Abstract
Background:
Adjacent segment degeneration (ASD) following lumbar spine surgery occurs in up to 30% of cases, and descriptions of such changes are not new. Here, we review some of the older literature concerning the rate of ASD, typically more severe cephalad than caudad, and highly correlated with instrumented fusions. Therefore, for degenerative lumbar disease without frank instability, ASD would be markedly reduced by avoiding instrumented fusions.
Methods:
In a prior review, the newer literature regarding the frequency of ASD following lumbar instrumented fusions (e.g., transforaminal or posterior lumbar interbody fusions [TLIF/PLIF] fusions or occasionally, posterolateral fusions [PLFs]) was presented. Some studies cited an up to an 18.5% incidence of ASD following instrumented versus noninstrumented fusions/decompressions alone (5.6%). A review of the older literature similarly documents a higher rate of ASD following instrumented fusions performed for degenerative lumbar disease alone.
Results:
More frequent and more severe ASD follows instrumented lumbar fusions performed for degenerative lumbar disease without instability. Alternatively, this entity should be treated with decompressions alone or with noninstrumented fusions, without the addition of instrumentation.
Conclusions:
Too many studies assume that TLIF, PLIF, and even PLF instrumented fusions are the “gold standard of care” for dealing with degenerative disease of the lumbar spine without documented instability. It is time to correct that assumption, and reassess the older literature along with the new to confirm that decompression alone and noninstrumented fusion avoid significant morbidity and even potentially mortality attributed to unnecessary instrumentation.
Keywords: Adjacent level disease, lumbar fusions, unnecessary
INTRODUCTION
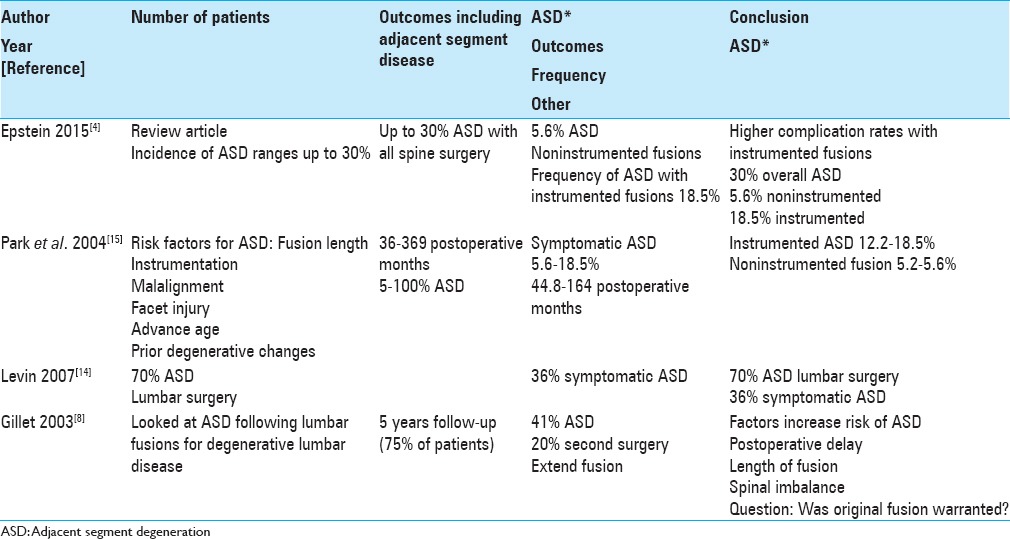
Adjacent segment degeneration (ASD) is defined as “the mobile segment next to a lumbar or lumbosacral spine fusion” [Tables 1–3].[15] Findings include adjacent segment disc pathology due to changes in biomechanical factors altering intradiscal pressures, greater stress on facet joints, greater mobility following fusions, new or progression of preexisting stenosis, plus other findings (e.g., synovial cyst extrusions, hypertrophy/ossification of the yellow ligament etc.). Epstein's prior review of ASD in 2015 showed the incidence of ASD following instrumented lumbar spine surgery predominantly for degenerative lumbar disease approached 30%.[4] This review offers not only a perspective on the older literature regarding the high frequency/severity of ASD with instrumented lumbar fusions, but also offers the alternative perspective that more lumbar degenerative disease should be treated with decompressions/noninstrumented fusions to reduce the frequency of ASD.
