator varies by cell-type and target promoter[15].
Through a proteomic analysis of NPM-RAR-interacting proteins, we have recently identified NPM-RAR as specifically binding to the tumor necrosis factor receptor type-I –associated DEATH domain protein TRADD[16]. TRADD is a scaffold protein downstream of the TNF receptor. Upon binding TNF, TNF-R recruits TRADD, which in turn recruits a series of mediators to activate caspase 3, NFκB , and JNK pathways that regulate apoptosis, cell survival, cell growth pathways[17].
We have shown that NPM-RAR blocks TNF-R mediated activation of the extrinsic apoptotic pathway. NPM-RAR interaction with TRADD inhibits TNF-dependent activation of the initiator caspase 3 and the effector capsase 8, cleavage of PARP, and initiation of apoptosis[16]. In this manuscript we describe the impact of NPM-RAR on the other pathways activated by TNF-R: NFκB and JNK. We find that NPM-RAR is permissive for TNF-mediated activation of both NFκB and JNK pathways. Both of these pathways contribute to accelerated cell growth. We propose that NPM-RAR selectively inhibits TNF-activation of the extrinsic apoptosis pathway while preserving TNF-activation of cell growth pathways.
MATERIALS and METHODS
Cell Culture and Reagents
HeLa, HEK293 and U937 cells were obtained from the American Type Culture Collection (ATCC; Bethesda, MD). HeLa and HEK293 cells were maintained in a humidified 5% CO2 atmosphere in Dulbecco’s modified Eagle’s medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% Fetal Bovine Serum (GIBCO, Grand Island, NY), 2 mM glutamine, 100 U/ml penicillin, and 100 microg/ml streptomycin. U937 cells were grown in RPMI 1640 (Mediatech) containing the same supplements. HeLa and HEK293 cells were transfected using Fugene Transfection Reagent (Promega, Madison, WI), per the manufacturer’s protocol. Stably transfected HeLa clones were selected in medium containing 1 mg/ml G418 (Gibco). The generation and characterization of the stably-transfected U937 cells has been previously described[18].
Antibodies and reagents
Rabbit polyclonal anti-RARA and rabbit polyclonal anti-p65 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin and anti-FLAG mouse monoclonal antibodies were purchased from Sigma (St. Louis, MO). Anti-lamin and anti-V5 antibodies were purchased from Invitrogen (Gran Island, NY). Anti-hsp90 antibody was obtained from Enzo Life Sciences (Farmingdale, NY). Human recombinant TNF-alpha was purchased form R&D Systems (Minneapolis, MN). NPM-RAR expression vector was generated by subcloning the NPM-RAR coding sequence into the multiple cloning site of pSG5 (Agilent Technologies, Santa Clara, CA). The coding region of TRADD was subcloned into pcDNA3/V5-His (Invitrogen) in the appropriate reading frame to generate fusion proteins bearing a V5-His tag at the C terminus. Fidelity was confirmed by DNA sequencing and Western blotting.
NFκB Reporter Assays
The NFκB -responsive MnSOD2-luciferase reporter construct was generated by subcloning the human MnSOD2 promoter (−555 to +24) along with the enhancer element from the second intron (+1742 to +2083) into the PGL4.26 luciferase vector (Promega, Madison, WI). The NFκB responsive HIV p65 luciferase vector was a gift form Robert Ferris (University of Pittsburgh). Luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions. Renilla Luciferase Reporter pRL-TK (Promega) was used as transfection control.
JNK Kinase Assay
SAPK/JNK Kinase Assay Kit (Cell Signaling, Beverly, MA) was used according to the manufacturer’s instructions. Briefly, 1 × 106 cells were treated with 10 ng/ml TNF alpha for 3 min. (u937) or 5 min (HeLa) before lysis. The lysate was incubated with anti-phospho SAPK/JNK antibody (Thr183/Tyr185) conjugated beads overnight. After washing, the captured JNK kinase was incubated with 1 microgram c-Jun fusion protein in kinase buffer containing 200 microM ATP for 30 min. The reaction was terminated and phospho c-Jun was separated on 8% SDS-PAGE. Immunoblots were probed with anti-phospho c-Jun (Ser63) antibody and developed with the ECL detection system (Invitrogen).
