Abstract
Background & objectives:
There is limited information available about the drug resistance patterns in extrapulmonary tuberculosis (EPTB), especially from high burden countries. This may be due to difficulty in obtaining extrapulmonary specimens and limited facilities for drug susceptibility testing. This study was undertaken to review and report the first and second-line anti-TB drug susceptibility patterns in extrapulmonary specimens received at the National Institute for Research in Tuberculosis (NIRT), Chennai, India, between 2005 and 2012.
Methods:
Extrapulmonary specimens received from referring hospitals were decontaminated and cultured using standard procedures. Drug susceptibility testing (DST) for Mycobacterium tuberculosis was done by absolute concentration or resistance ratio methods for the first and the second line anti-TB drugs.
Results:
Between 2005 and 2012, of the 1295 extrapulmonary specimens, 189 grew M. tuberculosis, 37 (19%) cases were multidrug resistant (MDR) while one was extensively drug resistant (XDR). Specimen-wise MDR prevalence was found to be: CSF-10 per cent, urine-6 per cent, fluids and aspirates-27 per cent, pus-23 per cent, lymph nodes-19 per cent. Resistance to isoniazid and ethionamide was found to be high (31 and 38%, respectively).
Interpretation & conclusions:
Drug resistance including MDR-TB was observed in a significant proportion of extrapulmonary specimens referred for DST. Access to culture and DST for extrapulmonary specimens should be expanded. Guidelines for MDR-TB management should have explicit sections on extra-pulmonary tuberculosis and training on laboratory techniques is urgently required.
Keywords: Extrapulmonary tuberculosis, Mycobacterium tuberculosis, MDR-TB, XDR-TB, drug resistance
India bears 24 per cent of the global burden of tuberculosis (TB) and is estimated to have 2 - 2.3 million new cases each year1. Of the 1.5 million cases reported to the Revised National Tuberculosis Control Programme (RNTCP), 10-15 per cent are extrapulmonary tuberculosis (EPTB), mostly TB lymphadenitis and pleural effusion2,3. EPTB generally constitutes 15-20 per cent of all cases of tuberculosis among immune-competent individuals and up to 50 per cent in HIV-infected patients4. Depending on the site of disease, specimens may be difficult to obtain and the lesions are usually paucibacillary; hence bacteriologic confirmation is the exception rather than the rule. In general, extrapulmonary tuberculosis (TB lymph node, pleural effusion, abdominal TB, skin TB, etc.) is treated with the same regimens that are used for pulmonary disease, with clinical trials confirming the efficacy of short-course regimens5. Exceptions are bone and joint TB and tuberculous meningitis, for which there are inadequate data to support 6-month therapy; thus 9-12 months of treatment is recommended5.
There is limited information in the literature regarding prevalence of drug resistance in EPTB especially from high burden settings like India6. The reasons for this include the difficulty in obtaining diagnostic specimens and the limited number of laboratories in the country having the facility to perform culture and drug susceptibility testing (DST) for Mycobacterium tuberculosis from extrapulmonary specimens. Clinical symptoms are the main prognostic indicators in the case of EPTB and physicians suspect drug resistance only after failure or non-response to first line therapy.
However, capacity for identification of drug resistance has rapidly expanded in 2013. In 2013 alone, 20,763 patients were diagnosed with MDR-TB in India, almost all with pulmonary disease7. State-wide drug resistance prevalence surveys conducted in Gujarat and Tamil Nadu found rates of primary drug resistance in pulmonary TB ranging from 10-15 per cent for isoniazid and 2-3 per cent for MDR-TB8. Among previously treated patients, MDR-TB occurs in approximately 12-17 per cent of patients8. Reported MDR-TB among new cases in the state of Gujarat (2007-2008), Maharashtra (2008) and Andhra Pradesh (2009) were 2.4, 2.7 and 1.8 per cent, respectively9. National Institute for Research in Tuberculosis (NIRT) is a national and supranational reference laboratory for mycobacteriology and receives specimens from different parts of the country for culture and DST. A large number of extrapulmonary specimens are received and subjected to smear, culture, drug susceptibility testing and species identification. Here, we report drug susceptibility patterns observed in extrapulmonary specimens received in our laboratory during the period 2005 to 2012.