Table 1.
Patient series (>100) regarding frequency of adjacent segment degeneration
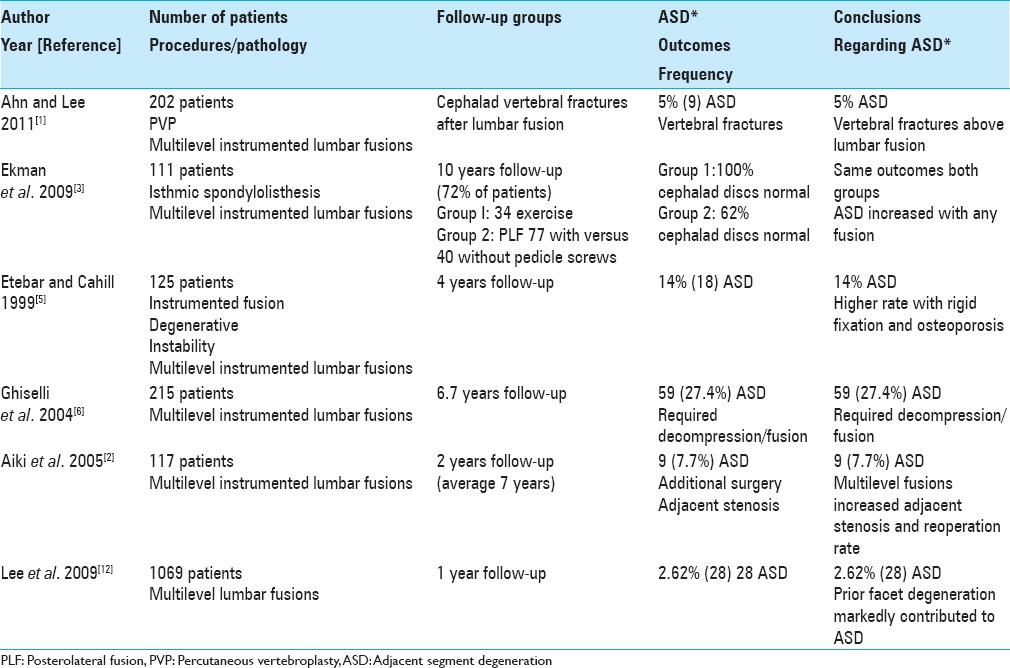
Table 3.
Review articles; adjacent segment degeneration
FREQUENCY OF ASD AFTER LUMBAR FUSION
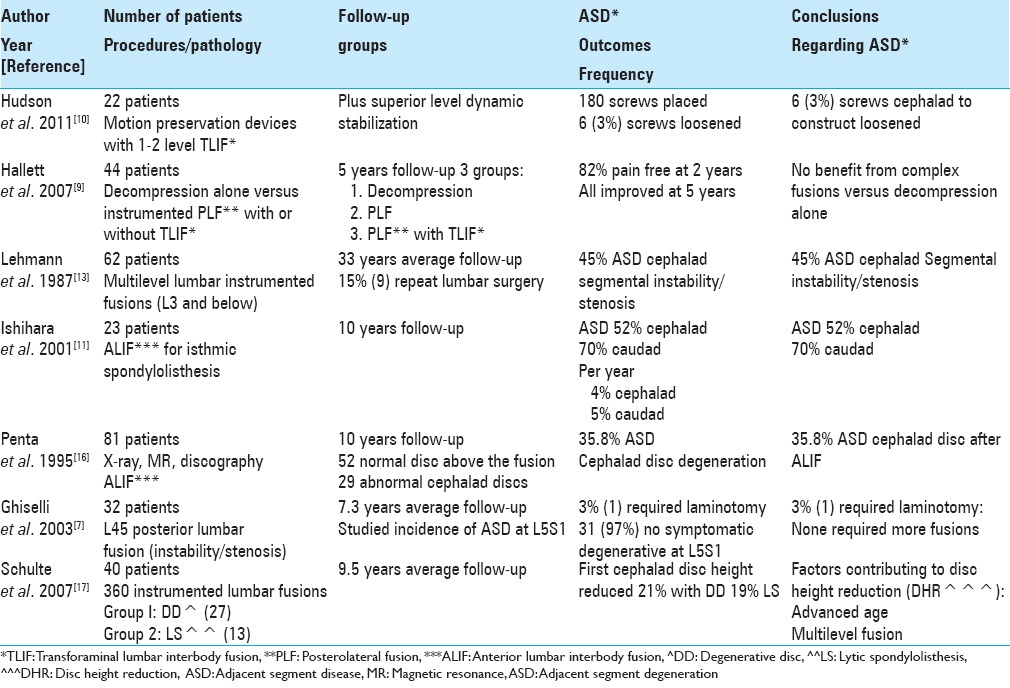
Varying frequencies of ASD have been reported following lumbar fusions, typically utilizing instrumentation (e.g.,14–70%) [Tables 1–3].[4,5,11,13,16] Lehmann et al. in 1987 followed 62 patients over an average of 33 years following lumbar fusions; ASD (segmental instability) was noted above the fusion in 45% of patients (X-rays) [Table 2].[13] In 1995, Penta et al. evaluated plain X-rays and magnetic resonance (MR) studies looking at the frequency of ASD over a minimum 10 years following anterior lumbar interbody fusions (ALIFs) and asked whether the fusion length impacted outcomes [Table 2].[16] They assessed 52 patients whose discs above the fusion levels were originally normal; 32% exhibited new degenerative changes at the cephalad disc level at 10 postoperative years, but this finding did not directly reflect the length of the fusion construct. Subsequently, in 1999, Etebar and Cahill looked at risk factors for adjacent segment failure after instrumented (rigid) fusions for patients with degenerative instability.[5] They performed a retrospective analysis of 125 consecutive patients undergoing such fusions with a 4 year follow-up: 18 (14%) developed ASD [Table 1]. They concluded the risk of ASD was greater utilizing rigid fixation for treating degenerative instability and