Cell Fractionation
Cell pellets were resuspended in hypotonic buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 1x Protease Inhibitor Cocktail (Sigma-Aldrich Corp. St. Louis, MO). Cells were allowed to swell for 15 min on ice before addition of 10% NP-40. The homogenate was centrifuged at 1000g for 5 minutes. The supernatant was stored (cytoplasmic fraction) and the pellet, containing the nuclear fraction, was further washed with 20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM PMSF, and 1X Protease Inhibitor Cocktail. The nuclear fraction was centrifuged at 14,000 g for 5 minutes, and the supernatant (nuclear fraction) stored. Protein concentration was determined using the Bio-Rad DC Protein Assay kit (Bio-Rad, Hercules, CA).
Western blotting
Lysates were cleared by centrifugation. 50 μg of lysate were resolved by 8% or 12% SDS-PAGE and transferred onto Nitrocellulose membranes (Bio-Rad). After blocking with 5% non-fat milk, membranes were incubated with primary antibodies as recommended by the supplier. The immune complexes were detected by the ECL detection system (Invitrogen).
RESULTS
TNF-Induced Activation of NFκB
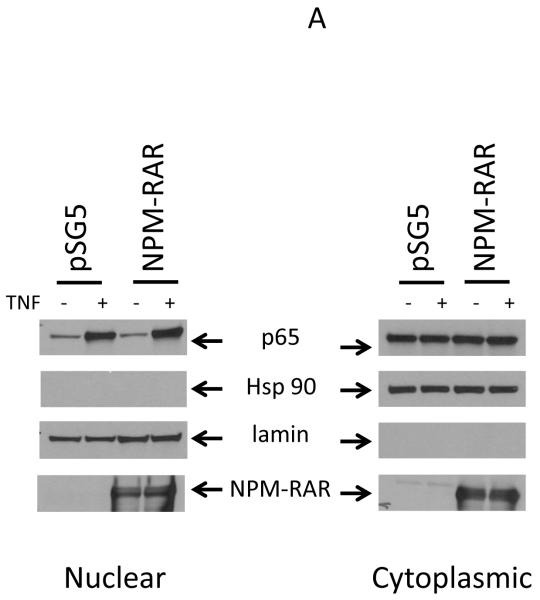
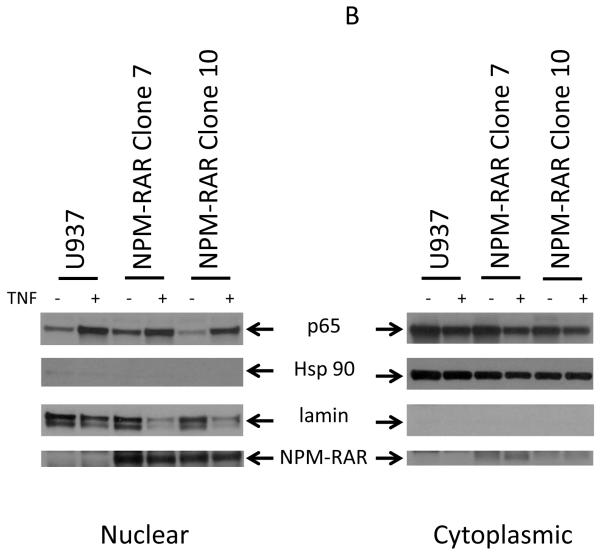
Activation of the canonical NFκB pathway leads to degradation of IkB to allow cytoplasmic NFκB to migrate into the nucleus, where it can activate transcription[19]. To determine whether NPM-RAR altered the ability of TNF and TNF-R to activate this pathway through binding to TRADD, we analyzed the subcellular distribution of the NFκB component protein p65 after treatment of NPM-RAR-expressing HeLA cells with TNF. Cytoplasmic and nuclear extracts were prepared, and immunoblots probed with antibodies against p65 (Figure 1A). Expression of HSP90 was assessed as a cytoplasmic control. Expression of lamin was assessed as a nuclear control. The anti-RARA antibody recognizes the C-terminal domains of NPM-RAR, and confirms expression of NPM-RAR in the cells (in both cytoplasm and nucleus – see also Figure 4). Figure 1A indicates that TNF induced an increase in p65 in the nuclear fraction consistent with TNF-induced activation and translocation of the NFκB subunit. Expression of NPM-RAR did not impact NFκB activation. To confirm these results in a different cell line, we analyzed the effects of TNF stimulation on parental U937 cells and two stable NPM-RAR-expressing U937 subclones (Figure 1B). Subcellular distribution of P65 and NPM-RAR were assessed by immunobloting of the cytoplasmic and nuclear extracts. Lamin expression was assessed as a nuclear control; HSP90 as a cytoplasmic control. The U937 and NPM-RAR-clone nuclear fractions show an increase in p65. The degree of nuclear translocation is similar in both parental and the NPM-RAR expressing clones, indicated a lack of inhibitory effect by NPM-RAR on NFκB activation by TNF.