Material & Methods
Study population: Extrapulmonary specimens selectively referred by physicians for drug susceptibility testing, including patients who were not responding to treatment, were processed using standard methods10,11. A total of 1248 extrapulmonary specimens were from Chennai, 15 samples from Puducherry, three from Bengaluru, four from Vellore, three from Goa, seven from Gangtok, six from Bhubaneswar and nine from Tirupathi. Two hundred forty pulmonary specimens other than sputum, including gastric aspirates and bronchial wash, were received during the same period. Minimal patient details were provided by the referring physicians and no treatment or follow up data were available.
Specimen processing: Specimens were accepted only if properly transported11. Briefly, specimens were centrifuged at 3000 g for 15 min and decontaminated using 4 per cent sulphuric acid. Same procedure was followed for tissues after homogenization by cutting into small pieces and grinding with tissue grinder. In the case of cerebrospinal fluid (CSF) the specimen was inoculated before the decontamination onto Lowenstein-Jensen (L-J) medium in addition to the above procedure. The processed sediment was used to prepare a smear for acid fast bacilli (AFB) staining by auramine and was also inoculated on two slopes each of plain L-J medium and L-J with sodium pyruvate as well as in selective liquid Kirchner's medium. The slopes were read every week for eight weeks. Further, after six weeks of incubation, the liquid culture was sub-cultured on L-J medium after decontamination by modified Petroff's method12 and the L-J slants were read every week, thereafter for another eight weeks. The cultures were identified as M. tuberculosis by niacin production and observing the growth pattern in p-nitrobenzoic acid containing L-J slants. Non-tuberculous mycobacteria (NTM) were identified using Runyon classification12 and speciated by HPLC12, but this was not performed for all the NTMs due to non availability of resources.
Drug susceptibility testing: The DST was performed for streptomycin (SM) by resistance ratio method and for isoniazid (INH), rifampicin (RMP), ethambutol (EMB), kanamycin (KM), ethionamide (ETH) and ofloxacin (OFX) by absolute concentration methods11. Standard definitions of drug resistance were used (Table I)13,14.
Table I.
Drugs and the standard definition of resistance
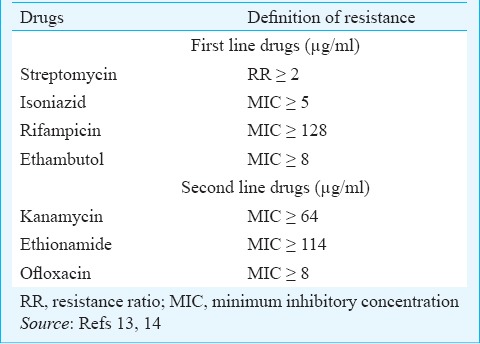
The strains were then classified as follows: (i) SHRE sensitive: Isolate susceptible to SM, INH, RMP, EMB; (ii) Mono-resistance: Isolate resistant to only one drug; (iii) MDR: Resistance to at least INH and RMP; (iv) XDR: Resistance to INH, RMP, KM and OFX; and (v) Polyresistance: All other patterns, showing resistance to at least two drugs, but not in any of the above combinations
Results
A total of 1295 extrapulmonary specimens were received between October 2005 and May 2012. Of these, smear was performed on 1015 specimens (urine specimens excluded for smear), 342 were smear positive while 189 specimens were culture positive for M. tuberculosis and these were processed for DST. An additional 72 specimens yielded NTM and these specimens were excluded from further analysis. Seventy eight (41%; 95% CI 34-48) M. tuberculosis isolates were fully susceptible to first line anti-TB drugs tested, whereas 61 (37.6%; 95% CI 31.4-43.9) were susceptible to all the drugs tested (SM, INH, RMP, EMB, KM, ETH and OFX). Resistance to SM, INH, RMP, EMB, KM, ETH and OFX individually or in combination with other drugs was 17.7, 30.7, 24.6, 14.2, 2.1, 37.6 and 15.5 per cent, respectively.
As listed in Table II, of the 189 isolates tested, one was an XDR-TB isolate (0.4%), whereas 37 isolates (19%; 95% CI 13.4-24.6) were multidrug resistant. Mono resistance to OFX, RMP, INH and SM was seen in 9 (4.8%), 5 (2.6%), 3 (1.6%) and 2 (1%) cases, respectively. Nine patients (17.5%) had MDR-TB in respiratory specimens other than sputum (Table II).