that this frequency was substantially increased in postmenopausal females (83%). Later, in 2001, Ishihara et al. looked at ALIF utilized to treat isthmic spondylolisthesis; the 23 patients in the series were followed for over 10 years [Table 2].[11] X-rays showed new ASD; 52% cephalad and 70% caudad. They determined the yearly rates for cephalad ASD was 4% and for caudad ASD was 5%. Of interest, follow up MR scans in 11 patients showed a 73% incidence of new disc degeneration at the upper and 100% at the lower disc levels.
Table 2.
Patient series (<100 patients) adjacent segment degeneration
Comments
The frequency of ASD following instrumented lumbar fusions varies markedly from 14% to 70% as acknowledged above [Tables 1–3].[4,5,11,14,16] Of interest, one particular study focused on the 4% yearly rate of cephalad and 5% yearly rate of caudad ASD progression following instrumented spinal fusions.
THE FATE OF THE ADJACENT MOTION SEGMENTS AFTER LUMBAR FUSION
In 2003, Gillet followed outcomes of lumbar fusions addressing degenerative lumbar disease over a 2–15 postoperative interval; 75% of patients had a minimum 5-year follow-up [Table 3].[8] They observed that 41% of patients developed ASD, with 20% requiring extension of fusions. Factors that appeared to increase the risk for ASD included postoperative delay, length of fusion, and spinal imbalance. They further questioned whether the relatively high frequency of ASD warranted the original performance of a fusion if there was no evidence of instability accompanying degenerative changes.
