Abstract
Background & objectives:
Multidrug-resistant tuberculosis (MDR-TB) is a public health problem of great significance in India. In the present study an attempt was made to analyse the progression of MDR-TB pattern during a course of 13 years (2000-2012) among the patient population at a tertiary care centre in New Delhi, India.
Methods:
Mycobacterial isolates obtained on Lowenstein-Jensen (L-J) medium/BacT/ALERT 3D were identified using AccuProbe molecular identification system, routine biochemical tests or GenoType Mycobacteria CM. Antimycobacterial susceptibility testing was performed using resistance ratio method on L-J medium (2000-2004) and one per cent proportion method on BacT/ALERT 3D system (2005-2012).
Results:
Of the total 14,849 samples subjected to mycobacterial culture, 6569 pulmonary and 8280 extrapulmonary, 2364 were detected positive for mycobacteria. The average percentage positivity rate was 15.9 per cent (18.9 and 13.6% in case of pulmonary and extrapulmonary samples, respectively). Our study revealed a significant increase (P<0.001) in multidrug resistance by 12 per cent (4.7% in 2000 to 19.8% in 2012). MDR-TB was more in case of pulmonary (28.2%) than extrapulmonary (11.6%) TB (P<0.001). Only 6.5 per cent (154) of mycobacterial isolates were non-tuberculous mycobacteria and rapid growers represented by Mycobacterium fortuitum and M. abscessus were the most commonly isolated species.
Interpretation & conclusions:
Increase in prevalence of MDR-TB by 12 per cent in the past 13 years is alarming. Policy modifications may have to be done to strengthen the existing TB control programmes to encourage contact tracing and culture and drug susceptibility testing for all smear positive pulmonary cases to ensure early and appropriate therapy.
Keywords: Extrapulmonary TB, MDR-TB, non-tuberculous mycobacteria
India is one of the highest tuberculosis burden countries in the world and is likely to harbour the largest number of multi-drug resistant tuberculosis (MDR-TB) cases. Globally, there were an estimated 9.0 million incident cases and 1.5 million deaths due to tuberculosis in 20131. Of these, the largest number of cases were from India and accounted for an estimated one quarter of all TB cases worldwide. India, China and Russian Federation together account for more than half of the estimated global burden of new MDR-TB cases1.
The phenomenon of MDR-TB emerged as a clinical entity in the early 1990s after a couple of decades of widespread use of rifampicin. Ever since, the prevalence of MDR-TB is increasing throughout the world2. Currently, MDR-TB accounts for 3.5 per cent among new cases and 20.5 per cent among previously treated cases, globally1. But countrywide surveillance data are lacking from India particularly that of extrapulmonary TB. Observations from accredited mycobacteriology laboratories from India suggest that the prevalence of MDR-TB is <3 per cent in new cases and 15-30 per cent among previously treated cases3.
The inadequate ability to rapidly diagnose MDR-TB in developing countries remains the major obstacle in the control of the disease. In the absence of countrywide surveillance studies, MDR-TB is likely to go under-reported in our country. In this study, an attempt was made to analyse the pattern of progression of drug resistance in tuberculosis over a period of 13 years in a tertiary care centre in New Delhi, India, utilizing various techniques accepted as the gold standard in the diagnosis of the drug resistant tuberculosis.
Material & Methods
In this retrospective study, conducted in the department of Microbiology and Immunology, Sir Ganga Ram Hospital, New Delhi, mycobacterial culture and drug susceptibility data were analysed for the past 13 years (2000-2012). The study population included all consecutive patients who had submitted sample for mycobacterial culture. Both admitted patients as well as patients attending out patient department were included. Repeat samples of the same patient, whether from the same site or a different site were excluded. Most of the patients belonged to Delhi and the National Capital Region, which included the neighbouring towns of Delhi from Haryana, Uttar Pradesh and Rajasthan. The specimens processed included pulmonary and extrapulmonary samples including sputum, endotracheal aspirate, bronchoalveolar lavage fluid, pleural, peritoneal and pericardial fluids, synovial fluid, CSF, tissue samples, pus, fine needle aspiration cytology (FNAC) samples from lymph nodes, blood and bone marrow and gastric aspirates. The study protocol was approved by the hospital ethics committee.