Table II.
Drug susceptibility patterns of M. tuberculosis identified from different pulmonary and extrapulmonary specimens. Percentage resistance is out of culture positive specimens only
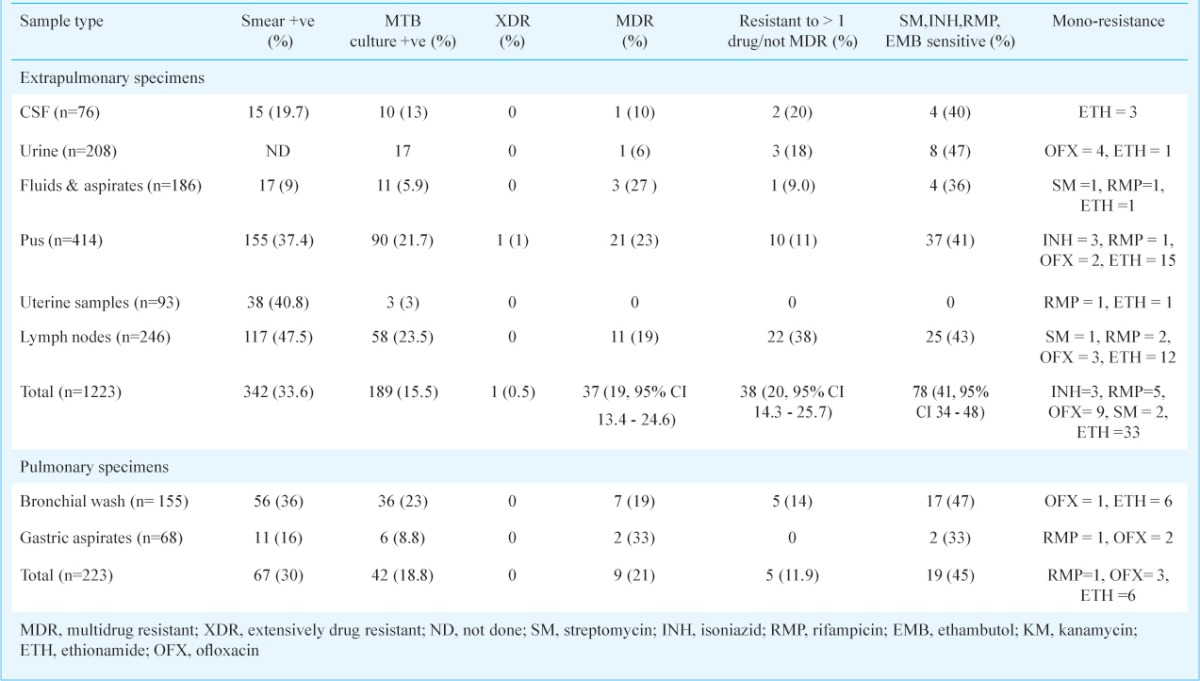
Analyzed by age group, 67 (51.5%) culture positive patients were in the age group of 15 to 45 yr, 43 (33%) were less than 15 yr and 20 (15.35%) were over 45 yr of age. Among patients <15 yr, the proportion of specimens that had multidrug resistance or RMP mono-resistance was 28 per cent, in the 15-45 yr age group it was 39 per cent and in patients >45 yr it was 30 per cent.
Of the various extrapulmonary specimens received, pus (from various sites), lymph node aspirates, tissue biopsies and body fluids made up the largest group. MDR-TB ranged from 6 per cent in urine to 27 per cent in exudates, the average being 20 per cent. Another 18.6 per cent of specimens had poly drug resistance, which excludes RMP resistance. Of the 93 endometrial specimens, three were M. tuberculosis positive and 27 were NTM isolates (Data not shown). Of the 58 cases with M. tuberculosis isolated from lymph nodes, 11 (19%) were MDR, 25 (43%) were susceptible to SM, INH, RMP, EMB and 22 (38%) were resistant to one or more drugs (2 and 3 were R and OFX mono resistant, respectively). Similarly, a total of 90 pus samples yielded M. tuberculosis isolates. Among these, one was found to harbour XDR, while 21 (23%) were MDR, 37 (41%) were susceptible to SM, INH, RMP, EMB and 10 (11%) were resistant to one or more drugs (3, 1 and 2 were INH, RMP and OFX mono resistant, respectively).