Comments
This 5 year study following instrumented lumbar fusions performed for degenerative lumbar disease, noted a 41% incidence of ASD and 20% frequency of secondary surgery requiring extension of fusions.[8] The real question here, as asked also by Gillet, was why perform a fusion in the first place?[8]
CLINICAL OUTCOME ANALYSIS OF ASD AT L5S1 FOLLOWING AN L4–L5 ISOLATED FUSION
In 2003, Ghiselli et al. retrospectively evaluated the caudad ASD motion at L5–S1 following focal L4–5 posterior lumbar fusions (average follow-up 7.3 years; range 2.3–12.4 years) [Table 2].[7] The 32 consecutive patients in the study averaged 56.4 years of age and underwent single-level L4–L5 posterior spinal fusions addressing instability with stenosis. Of these, 31 (97%) patients had no symptomatic degeneration at L5–S1, only 1 (3%) patient required a focal L5–S1 foraminotomy/laminotomy, and none required additional fusions. In their larger series in 2004, Ghiselli et al. next evaluated a total of 215 patients undergoing posterior lumbar fusions followed for an average of 6.7 years, and now noted a much higher number of patients (e.g., 59 patients [27.4%]) who warranted decompression/fusion to address ASD [Table 1].[6]
Comments
For the initial small group of just 32 patients in 2003, the incidence of ASD at the L5–S1 level below an L4–L5 fusion was just 3%.[7] However, when a larger series of 215 patients was studied in 2004, now 27.4% (59 patients) exhibited ASD requiring decompression/fusion.[6] This study highlights how larger series are often needed to confirm early findings based on smaller samples, and how greater experience with more patients undergoing posterior lumbar fusions yielded a much higher number requiring additional ASD surgery. Again, this raises the question, if there is no overt instability, why fuse?
HIGH FREQUENCY/SEVERITY OF ASD FOLLOWING INSTRUMENTED LUMBAR FUSIONS FOR DEGENERATIVE LUMBAR DISEASE
In 2004, Park et al. reviewed, over 44.8–164 postoperative months, the frequency/indications and risk factors resulting in adjacent segment degeneration in the lumbar spine following decompressions alone or noninstrumented (5.2–5.6%) fusions versus pedicle screw instrumented fusions (12.2–18.5%) for patients with degenerative spine disease without instability [Table 3].[15] The following risk factors contributed to ASD; “instrumentation, fusion length, capital malalignment, facet injury, age, maintaining sagittal balance, and preexisting degenerative changes.”
Comments
In 2004, Park et al. determined the frequency of ASD following decompressions alone/noninstrumented (5.2–5.6%) fusions was much less than for pedicle screw instrumented fusions (12.2–18.5%) in patients with degenerative spine disease without instability.[15] Therefore, more decompressions/noninstrumented fusions would markedly limit postoperative ASD.
SYMPTOMATIC ADJACENT SEGMENT STENOSIS FOLLOWING LUMBAR FUSIONS REQUIRING SECONDARY SURGERY
In 2005, Aiki et al. observed that although ASD is frequently noted following lumbar fusions, few patients are typically sufficiently symptomatic to warrant secondary surgery [Table 1].[2] Following patients for a minimum of 2 postoperative years (average 7 years), they specifically looked at adjacent segment stenosis responsible for new neurological deficits in 9 (7.7%) of 117 patients who warranted secondary surgery. Notably, only the performance of a multilevel fusion significantly increased the reoperation rate.
Comments
In 2005, Aiki et al. observed that 9 (7.7%) of 117 patients initially undergoing lumbar fusions developed symptomatic adjacent level stenosis requiring secondary surgery.[2] Furthermore, the more levels originally fused, the more likely ASD/stenosis would develop. Here again, fewer and less extensive lumbar fusions would limit the incidence of ASD warranting secondary surgery.
DISC HEIGHT REDUCTION AT ADJACENT SEGMENTS AND CLINICAL OUTCOME 10 YEARS AFTER LUMBAR 360 FUSIONS
In 2007, Schulte et al. looked at the frequency of ASD following 40 lumbar fusions (360 instrumented procedures); there were 27 patients in Group 1 (degenerative disc disease), and 13 in Group 2 (lytic spondylolisthesis) who were evaluated over 114 postoperative months [Table 2].[17] Clinically, patients showed a 44.6% incidence of improvement on the Oswestry Disability Index scale and 43.8% frequency of improvement on the visual analog scale (VAS). Using postoperative X-rays, first cephalad disc heights were reduced by an average of 21% and 19%, respectively; at second cephalad levels, the reductions were a lesser 16% and 14%, respectively. Notably, the overall fusion rate was 95%. Advanced age and multilevel fusions contributed to greater disc height reduction at the first versus the second cephalad adjacent segments.