Sample processing: All respiratory samples, urine and tissue samples were subjected to digestion and decontamination by N-acetyl-L-cystine NaOH method4. Other samples from sterile sites were subjected to decontamination only if a Gram stain or routine bacterial culture showed the presence of any organism, otherwise processed directly. Blood (5 ml) and bone marrow specimens were inoculated directly in to the culture bottle (Lowenstein-Jensen medium or BacT/ALERT MB bottle) under aseptic precautions (after disinfecting the top of BacT/ALERT MB bottle with an alcohol swab) as per the manufacturer's instructions5. All samples were subjected to culture on L-J medium till 2004 and by BacT/ALERT 3D (bioMerieux, Durham, North Carolina, USA) and L-J medium from 2005 onwards as per the standard protocol4.
Identification of mycobacteria: Positive growth in either medium was identified as M. tuberculosis using AccuProbe molecular identification system (GenProbe, San Diego, California, USA)4. For the identification of non-tuberculous mycobacteria (NTM), AccuProbe identification test (identifies M. tuberculosis, M. avium, M. intracellulare, M. gordonae, M. avium complex and M. kansasii), phenotypic traits (e.g. growth on Mac Conkey agar and pigmentation) along with routine biochemical tests like catalase test (68°C), niacin test and nitrate test (for rapid growers) were used till 20084. AccuProbe culture identification test was performed as described by the manufacturer. Briefly, one loopful of growth from L-J medium or 1.5-2 ml of positive broth from BacT/ALERT MP bottle, after centrifugation and pelleting, was sonicated in tubes containing glass beads and lysing reagents in a sonication bath for 15 min. It was subsequently heated at 95°C for 10 min. Then, 100 µl of the lysates was incubated for 15 min at 60°C in a water bath with the lyophilized DNA probe, and 300 µl of a selection reagent was added. The tube was mixed well, further incubated at 60°C for 5 min, and kept at room temperature for 5 min. The assay results were read on a luminometer (Leader 50; Gen-Probe). Results were expressed as relative light units (RLU). Samples producing signals greater than or equal to 30,000 RLU were considered positive.
From 2009 onwards GenoType Mycobacteria CM (Hain Lifescience GmbH, Nehren, Germany) was introduced. This assay permits the simultaneous molecular genetic identification of the M. tuberculosis complex and 14 of the most common NTM species (M. avium ssp., M. chelonae, M. abscessus, M. fortuitum, M. gordonae, M. intracellulare, M. scrofulaceum, M. interjectum, M. kansasii, M. malmoense, M. peregrinum, M. marinum/M. ulcerans, the M. tuberculosis complex and M. xenopi) from cultivated samples. The GenoType Mycobacteria CM assay was performed as recommended by the manufacturer6. Briefly, the amplification mixture consisted of 35 µl primer nucleotide mix, 5 µl of PCR buffer with, 2 µl of 25 mM MgCl2, 1 U of HotStar Taq DNA polymerase from Qiagen, 3 µl of molecular biology grade water and 5 µl DNA supernatant in a final volume of 50 µl. Amplification was done in a thermal cycler (MyCycler, Bio-Rad Laboratories, USA) using the amplification profile: denaturation of 15 min at 95°C, followed by 10 cycles of 30 sec at 95°C and 2 min at 58°C, 20 cycles of 25 sec at 95°C, 40 sec at 53°C and 40 sec at 70°C and the extension step of 8 min at 70°C. Hybridization was performed using a pre-programmed TwinCubator (Hain Lifescience GmbH, Nehren, Germany). After denaturation, the biotin labelled amplicons were hybridized to the single stranded membrane-bound probes. After a stringent washing, as streptavidin-alkaline phosphate conjugate was added to the strips, an alkaline phosphatase mediated staining reaction was observed as bands where the amplicon and the probe hybridized.