Discussion
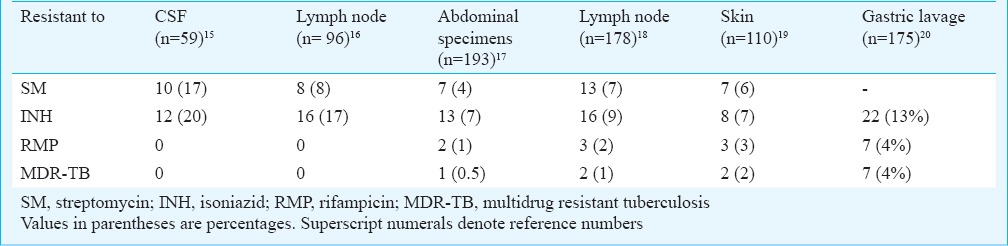
Controlled clinical trials for extrapulmonary tuberculosis conducted in our centre reported isoniazid resistance of < 20 per cent and MDR < 4 per cent till 2008 (Table III)15,16,17,18,19,20. In these trials mostly patients with little or no prior exposure to anti-TB drugs were enrolled, which accounted for the relatively low percentage of drug resistance. In the present retrospective analysis our finding of high rates of drug resistance in most specimen types received is of concern, with less than half of isolates showing susceptibility to first line drugs. A study conducted by Maurya et al6 in northern India reported MDR-TB of 13.4 per cent among EPTB patients, with an overall resistance to anti-TB drugs of 40 per cent. The isolates were of diverse nature in the Mumbai population with a preponderance of Beijing genotype as reported, implying an urgent need for better epidemiological surveillance21. Vadwai et al21 also reported MDR-TB in 29 per cent among extrapulmonary specimens.
Table III.
Drug resistance patterns in various extrapulmonary TB studies undertaken at NIRT
L-J-based culture positivity for M. tuberculosis for extrapulmonary samples was 10.5 and 9 per cent in studies conducted in Nepal and Delhi22,23. In our analysis, we reported positive cultures in 15.5 per cent (21% including atypical mycobacteria). The higher isolation rate in our laboratory might be due to the processing methods and use of multiple media consisting of plain L-J, L-J with sodium pyruvate and liquid Kirschner ‘ s medium.
In India, EPTB forms 10-15 per cent of all TB, mostly TB lymphadenitis and pleural effusion3. Patients with extrapulmonary manifestations need specialized investigations and the diagnosis is usually based upon clinical, radiographic or histopathological findings, rather than bacteriologic evidence. Therefore, there are very few reports available on drug susceptibility patterns of EPTB as these patients are usually not included in DR surveys, which focus mainly on pulmonary TB.
The treatment history of the patients in our study was not known. Physicians often send the samples of chronically ill patients not responding to therapy and so there is a potential bias in the sampling, which could explain the high rates of MDR among the samples analysed. Earlier, in a similar analysis of referred sputum specimens collected between 2001 and 2004 in this centre, 53 per cent were identified as having MDR-TB, 32.7 per cent had resistance to ETH, 16.4 per cent to OFX and 11.3 per cent to KM; 4.6 per cent were XDR-TB24. Surveillance of drug resistance in the State of Gujarat in India showed that 17.4 per cent of M. tuberculosis isolates were MDR and 37 per cent had any INH resistance (including mono-resistance) among previously treated pulmonary tuberculosis patients8. In Maharashtra and Andhra Pradesh 14 and 11.8 per cent of previously treated patients had MDR-TB, respectively9. Pawar et al25 detected 25 MDR cases among 238 patients with culture confirmed TB spine. These rates are comparable to our findings in the present report (19% MDR-TB and 30% any INH resistance). Further resistance to ETH (37.6%) was found to be high compared to other drugs and was similar to INH. This could be because the presence of mutations in inhA coding region which can lead to the development of cross-resistance with ETH, though this was not tested in this study26.
