INTRODUCTION
The incidence of congenital heart disease among all live births in India is reportedly 0.5–0.8% of which tetralogy of Fallot (TOF) is the second most common (17.86%).[1] Paraganglioma accounts for approximately 0.1% of the hypertensive Indian population with an overall incidence of 1–2/million.[2] Both diseases are relatively uncommon and their concurrent occurrence is exceptionally rare. Only two cases of phaeochromocytoma with uncorrected TOF have been reported from India.[1,3] We have described the pertinent anaesthetic considerations in the perioperative management of surgical excision of phaeochromocytoma with unrepaired, unpalliated TOF.
CASE REPORT
A 32-year-old female weighing 40 kg with complaints of abdominal pain on and off, paroxysmal headache and palpitations since a year was scheduled for open adrenalectomy. She was a diagnosed case of TOF since childhood with past history of cyanotic spells. She had refused corrective surgery and was on oral propranolol (10 mg OD) prophylaxis. There was no past history of hospital admissions.
On physical examination, pulse rate was 90 beats/min and blood pressure (BP) was 164/104 mmHg with room air oxygen saturation (SpO2 ) of 85%. Mucous membrane cyanosis, Grade III digital clubbing and jugular venous distension were present. Systemic examination was notable for a parasternal heave, ejection systolic murmur in the pulmonary area and systolic murmur in left parasternal region.
Laboratory investigations showed haemoglobin as 17.7 g% with a haematocrit of 53.93% and routine liver and renal function tests within normal limits. Blood glucose level was normal. Arterial blood gas analysis revealed partial pressure of oxygen (PaO2 ) of 56 mmHg and arterial oxygen saturation (SaO2 ) of 85% on room air. Chest X-ray displayed cardiomegaly and a right-sided aortic arch with normal lung fields. Two-dimensional echocardiogram revealed TOF with infundibular pulmonary stenosis and a pressure gradient of 104 mmHg across the right ventricular outflow tract. Computed tomography (CT)-scan abdomen showed a 3.8 cm × 4.6 cm × 4.4 cm well-defined, lobulated, enhancing retroperitoneal mass displacing renal vessels and indenting the upper pole of right kidney. Histopathological examination of CT-guided biopsy specimen confirmed it to be a neuroendocrine tumour. Her plasma free normetanephrine levels (4665.3 pg/mL) were significantly raised.
The patient was started on tablet prazosin 4 mg/day, which was gradually increased to 8 mg/day over next 10 days under supervision. On the immediate pre-operative day, we performed right internal jugular vein cannulation under local anaesthesia for overnight hydration with central venous pressure (CVP) monitoring. In the operation room, monitors as per American Society of Anaesthesiologists' standards were attached. The patient was premedicated with intravenous fentanyl 150 μg, midazolam 1 mg and 0.5 μg/kg/h dexmedetomidine infusion over 20 min. Lumbar epidural catheter was inserted at T12–L1 level and fixed at 6 cm within. Right femoral arterial cannulation was performed after injecting local anaesthesia. General anaesthesia was induced with etomidate 5 mg intravenously and tracheal intubation facilitated with rocuronium 40 mg. The patient tolerated anaesthesia induction well with minimal haemodynamic fluctuations.
Anaesthesia was maintained with dexmedetomidine infusion at 0.25 μg/kg/h and isoflurane in oxygen and air. Muscle relaxation was maintained with intermittent top ups of rocuronium. Epidural infusion of 0.25% bupivacaine was titrated between 4 and 6 mL/h. CVP was maintained at 6–8 cm H2O by adequate fluid infusion. The haemodynamic perturbations [Figure 1] during tumour dissection when BP shot up to 210/110 mmHg were controlled by adjusting the anaesthetic depth, sodium nitroprusside (SNP) infusion titrated at 0.5–5 μg/kg/min and 0.5 mg esmolol boluses. After ligation of adrenal vessels, BP dropped to 90/64 mmHg and all hypotensive agents were discontinued. This was managed with fluid boluses and noradrenaline infusion titrated at 0.05–0.1 μg/kg/min to maintain systolic BP above 100 mmHg.