Figure 1.
TNF-induced nuclear/cytoplasmic redistribution of NFκB . Panel A. HeLa cells were transiently transfected with NPM-RAR. 32 hours after transfection cells were exposed to vehicle control or 10 ng/ml TNF for 16 hours. Immunoblots of nuclear and cytoplasmic fractions were probed with antibodies for p65. As controls the blots were probed with antibodies for RARA, lamin, and HSP90. Panel B. U937 cells or stably transfected NPM-RAR expressing clones were exposed to 10 ng/ml TNF for 16 hours. Immunoblots of nuclear and cytoplasmic extracts were probed with antibodies for p65, RARA, lamin or HSP90.
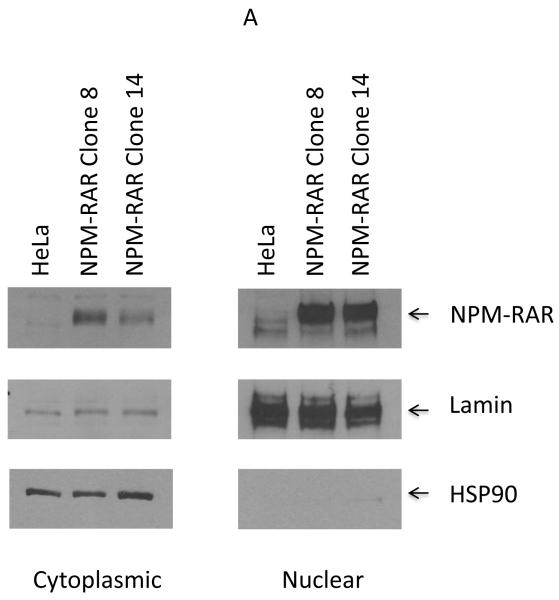
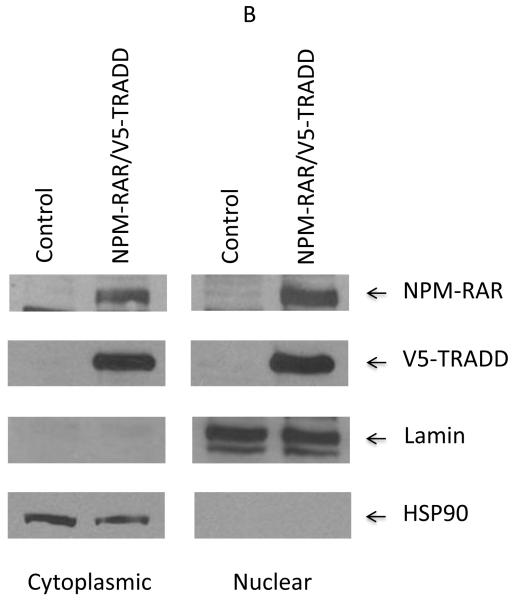
Figure 4.
Nuclear/cytoplasmic distribution of NPM-RAR and TRADD. Panel A. Nuclear and cytoplasmic extracts were prepared from HeLa cells or stably expressing NPM-RAR expressing clones, and run on SDS-PAGE gels. Immunoblots were probed with antibodies against RARA (which recognize NPM-RAR as well as wild-type RARA), lamin and HSP90. Panel B. HEK293 cells were transiently transfected with expression plasmids for NPM-RAR and V5-TRADD. 48 hours after transfection nuclear and cytoplasmic extracts were prepared and run on SDS-PAGE gels. Immunoblots were probed with antibodies against RAR, V5, lamin and HSP90.