In the present analysis, all specimen types showed similar rates of drug resistance, except urine, which had lower MDR but a higher rate of fluoroquinolone resistance, which could be due to the frequency of use of this class of antibiotics for urinary tract infections. Irrational use of antibiotics by the general physicians, self medication or non-compliance by patients may be some of the reasons for the high rates of drug resistance. It is important to offer DST for patients with EPTB, using criteria similar to what is now recommended for pulmonary TB (Programmatic Management of Drug Resistant TB guidelines-PMDT)27. Rapid DST methods are now available and it is important that all TB laboratories be equipped to process EPTB specimens which will enable these patients to access MDR-TB treatment, if required.
Another interesting fact was that smear positivity was more common than culture positivity in all the extrapulmonary samples; only 172 of the cultures were positive for M. tuberculosis out of 342 positive for AFB by smear microscopy (excluding culture positives from urine, as no smears were made for urine samples). Sinha et al28 reported a rate of 39.8 and 27.9 per cent smear and culture positivity, respectively from fine needle aspirates, similar to our findings. Presence of dead bacilli or viable but non-culturable forms could be the reason for the lower culture positivity rate. Many of these patients may have been on treatment at the time specimens were sent for culture, explaining this phenomenon.
The main limitation of this study was inherent bias due to the fact that these specimens were selectively referred by physicians for drug susceptibility testing. Hence, these data cannot be used to project drug resistance rates in the general extrapulmonary TB population as a whole or to make any conclusions about risk factors. Our findings are important, however, because these underline the fact that drug resistant TB especially MDR-TB affects all body sites and that physicians need to be aware of this possibility. It would be worthwhile doing a more systematic study of drug resistance in EPTB, especially correlating it to demographic, clinical and other risk factors.
Footnotes
Conflicts of Interest: None.
References
- 1.World Health Organization. Global Tuberculosis Report 2014. [WHO/HTM/TB/2014.08] 2014. [accessed on July 22, 2014]. Available from: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf .
- 2.RNTCP. Response to challenges of drug resistant TB in India January 2012 (Update) [accessed on July 22, 2014]. Available from: http://www.tbcindia.nic.in/pdfs/RNTCP%20Response%20DR%20TB%20in%20India%20-%20Jan%202012%20update.pdf .
- 3.Wares F, Balasubramanian R, Mohan A, Sharma SK. Extrapulmonary tuberculosis: management and control. In: Agarwal SP, Chauhan LS, editors. Tuberculosis control in India. New Delhi: Directorate General of Health Services. Ministry of Health and Family Welfare; 2005. pp. 95–114. [Google Scholar]
- 4.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]
- 5.4th ed. Geneva, Switzerland: WHO; 2010. World Health Organization (WHO). Treatment of tuberculosis: guidelines. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 6.Maurya AK, Kant S, Nag VL, Kushwaha RA, Dhole TN. Trends of anti-tuberculosis drug resistance pattern in new cases and previously treated cases of extrapulmonary tuberculosis cases in referral hospitals in northern India. J Postgrad Med. 2012;58:185–9. doi: 10.4103/0022-3859.101379. [DOI] [PubMed] [Google Scholar]
- 7.Central TB Division. TB India 2014, Revised National TB Control Programme, Annual Status Report. 2014. [accessed on October 22, 2015]. Available from: http://www.tbcindia.nic.in/pdfs/tb%20India%202014.pdf .
- 8.Ramachandran R, Nalini S, Chandrasekar V, Dave PV, Sanghvi AS, Wares F, et al. Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis. 2009;13:1154–60. [PubMed] [Google Scholar]
- 9.Central TB Division. National Scale-up Plan for the Programmatic Management of Drug-Resistant Tuberculosis (PMDT) 2011-2012. [accessed on July 22, 2014]. Available from: http://tbcindia.nic.in/pdfs/National%20PMDT%20Scale-up%20Plan%20-%20India%20-%202011-12.pdf .
- 10.Geneva, Switzerland: WHO; 1998. World Health Organization (WHO). Laboratory services in tuberculosis control. WHO/TB/98.258. [Google Scholar]
- 11.Mitchison DA, Aber VR. Culture of specimens other than sputum for mycobacteria. J Clin Pathol. 1974;27:883–7. doi: 10.1136/jcp.27.11.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute for Research in Tuberculosis, Department of Bacteriology. 2010. [accessed on July 22, 2014]. Available from: http://ijmr.org.in/contributors.asp .