The NTM isolates were reported in accordance to American Thoracic Society guidelines for respiratory isolates7 and after correlating with clinical, radiological and histopathological evidences and repeat isolation of the same organism wherever possible, in case of extrapulmonary samples, to limit the reporting of only pathogenic isolates.
Drug susceptibility testing: Drug susceptibility testing was done using the resistance ratio method8 on L-J media during 2000-2004. From 2005 onwards susceptibility testing was carried out by the BacT/ALERT 3D system using one per cent proportion method according to manufacturer's instructions9. Briefly, the drug stock solution (0.5 ml) and 0.5 ml of the suspension containing M. tuberculosis were added to a BacT/ALERT MP bottle. For each specimen tested, one direct growth control which did not contain any drugs and one proportional growth control (10-2 of growth control) were included. Drug susceptibility testing sets were entered into the BacT/ALERT instrument and continuously monitored until a positive or negative result was obtained. The critical concentrations used were 0.1µg/ml for isoniazid and 1 µg/ml for rifampicin. An organism was determined to be susceptible when the antibiotic-containing bottle was not positive or had a positive time to detection greater than that of the 10-2 control. An organism was determined to be resistant when the antibiotic-containing bottle had a positive time to detection that was equal to or less than that of the 10-2 control. Standard strain H37Rv was used for the quality control.
Among the NTMs, only the rapidly growing mycobacteria (RGM) were subjected to susceptibility testing, from 2009-2012, using E-test (bioMerieux, France) on Mueller-Hinton agar plates10. 0.5 McFarland standard suspension was further diluted to 1:1000 with saline and was used as the seed culture (1.5x105 organism/ml), inoculated onto the agar plates, E-test strips placed on the surface and were incubated in 5 per cent CO2 at 37°C. The antimicrobials tested were amikacin, ciprofloxacin, clarithromycin, sulphamethoxazole, doxycycline and imipenem. The minimum inhibitory concentration (MIC, end point) was read after 72 h from the scale on the strip. All antibiotics read at complete inhibition of growth, except sulphamethoxazole which was read at 80 per cent inhibition. Staphylococcus aureus ATCC 29213 was used for quality control of the E-test strips.
Statistical methods: Time trend analysis of MDR-TB prevalence was done by fitting the time trend function Y=aebt11. In this b is the percentage growth rate per year. The growth rate was tested for its significance using student t test. Categorical data were analysed using chi-square test.
Results
A total of 14,849 samples were subjected to mycobacterial culture during 2000 to 2012. The year-wise distribution of samples and their culture positivity status is given in Table I. There was a gradual and steady increase in sample size from 512 in 2000 to 2309 in 2012. The culture positivity improved from 9.7 per cent during 2000-2004 to 16.4 per cent during 2005-2012 after the introduction of automated liquid culture system BacT/ALERT 3D. The introduction of hospital information system in Mycobacteriology in the hospital from 2009 gave an option of dissociating sensitivity from mycobacterial culture. Though this significantly increased the total number of samples for culture alone from 2009 onwards, susceptibility testing requests decrease presumably due to high cost.
Table I.