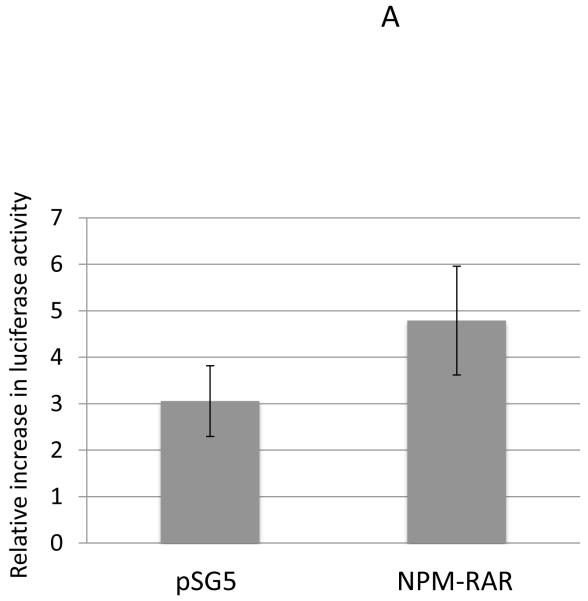
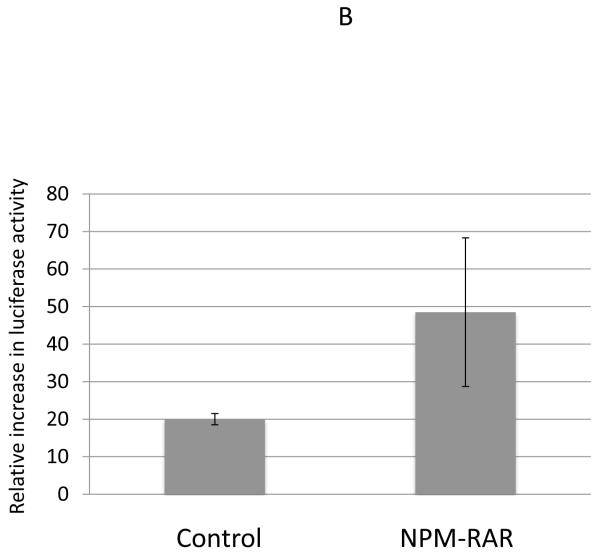
To confirm these results, we also assessed the ability of TNF to activate transcription of NFκB -responsive reporter constructs. We tested this using two different reporter constructs that incorporated the NFκB response elements from the MnSOD2 promoter and enhancer[20,21] (Figure 2A), and the HIV promoter[22] (Figure 2B). MnSOD and HIV luciferase constructs were expressed in HeLa cells, and the ratio of luciferase expression (normalized for transfection efficiency) in cells treated with TNF vs. vehicle control determined. For both reporter promoters we found no evidence for diminution of induction of NFκB transcriptional activity by NPM-RAR (Figure 2). Indeed, though the error bars are broad, there is a suggestion that NPM-RAR expression may enhance NFκB activity.
Figure 2.
TNF-induced expression of NFκB reporter constructs. HeLa cells were transiently transfected with expression plasmids for MnSOD2-luciferase (Panel A) or p65-HIV-luciferase (Panel B) along with Renilla luciferase as transfection control and NPM-RAR-pSG5 or pSG5 control. 48 hours after transfection 20 ng/ml TNF was added to the cultures. Lysates were prepared at 72 hours, and luciferase activity normalized for Renilla expression. The ratio of normalized luciferase activity induced by TNF compared to vehicle control was determined. Shown are the mean and standard error for seven (Panel A) and three (Panel B) independent experiments.