Figure 1.
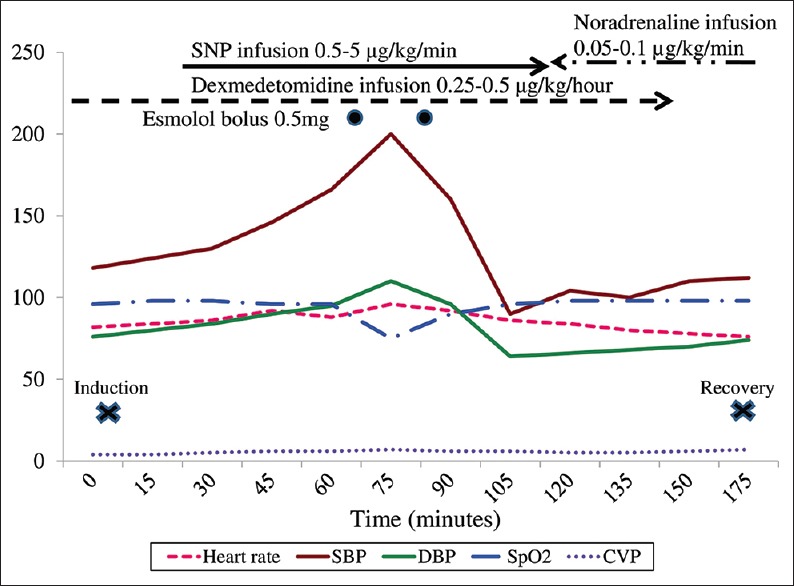
Intraoperative haemodynamic fluctuations
The total duration of surgery was 150 min and blood loss was 500 mL. Intraoperative sugar was within normal range. At the end neuromuscular blockade was reversed, trachea extubated and patient shifted to the recovery room. Post-operative analgesia comprised of epidural boluses of 0.125% bupivacaine with 2 μg/mL fentanyl 6–8 hourly. The immediate post-operative period was uneventful except for a hypoglycaemic episode the next morning, despite 4–6 hourly sugar monitoring. Blood sugar dropped to 60 mg% and SpO2 became 75% as the patient became unresponsive in the post-anaesthesia care unit. This was managed promptly with 100 mL 25% dextrose, 50 mEq sodium bicarbonate and 0.5 mg esmolol boluses intravenously. Thereafter, the patient made an uneventful recovery and was discharged on 10th post-operative day.
DISCUSSION
Very few case reports describing the perioperative anaesthetic management for a patient with phaeochromocytoma and uncorrected TOF exist in literature. Balakrishnan et al. reported successful surgeries for both; phaeochromocytoma in the first sitting followed by corrective repair of TOF 5 days later.[3]
The first international symposium on phaeochromocytoma advocates that α-adrenergic blockade should be initiated preoperatively to protect against haemodynamic instability.[4] Our patient was already on propranolol for prevention of infundibular spasm. We added α1-blocker prazosin which has advantages over phenoxybenzamine such as shorter duration of action, decreased post-operative hypotension and lesser reflex tachycardia. In the setting of uncorrected TOF and significant pulmonary hypertension, α-blockade and ensuing systemic afterload reduction could decrease pulmonary blood flow and worsen the existing cyanosis.
Normalisation of intravascular volume and BP prior to surgery assisted in keeping the patient's haemodynamic status stable during tumour resection. The open approach was selected for tumour excision as intra abdominal insufflation and pneumoperitoneum could cause a deleterious rise in pulmonary hypertension, deteriorating the shunt fraction of TOF. Establishment of central venous and arterial cannulation, as well as an epidural catheter, was done under local anaesthesia with mild sedation by an experienced anaesthesiologist. The foremost anaesthetic goals were to avoid sympathetic stimulation and to maintain systemic vascular resistance (SVR) to pulmonary vascular resistance (PVR) ratio near pre-operative value. Factors which can raise PVR such as hypoxia, hypercarbia, hypothermia, acidaemia and pain were avoided while defending SVR to near normal.