Year-wise distribution of samples and mycobacteria isolates
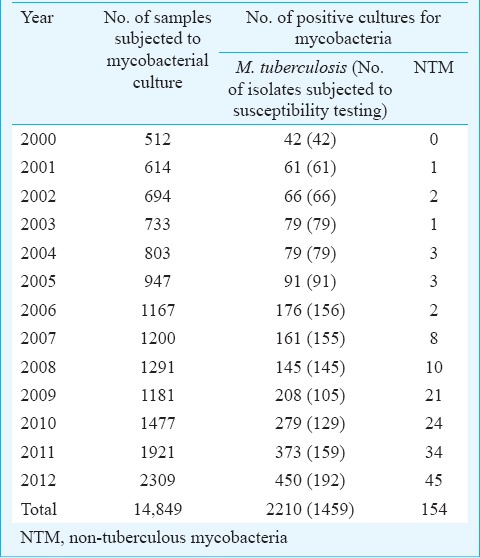
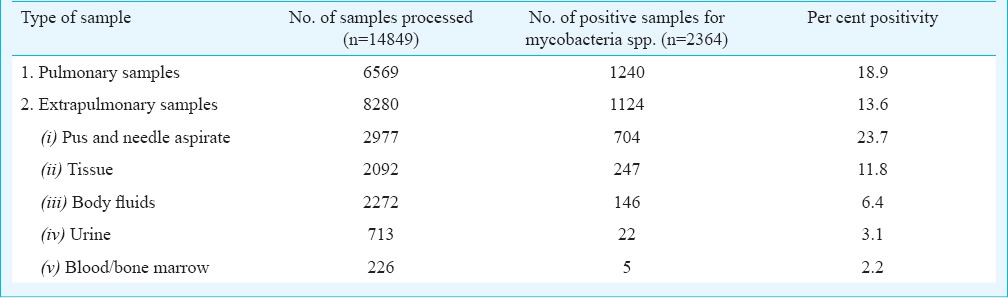
Of the total 14,849 samples, 6569 were pulmonary samples and 8280 were extrapulmonary samples. The average percentage positivity of mycobacterial culture was estimated to be 15.9 per cent (2364/14849). When analysed separately for pulmonary and extrapulmonary samples, the positivity rates were 18.9 and 13.6 per cent, respectively. The break-up of the samples received and their culture positivity status is given in Table II. Pus and needle aspirate samples which included mainly lymph node aspirates showed the highest positivity (23.7%).
Table II.
Distribution of various samples and per cent positivity
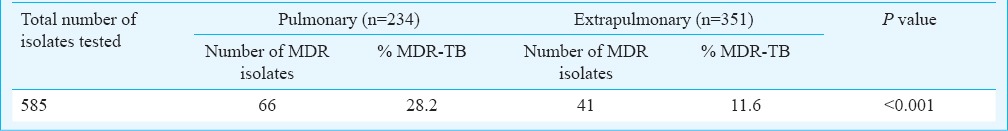
Of the total 2210 M. tuberculosis isolates, 1459 were subjected to susceptibility testing for isoniazid and rifampicin. Of these, 214 (14.7%) were MDR-TB, 92 isolates were isoniazid mono-resistant (6.3%) and five (0.34%) were rifampicin mono-resistant. The percentage of MDR-TB isolates during the course of the study period is depicted in the Figure. The percentage of MDR-TB showed a gradual and significant increase from 4.7 per cent in 2000 to 19.8 per cent in 2012 (P<0.001). However, the delineation of the drug resistance data in to pulmonary and extrapulmonary TB was available separately for four years only between 2009 to 2012 (Table III). MDR-TB was significantly higher (P<0.001) in pulmonary TB (28.2%) compared to extrapulmonary TB (11.6%) cases.
Figure.
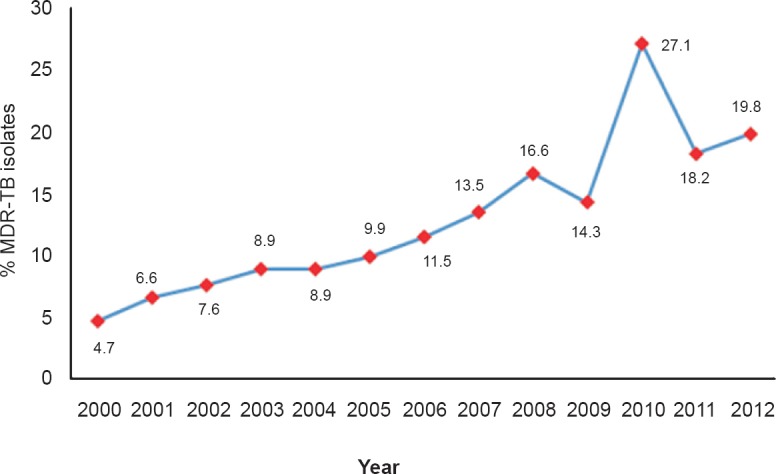
Percentage of MDR-TB isolates over a period of 13 years (n=1459).
Table III.