TNF-Inducted Activation of JNK
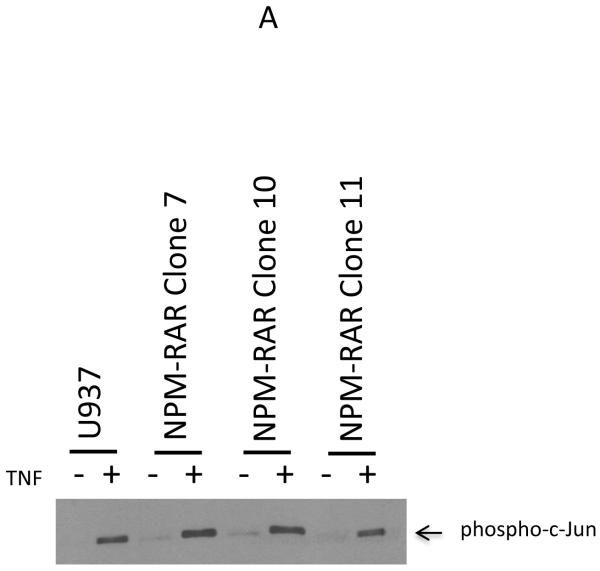
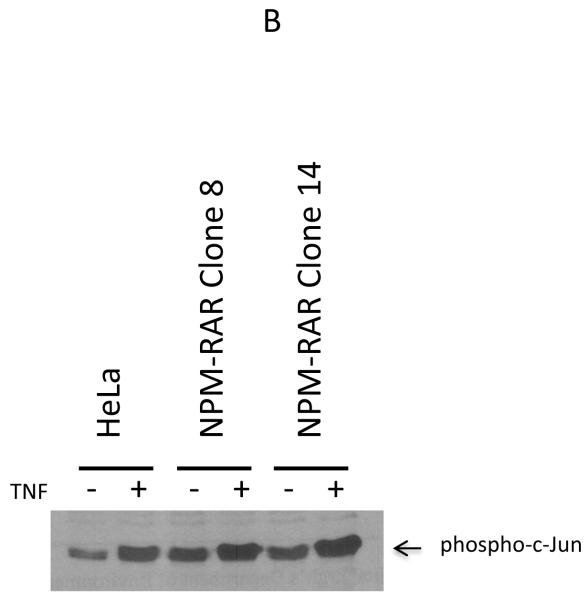
Binding of TNF by its receptor initiates a cascade that activates the JNK kinase pathway[17]. To investigate whether NPM-RAR expression alters JNK activation, we studied NPM-RAR expressing U937 and HeLa clones. JNK activation was determined by immunoprecipitating JNK from cell lysates, and assessing the ability of the pellets to phosphorylate c-Jun substrate. Figure 3 indicates that, in a panel of either U937 (Figure 3A) or HeLa clones (Figure 3B) expressing NPM-RAR, the ability to activate JNK was equivalent to controls.
Figure 3.
TNF-induced JNK activation. Panel A. U937 cells or three NPM-RAR-stably expressing clones were treated with 10 ng/ml TNF or vehicle control for 3 minutes before cells were lysed and assayed for activated JNK kinase activity with SAPK/JNK Kinase Assay Kit. Panel B. HeLa cells or two NPM-RAR-stably expressing clones were treated with 10 ng/ml or vehicle control for 5 minutes before cells were lysed and assayed for activated JNK kinase activity with SAPK/JNK Kinase Assay Kit.
Cellular Localization of NPM-RAR
TRADD has been reported to be localized primarily in the cytoplasm, but also in the nucleus. Nuclear TRADD has been implicated in regulating an ill-defined caspase 9-dependent, capase 8-independent apoptotic pathway. Our prior immunofluorescent localization studies indicated that NPM-RAR was expressed primarily in the nucleus, but to a lesser extent in the cytoplasm as well[16]. We speculated that the cytoplasmic component was responsible for the interaction with TRADD, but it is possible that the nuclear component could also interact with nuclear TRADD. To further analyze the subcellular localization of NPM-RAR, we isolated nuclear and cytoplasmic extracts from either stably transfected NPM-RAR expressing HeLa clones (Figure 4A), or transiently transfected HEK293 cells (Figure 4B). Both experiments indicate that NPM-RAR is present in both cytoplasmic and nuclear fractions. This was also seen in Figure 1A and 1B. To confirm the prior reports that TRADD is expressed both in the cytoplasm and nucleus[23,24], we included in the HEK293 transient transfection experiment an expression vector encoding epitope-tagged TRADD: immunobloting demonstrates that TRADD is found in both cytoplasm and nucleus (Figure 4B). This raises the possibility that NPM-RAR could interact with TRADD in both the cytoplasm and the nucleus.