Comments
In 2007, Schulte et al. looked at the frequency of ASD following 40 lumbar fusions (360 instrumented procedures performed for degenerative disc disease 27 patients) versus lytic spondylolisthesis (13 patients) over 114 postoperative months.[17] This study highlights the greater incidence of adjacent level disc degeneration at the first rather than the second cephalad level above an instrumented lumbar fusion. Of course, one should strongly consider avoiding fusion entirely for degenerative disc disease whereas fusion would be warranted for lytic spondylolisthesis.
ADJACENT SEGMENT DEGENERATION FOLLOWING SPINAL FUSION FOR DEGENERATIVE DISC DISEASE
In 2007, Levin et al. noted the incidence of ASD in the cervical spine after a fusion approaches 50%, while in the lumbar spine it is 70% at 10 postoperative years [Table 3].[14] Nevertheless, the frequency of symptomatic ASD is a lesser 25% in the cervical and a reduced 36% in the lumbar spine over this decade duration. The etiology of these changes may be variously attributed to the normal aging process versus postoperative biomechanical factors.
Comments
In 2007, Levin et al. noted the frequency of radiographic ASD 10 years following both cervical (50%) and lumbar (70%) surgery, and correctly emphasized that symptomatic disease occurs in fewer patients, respectively, 25% and 36%.[14]
FORAMINAL STENOSIS AND SINGLE-LEVEL DISC DISEASE: DECOMPRESSION VERSUS DECOMPRESSION/INSTRUMENTED FUSION
In 2007, Hallett et al. studied the 5 year outcomes for 44 patients randomly divided into three surgical groups; spinal decompressions alone (Group 1), decompression and instrumented posterolateral fusion (PLF) (with pedicle screw fixation) (Group 2), and instrumented PLF (pedicle/screw fixation) plus transforaminal lumbar interbody fusion (TLIF) using titanium cages filled with autograft (Group 3) [Table 2].[9] They evaluated several variables; spinal disability, quality of life, and pain preoperatively and up to 1, 2, and 5 years postoperatively. At 5 postoperative years, all three groups demonstrated some improvement on the three outcomes scores (low back outcome score, short form-36 physical functioning, and Roland Morris score). One patient from Group 1 required a secondary fusion for chronic pain, while 1 patient from each of Groups 2 and 3 required secondary surgery for adjacent level stenosis. Notably, none required revision surgery for “instrumentation failure, cage displacement, or pseudarthrosis,” and 95% of the latter patients showed at least unilateral fusion (Groups 2 and 3).
Comments
In 2007, Hallett et al. studied the 5 year outcomes for 44 patients randomly divided into those undergoing decompressions alone, decompression and instrumented PLF (with pedicle screws), and instrumented PLF with pedicle screws plus TLIF using titanium cages filled with autograft (Group 3).[9] The authors came to the right conclusions; after 5 postoperative years, virtually all patients showed improvement, and there was no superior benefit for the more complex instrumented fusions.
LONG-TERM EFFECT OF LUMBAR FUSION ON ADJACENT DISC DEGENERATION
In 2009, Ekman et al. noted that randomized controlled trials (RCTs) previously documented that ASD is accelerated following lumbar fusions [Table 1].[3] In this randomized controlled prospective study, 111 patients, aged 18–55, with isthmic spondylolisthesis were randomized into two treatment groups: exercise alone (EX, n = 34) versus PLF (n = 77) with (n = 37) or without pedicle screw instrumentation (n = 40). In this series, the minimum 10-year follow-up rate was 72%. The average follow-up was 12.6 years (range 10–17 years). They utilized different methods to assess ASD: two digital radiographs and the semi quantitative University of California Los Angeles (UCLA) grading scale. The average mean disc height at the adjacent level was reduced by 2% in the EX group and by 15% in the PLF group (P = 0.0016). Those using the UCLA grading scale demonstrated normal cephalad discs in 100% of patients in the EX group versus 62% in the PLF group (P = 0.026). Notably, there were no significant differences for those undergoing instrumented versus noninstrumented PLF. In addition, there was a greater frequency of ASD for those undergoing laminectomy versus those without laminectomy.
Comments
In 2009, Ekman et al. noted that RCTs previously documented that ASD is accelerated following lumbar fusions [Table 1].[3] This study demonstrated that fusions whether instrumented or noninstrumented and laminectomy alone all “accelerated degenerative changes at the adjacent level compared with the natural history” of the disease.