- 13.Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 14.Sulochana S, Rahman F, Paramasivan CN. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J Chemother. 2005;17:169–73. doi: 10.1179/joc.2005.17.2.169. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran P, Duraipandian M, Nagarajan M, Prabhakar R, Ramakrishnan CV, Tripathy SP. Three chemotherapy studies of tuberculous meningitis in children. Tubercle. 1986;67:17–29. doi: 10.1016/0041-3879(86)90028-0. [DOI] [PubMed] [Google Scholar]
- 16.Jawahar MS, Sivasubramanian S, Vijayan VK, Ramakrishnan CV, Paramasivan CN, Selvakumar V, et al. Short course chemotherapy for tuberculous lymphadenitis in children. BMJ. 1990;301:359–62. doi: 10.1136/bmj.301.6748.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasubramanian R, Nagarajan M, Balambal R, Tripathy SP, Sundararaman R, Venkatesan P, et al. Randomised controlled clinical trial of short course chemotherapy in abdominal tuberculosis: a five-year report. Int J Tuberc Lung Dis. 1997;1:44–51. [PubMed] [Google Scholar]
- 18.Jawahar MS, Rajaram K, Sivasubramanian S, Paramasivan CN, Chandrasekar K, Kamaludeen MN, et al. Treatment of lymph node tuberculosis - a randomized clinical trial of two 6-month regimens. Trop Med Int Health. 2005;10:1090–8. doi: 10.1111/j.1365-3156.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 19.Umapathy KC, Begum R, Ravichandran G, Rahman F, Paramasivan CN, Ramanathan VD. Comprehensive findings on clinical, bacteriological, histopathological and therapeutic aspects of cutaneous tuberculosis. Trop Med Int Health. 2006;11:1521–8. doi: 10.1111/j.1365-3156.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 20.Swaminathan S, Datta M, Radhamani MP, Mathew S, Reetha AM, Rajajee S, et al. A profile of bacteriologically confirmed pulmonary tuberculosis in children. Indian Pediatr. 2008;45:743–7. [PubMed] [Google Scholar]
- 21.Vadwai V, Shetty A, Supply P, Rodrigues C. Evaluation of 24-locus MIRU-VNTR in extrapulmonary specimens: study from a tertiary centre in Mumbai. Tuberculosis (Edinb) 2012;92:264–72. doi: 10.1016/j.tube.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Gurung R, Bhattacharya SK, Pradhan B, Gurung S, Singh YI. Phenotypic characterisation and drug sensitivity testing of mycobacteria isolated from extra-pulmonary tuberculosis. Kathmandu Univ Med J (KUMJ) 2010;8:57–61. doi: 10.3126/kumj.v8i1.3223. [DOI] [PubMed] [Google Scholar]
- 23.Sachdeva R, Gadre DV, Talwar V. Characterisation & drug susceptibility patterns of extrapulmonary mycobacterial isolates. Indian J Med Res. 2002;115:102–7. [PubMed] [Google Scholar]
- 24.Paramasivan CN, Rehman F, Wares F, Sundar Mohan N, Sundar S, Devi S, et al. First- and second-line drug resistance patterns among previously treated tuberculosis patients in India. Int J Tuberc Lung Dis. 2010;14:243–6. [PubMed] [Google Scholar]
- 25.Pawar UM, Kundnani V, Agashe V, Nene A. Multidrug-resistant tuberculosis of the spine--is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine. 2009;34:E806–10. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 26.Schaaf HS, Victor TC, Venter A, Brittle W, Jordaan AM, Hesseling AC, et al. Ethionamide cross- and co-resistance in children with isoniazid-resistant tuberculosis. Int J Tuberc Lung Dis. 2009;13:1355–9. [PubMed] [Google Scholar]
- 27.Central TB Division. Guidelines on Programmatic Management of Drug Resistant TB (PMDT) in India. 2012. [accessed on July 22, 2014]. Available from: http://www.tbcindia.nic.in/pdfs/Guidelines%20for%20PMDT%20in%20India%20-%20May%202012.pdf .
- 28.Sinha SK, Chatterjee M, Bhattacharya S, Pathak SK, Mitra RB, Karak K, et al. Diagnostic evaluation of extrapulmonary tuberculosis by fine needle aspiraton (FNA) supplemented with AFB smear and culture. J Indian Med Assoc. 2003;101:588, 590–1. [PubMed] [Google Scholar]