DISCUSSION
We have previously shown that NPM-RAR interacts with TRADD, and inhibits TNF-R mediated activation of the extrinsic apoptosis pathway[16]. We now show that NPM-RAR does not impede TNF-mediated activation of either NFκB or JNK pathways.
The transcription factor NFκB controls expression of a panoply of genes, many of which are important for cell growth (reviewed in [25]). NFκB is a dimeric transcription factor composed of members of the Rel family – p50, p65 (RelA), c-Rel, p52, and RelB. Activation of NFκB is dependent upon IkB, which binds to the NFκB dimers and sequesters them in the cytoplasm. Upon phosphorylation, IkB undergoes ubiqination and degradation, freeing NFκB to migrate into the nucleus, bind to DNA, and initiate transcription of target genes.
JNKs are master protein kinases that regulate cell proliferation, differentiation, survival, and cell death (reviewed in [26]). Upon activation, JNKs phosphorylate the transcription factors AP-1 (actually a family of dimers of c-Jun, JunB, JunD, and c-Fos, FosB, Fra-1, and Fra-2) as well as ATF-2, c-Myc, p53, Elk1, NFAT. In addition JNKs phosphorylate regulators of the Bcl-2 family. Through these downstream mediators, JNK activity impacts cell proliferation, differentiation, apoptosis, and survival. JNKs become activated through the MAP3kinase cascade; hyperactivation of JNKs has been reported as a contributing factor in leukemogenesis[27].
Upon ligand activation, TNF-R binds the N-terminus of TRADD, which serves as a scaffold for activation of a series of mediator proteins[28]. RIP1 binds to the C-terminal death domain of TRADD and TRAF2 interacts with the N-terminal domains of TRADD to quickly form Complex I, a scaffold that recruits TAK1, c-IAP1, and UBC13. This complex activates MAP3K leading to activation of JNK, triggers IKKinases to target IkB for degradation and initiate the canonical NFκB pathway, and recruits c-IAP to inhibit apoptosis. After 30-60 minutes, TRADD, TRAF2, and RIP1 dissociate from the receptor, to form the backbone of Complex II, which recruits FADD and caspase 8 to initiate the extrinsic apoptosis pathway[28].
Our work suggests that NPM-RAR inhibits TNF-R’s potential to induce apoptosis while preserving it ability to activate the cell survival and proliferation pathways controlled by NFκB and JNK. We propose a model in which NPM-RAR is permissive for formation of TRADD Complex I on TNF-R, to allow activation of NFκB and JNK, but disallows formation of Complex II, and hence inhibits the apoptotic-inducing activity of TNF-R. Experiments to directly test this model are ongoing.
In many ways this model is analogous to that which has been generated to explain the function of the Epstein Barr Virus transforming protein LMP1[29,30]. LMP1 is not dependent upon TNF-R, but itself binds to the cell membrane. It binds TRADD, and inhibits formation of the proapoptotic complex. In contradistinction to TNF-R, LMP-1 interacts with members of the TRAF family directly. These interactions are not dependent upon TRADD. Hence, LMP-1 is able to independently activate the cascades leading to activation of NFκB and JNK/AP-1 while inhibiting TRADD mediated activation of apoptosis[29,30].
Though primarily localized in the cytoplasm, TRADD also has been found in the nucleus, where it plays a role in a poorly defined caspase -9 dependent, caspase 8 independent pathway for apoptosis[23,24]. Our prior immunofluorescent studies indicate that NPM-RAR is localized not only to the nucleoplasm but also in the cytoplasm. Our cellular localization experiments confirm the presence of both proteins in both compartments, raising the possibility that they interact in both cytoplasm and nucleus. Though we have not formally demonstrated it, it is very possible that NPM-RAR might also inhibit the nuclear TRADD caspase-9 dependent apoptotic pathway.