RISK FACTORS FOR ASD AFTER LUMBAR FUSION
In 2009, Lee et al. utilizing MR studies, looked at the frequency and clinical risk factors contributing to ASD 1 year following lumbar/lumbosacral fusions performed for degenerative disease (1995–2006) [Table 1].[12] Cephalad ASD required secondary surgery in 28 (2.62%) of 1069 patients who averaged 58.4 years of age (comparable to the average age of 58.2 for those not developing ASD). Caudad ASD was much lower in frequency. Notably, prior facet degeneration markedly contributed to the eventual risk of developing ASD after lumbar fusions.
Comments
In 2009, Lee et al. found a 2.62% risk of ASD in 1069 patients following lumbar/lumbosacral fusions performed for degenerative disease.[12] Again, these patients would likely have been adequately treated with decompressions alone, and only selectively with noninstrumented versus instrumented fusions.
VERTEBROPLASTY TO TREAT ADJACENT VERTEBRAL FRACTURES AFTER LUMBAR INTERBODY FUSION
In 2011, Ahn and Lee looked at the incidence of cephalad adjacent segment osteoporotic vertebral compression fractures following lumbar interbody fusions, and offered percutaneous vertebroplasty (PVP) to address these iatrogenic injuries [Table 2].[1] They evaluated 202 consecutive patients undergoing PVP for vertebral compression fractures (2007–2008); 9 occurred adjacent to prior lumbar instrumented fusions. These patients were compared with 50 controls that had vertebroplasty for one-level osteoporotic compression fractures alone. Variables studied included “age, height, body weight, body mass index, bone mineral density (BMD), VAS scores, and the rates of overall satisfaction.” The mean BMD (spine and femur) were significantly higher for the 9 study patients versus controls, the mean VAS score increased from 8.1 to 3.2 for the study patients, and the satisfaction rate was 88.9%. They concluded that ASD resulting in vertebral osteoporotic fractures occurring cephalad to instrumented fusions could be effectively managed with PVP.
Comments
Ahn and Lee concluded that ASD resulting in vertebral osteoporotic fractures occurring cephalad to instrumented fusions could be effectively managed with PVP.[1] Nevertheless, what should have been discussed was that instrumentation should have been avoided in the first place when treating patients with degenerative lumbar disease without instability.[1]
HYBRID LUMBAR DYNAMIC STABILIZATION WITH POSTERIOR SPINAL FUSION
In 2011, Hudson et al. mistakenly stated that instrumented lumbar fusions are the “gold standard” for treating lumbar degenerative disc disease; indeed this is an ill-founded premise [Table 2].[10] As they realized these instrumented fusions contributed to ASD, they looked at utilizing motion-preserving devices (e.g., a dynamic rods) to reduce load to cephalad adjacent segments. There were 22 patients in their prospective, consecutive, nonrandomized clinical trial; at 2 postoperative years, all patients underwent a “posterior lateral spinal fusion with single- or 2-level TLIF cages, with superior level posterior dynamic instrumentation.” Outcomes showed that none experienced device failure or screw breakage, range of motion averaged 2.5° at the surgical level and was unchanged at the cephalad level, and disc height at the adjacent level was preserved. Of 180 screws placed, only 6 (3%) showed radiographic loosening. The authors concluded that dynamic stabilization was effective when combined with posterior spinal fusion.
Comments
In 2011, Hudson et al. mistakenly stated that instrumented lumbar fusions are the “gold standard” for treating lumbar degenerative disc disease; indeed this is an ill-founded premise [Table 2].[10] However, they should have been assessing whether degenerative changes occurred cephalad to decompressions alone or noninstrumented fusions, and in these instances, could have avoided the use of dynamic rods entirely.