Dexmedetomidine is a novel agent for controlling hypertensive surges and reducing anaesthetic requirements by activation of presynaptic α2 receptors and subsequently decreased norepinephrine release.[5,6] Etomidate and rocuronium were chosen for their cardiovascular stability, minimal histamine release and insignificant fall in SVR or sympathetic stimulation. Vasodilators which are routinely used to control hypertensive surges during tumour handling can hamper oxygenation in TOF, so we preferred using ultrashort-acting agents such as SNP and esmolol. The hypotension seen after ligation of adrenal veins was effectively controlled by CVP guided titrated fluid as the first line of management. Vasopressors are ineffective in hypovolemic state and form the second line of treatment.[7] The combination of dexmedetomidine, isoflurane, epidural analgesia in titrated doses along with judicious CVP guided fluid therapy and ultrashort-acting vasodilators SNP and esmolol helped us achieve safe and effective anaesthesia in our patient.
Post-operatively, patients are highly vulnerable to develop hypotensive or hypoglycaemic episodes which can trigger cyanotic spells. Our patient had a hypoglycaemic episode the next day which can be attributed to the drastic rise in insulin levels after tumour resection owing to uninhibited stimulation of beta cells of the pancreas.[8]
CONCLUSION
Anaesthetic management of phaeochromocytoma with uncorrected TOF poses a challenge and necessitates a multidisciplinary team approach. Careful selection of appropriate anaesthetic agents available in our armamentarium plays a crucial role in proper perioperative management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Singh GD, Anuradha S, Sethi P, Singla S, Saran RK, Dewan R. Pheochromocytoma and tetralogy of Fallot: Coincidence or a rare association? Asian Cardiovasc Thorac Ann. 2014:pii: 0218492314545499. doi: 10.1177/0218492314545499. [DOI] [PubMed] [Google Scholar]
- 2.Krishnappa R, Chikaraddi SB, Arun HN, Deshmane V. Pheochromocytoma in Indian patients: A retrospective study. Indian J Cancer. 2012;49:188–93. doi: 10.4103/0019-509X.98951. [DOI] [PubMed] [Google Scholar]
- 3.Balakrishnan G, Ravikumar R, Rao S, Balakrishnan KR. Tetralogy of Fallot with pheochromocytoma: An unusual therapeutic challenge. Asian Cardiovasc Thorac Ann. 2013;21:464–6. doi: 10.1177/0218492312456979. [DOI] [PubMed] [Google Scholar]
- 4.Rich BS, Moo TA, Mark S, Scognamiglio T, Pecker MS, Sobol I, et al. Sympathetic paraganglioma in a patient with unrepaired tetralogy of Fallot: A case report and review of the literature. J Clin Endocrinol Metab. 2013;98:7–12. doi: 10.1210/jc.2012-1969. [DOI] [PubMed] [Google Scholar]
- 5.Domi R, Laho H. Management of pheochromocytoma: Old ideas and new drugs. Niger J Clin Pract. 2012;15:253–7. doi: 10.4103/1119-3077.100616. [DOI] [PubMed] [Google Scholar]
- 6.Dias R, Dave N, Garasia M. Dexmedetomidine for anaesthetic management of phaeochromocytoma in a child with von Hippel-Lindau type 2 syndrome. Indian J Anaesth. 2015;59:319–21. doi: 10.4103/0019-5049.156891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turkistani A. Anesthetic management of pheochromocytoma - A case report. Middle East J Anaesthesiol. 2009;20:111–3. [PubMed] [Google Scholar]
- 8.Bajwa SS, Bajwa SK. Implications and considerations during pheochromocytoma resection: A challenge to the anesthesiologist. Indian J Endocrinol Metab. 2011;15(Suppl 4):S337–44. doi: 10.4103/2230-8210.86977. [DOI] [PMC free article] [PubMed] [Google Scholar]