TRADD knockout mice have a high incidence of malignancies, indicating that TRADD serves as a tumor suppressor[31]. To our knowledge, LMP-1 and NPM-RAR are the only oncogenic proteins yet demonstrated to bind and subvert the activities of TRADD. We propose that this represents a novel pathway though which NPM-RAR contributes to leukemogenesis.
ACKNOWLEDGEMENTS
The authors would like to thank Richard Steinman MD PhD, Daniel Johnson PhD, and Preet Chaudhary MD PhD for valuable discussions and sharing of reagents. The HIV luciferase plasmid was a kind gift form Robert Ferris MD PhD. This work was supported by NIH R01 CA67346 and P30 CA047904.
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–3215. [PubMed] [Google Scholar]
- 2.Chen Z, Brand NJ, Chen A, et al. Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. The EMBO journal. 1993;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redner RL, Rush EA, Faas S, Rudert WA, Corey SJ. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood. 1996;87:882–886. [PubMed] [Google Scholar]
- 4.Wells RA, Catzavelos C, Kamel-Reid S. Fusion of retinoic acid receptor alpha to NuMA, the nuclear mitotic apparatus protein, by a variant translocation in acute promyelocytic leukaemia. Nature genetics. 1997;17:109–113. doi: 10.1038/ng0997-109. [DOI] [PubMed] [Google Scholar]
- 5.Arnould C, Philippe C, Bourdon V, Gr goire MJ, Berger R, Jonveaux P. The signal transducer and activator of transcription STAT5b gene is a new partner of retinoic acid receptor alpha in acute promyelocytic-like leukaemia. Human molecular genetics. 1999;8:1741–1749. doi: 10.1093/hmg/8.9.1741. [DOI] [PubMed] [Google Scholar]
- 6.Catalano A, Dawson MA, Somana K, et al. The PRKAR1A gene is fused to RARA in a new variant acute promyelocytic leukemia. Blood. 2007;110:4073–4076. doi: 10.1182/blood-2007-06-095554. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Tsuzuki S, Tsuzuki M, Handa K, Inaguma Y, Emi N. BCOR as a novel fusion partner of retinoic acid receptor alpha in a t(X;17)(p11;q12) variant of acute promyelocytic leukemia. Blood. 2010;116:4274–4283. doi: 10.1182/blood-2010-01-264432. [DOI] [PubMed] [Google Scholar]
- 8.Kondo T, Mori A, Darmanin S, Hashino S, Tanaka J, Asaka M. The seventh pathogenic fusion gene FIP1L1-RARA was isolated from a t(4;17)-positive acute promyelocytic leukemia. Haematologica. 2008;93:1414–1416. doi: 10.3324/haematol.12854. [DOI] [PubMed] [Google Scholar]
- 9.Won D, Shin SY, Park CJ, et al. OBFC2A/RARA: a novel fusion gene in variant acute promyelocytic leukemia. Blood. 2013;121:1432–1435. doi: 10.1182/blood-2012-04-423129. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Li S, Zhou C, et al. TBLR1 fuses to retinoid acid receptor alpha in a variant t(3;17)(q26;q21) translocation of acute promyelocytic leukemia. Blood. 2014 doi: 10.1182/blood-2013-10-528596. [DOI] [PubMed] [Google Scholar]
- 11.Redner RL, Corey SJ, Rush EA. Differentiation of t(5;17) variant acute promyelocytic leukemic blasts by all-trans retinoic acid. Leukemia. 1997;11:1014–1016. doi: 10.1038/sj.leu.2400661. [DOI] [PubMed] [Google Scholar]
- 12.Du C, Redner RL, Cooke MP, Lavau C. Overexpression of wild-type retinoic acid receptor alpha (RARalpha) recapitulates retinoic acid-sensitive transformation of primary myeloid progenitors by acute promyelocytic leukemia RARalpha-fusion genes. Blood. 1999;94:793–802. [PubMed] [Google Scholar]
- 13.Rego EM, Ruggero D, Tribioli C, et al. Leukemia with distinct phenotypes in transgenic mice expressing PML/RAR alpha, PLZF/RAR alpha or NPM/RAR alpha. Oncogene. 2006;25:1974–1979. doi: 10.1038/sj.onc.1209216. [DOI] [PubMed] [Google Scholar]