SUMMARY
The frequency of ASD following instrumented spinal fusions (up to 18.5%) far exceeds that of ASD when decompressions alone and/or noninstrumented fusions (up to 5.6%) are performed.[15] In a review of the recent literature on ASD particularly addressing degenerative lumbar disease without instability, the frequency of ASD treated with instrumented fusions approached 30% in some series [Table 3].[4] Here, assessment of the older literature was still very variable ranging from 2.62% in some studies up to 70% in others [Tables 1–3].[11,12] The most critical question repeatedly raised was whether an instrumented fusion was warranted in the first place, and that performing fewer instrumented fusions would likely markedly reduce the largely iatrogenic disease called ASD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
REFERENCES
- 1.Ahn Y, Lee SH. Vertebroplasty for adjacent vertebral fracture following lumbar interbody fusion. Br J Neurosurg. 2011;25:104–8. doi: 10.3109/02688697.2010.508848. [DOI] [PubMed] [Google Scholar]
- 2.Aiki H, Ohwada O, Kobayashi H, Hayakawa M, Kawaguchi S, Takebayashi T, et al. Adjacent segment stenosis after lumbar fusion requiring second operation. J Orthop Sci. 2005;10:490–5. doi: 10.1007/s00776-005-0919-3. [DOI] [PubMed] [Google Scholar]
- 3.Ekman P, Möller H, Shalabi A, Yu YX, Hedlund R. A prospective randomised study on the long-term effect of lumbar fusion on adjacent disc degeneration. Eur Spine J. 2009;18:1175–86. doi: 10.1007/s00586-009-0947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein NE. Adjacent level disease following lumbar spine surgery. A review. Surg Neurol Int Spine. 2015 doi: 10.4103/2152-7806.170432. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90(2 Suppl):163–9. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 6.Ghiselli G, Wang JC, Bhatia NN, Hsu WK, Dawson EG. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am. 2004;86-A:1497–503. doi: 10.2106/00004623-200407000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Ghiselli G, Wang JC, Hsu WK, Dawson EG. L5-S1 segment survivorship and clinical outcome analysis after L4-L5 isolated fusion. Spine (Phila Pa 1976) 2003;28:1275–80. doi: 10.1097/01.BRS.0000065566.24152.D3. [DOI] [PubMed] [Google Scholar]
- 8.Gillet P. The fate of the adjacent motion segments after lumbar fusion. J Spinal Disord Tech. 2003;16:338–45. doi: 10.1097/00024720-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Hallett A, Huntley JS, Gibson JN. Foraminal stenosis and single-level degenerative disc disease: A randomized controlled trial comparing decompression with decompression and instrumented fusion. Spine (Phila Pa 1976) 2007;32:1375–80. doi: 10.1097/BRS.0b013e318064520f. [DOI] [PubMed] [Google Scholar]
- 10.Hudson WR, Gee JE, Billys JB, Castellvi AE. Hybrid dynamic stabilization with posterior spinal fusion in the lumbar spine. SAS J. 2011;5:36–43. doi: 10.1016/j.esas.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishihara H, Osada R, Kanamori M, Kawaguchi Y, Ohmori K, Kimura T, et al. Minimum 10-year follow-up study of anterior lumbar interbody fusion for isthmic spondylolisthesis. J Spinal Disord. 2001;14:91–9. doi: 10.1097/00002517-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Lee CS, Hwang CJ, Lee SW, Ahn YJ, Kim YT, Lee DH, et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18:1637–43. doi: 10.1007/s00586-009-1060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann TR, Spratt KF, Tozzi JE, Weinstein JN, Reinarz SJ, el-Khoury GY, et al. Long-term follow-up of lower lumbar fusion patients. Spine (Phila Pa 1976) 1987;12:97–104. doi: 10.1097/00007632-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Levin DA, Hale JJ, Bendo JA. Adjacent segment degeneration following spinal fusion for degenerative disc disease. Bull NYU Hosp Jt Dis. 2007;65:29–36. [PubMed] [Google Scholar]
- 15.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: Review of the literature. Spine (Phila Pa 1976) 2004;29:1938–44. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 16.Penta M, Sandhu A, Fraser RD. Magnetic resonance imaging assessment of disc degeneration 10 years after anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20:743–7. doi: 10.1097/00007632-199503150-00018. [DOI] [PubMed] [Google Scholar]
- 17.Schulte TL, Leistra F, Bullmann V, Osada N, Vieth V, Marquardt B, et al. Disc height reduction in adjacent segments and clinical outcome 10 years after lumbar 360 degrees fusion. Eur Spine J. 2007;16:2152–8. doi: 10.1007/s00586-007-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