- 14.Rush EA, Schlesinger KW, Watkins SC, Redner RL. The NPM-RAR fusion protein associated with the t(5;17) variant of APL does not interact with PML. Leukemia research. 2006;30:979–986. doi: 10.1016/j.leukres.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 15.Redner RL, Chen JD, Rush EA, Li H, Pollock SL. The t(5;17) acute promyelocytic leukemia fusion protein NPM-RAR interacts with co-repressor and co-activator proteins and exhibits both positive and negative transcriptional properties. Blood. 2000;95:2683–2690. [PubMed] [Google Scholar]
- 16.Chattopadhyay A, Hood BL, Conrads TP, Redner RL. Extrinsic Apoptosis Is Impeded by Direct Binding of the APL Fusion Protein NPM-RAR to TRADD. Molecular cancer research. 2014;12:1283–1291. doi: 10.1158/1541-7786.MCR-14-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell death and differentiation. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 18.Rush EA, Pollock SL, Abecassis I, Redner RL. Interaction with RXR is necessary for NPM-RAR-induced myeloid differentiation blockade. Leukemia research. 2013;37:1704–1710. doi: 10.1016/j.leukres.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wajant H, Scheurich P. TNFR1-induced activation of the classical NF-kappaB pathway. The FEBS journal. 2011;278:862–876. doi: 10.1111/j.1742-4658.2011.08015.x. [DOI] [PubMed] [Google Scholar]
- 20.Delhalle S, Deregowski V, Benoit V, Merville MP, Bours V. NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene. 2002;21:3917–3924. doi: 10.1038/sj.onc.1205489. [DOI] [PubMed] [Google Scholar]
- 21.Dhar SK, Lynn BC, Daosukho C, St Clair DK. Identification of nucleophosmin as an NF-kappaB co-activator for the induction of the human SOD2 gene. The Journal of biological chemistry. 2004;279:28209–28219. doi: 10.1074/jbc.M403553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretzschmar M, Meisterernst M, Scheidereit C, Li G, Roeder RG. Transcriptional regulation of the HIV-1 promoter by NF-kappa B in vitro. Genes & development. 1992;6:761–774. doi: 10.1101/gad.6.5.761. [DOI] [PubMed] [Google Scholar]
- 23.Bender LM, Morgan MJ, Thomas LR, Liu ZG, Thorburn A. The adaptor protein TRADD activates distinct mechanisms of apoptosis from the nucleus and the cytoplasm. Cell death and differentiation. 2005;12:473–481. doi: 10.1038/sj.cdd.4401578. [DOI] [PubMed] [Google Scholar]
- 24.Morgan M, Thorburn J, Pandolfi PP, Thorburn A. Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. The Journal of cell biology. 2002;157:975–984. doi: 10.1083/jcb.200204039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 26.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature reviews. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 27.Hartman AD, Wilson-Weekes A, Suvannasankha A, et al. Constitutive c-jun N-terminal kinase activity in acute myeloid leukemia derives from Flt3 and affects survival and proliferation. Experimental hematology. 2006;34:1360–1376. doi: 10.1016/j.exphem.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 29.Kieser A. Pursuing different ‘TRADDes’: TRADD signaling induced by TNF-receptor 1 and the Epstein-Barr virus oncoprotein LMP1. Biological chemistry. 2008;389:1261–1271. doi: 10.1515/BC.2008.144. [DOI] [PubMed] [Google Scholar]
- 30.Schneider F, Neugebauer J, Griese J, et al. The viral oncoprotein LMP1 exploits TRADD for signaling by masking its apoptotic activity. PLoS biology. 2008;6:e8. doi: 10.1371/journal.pbio.0060008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chio II, Sasaki M, Ghazarian D, et al. TRADD contributes to tumour suppression by regulating ULF-dependent p19(Arf) ubiquitylation. Nature cell biology. 2012;14:625–633. doi: 10.1038/ncb2496. [DOI] [PubMed] [Google Scholar]