MDR-TB isolates among pulmonary and extrapulmonary samples (2009-2012)
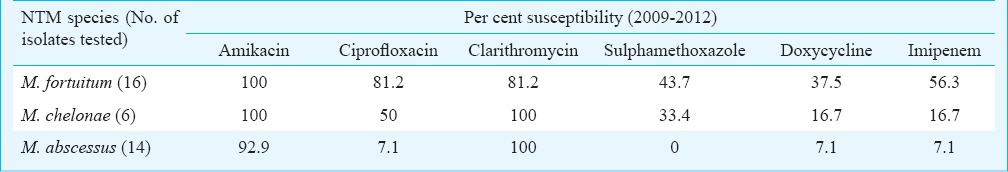
Of the total 2364 mycobacterial isolates, 154 (6.5%) were NTMs (Table IV). Rapid growers represented by M. fortuitum and M. abscessus were the most commonly isolated species. There was a gradual increase in the number of NTMs isolated from 0 in 2000 to 45 isolates in 2012. The drug susceptibility pattern of the rapidly growing mycobacteria during a period of four years (2009-2012) is shown in Table V. Clarithromycin and amikacin were found to be the best therapeutic options according to in vitro susceptibility testing.
Table IV.
Species distribution of non-tuberculous mycobacteria (NTM) isolates
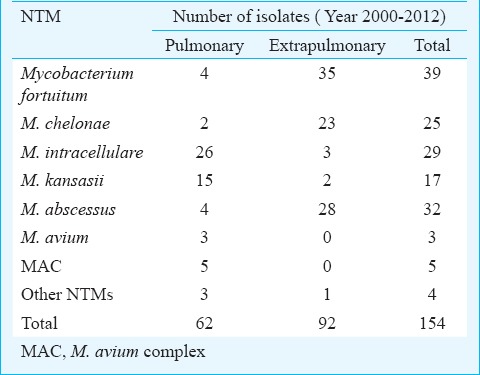
Table V.
Drug susceptibility pattern of rapidly growing Mycobacterium
Discussion
In the present study, the maximum number of mycobacteria isolates were from pulmonary samples (52.5%), but the proportion of extrapulmonary TB (EPTB) was also quite high (47.6%). Before HIV pandemic, extrapulmonary TB was seen as 15-20 per cent of all TB cases, but with global rise of HIV infection over the last decade, increasing association of EPTB in HIV infected individuals has been reported12,13. In an earlier review EPTB was estimated to constitute about 15 to 20 per cent of all cases of tuberculosis in immuno-competent patients in India, while a higher proportion of cases was documented in tertiary care centers (30-53%)12. The high percentage of extrapulmonary TB in our study may be contributed in part by the referral bias associated with a tertiary care centre. Another possible reason could be the lesser proportion of pulmonary samples subjected to culture and sensitivity and usually treated on the basis of a positive smear report alone.
Our data showed a higher isolation rate from pulmonary samples (18.9%) compared to extrapulmonary samples (13.6%). Among the extrapulmonary samples, the isolation rate from pus and tissue samples was higher compared to that of pulmonary samples. In case of body fluid samples, the positivity rate was low. The isolation of mycobacteria from body fluids remains a challenge owing to characteristically low bacterial count. The isolation rate from urine samples was 3.1 per cent in our study. Most of the studies reported an isolation rate varying from 1-5 per cent14,15, which was similar to our study. Blood and bone marrow are useful particularly in HIV positive patients for mycobacterial isolation5,16. In our study only 2.2 per cent blood/bone marrow samples yielded a positive culture and all of them grew MTB. The lower isolation rate might be due to the fact that the study was done in general patient population and not among a selected immuno-compromised group. A 10 year retrospective study by Duong et al17 also reported a low sensitivity of only 3 per cent positivity out of 432 acid fast cultures of bone marrow in a tertiary care facility.
We observed a steady increase in MDR-TB (4.7 to 19.8%) during the 13 years except an unexplainable peak in 2010. The increase in multidrug resistance was 12 per cent. Studies from different parts of our country reported a wide variation in the prevalence of MDR-TB, 0.6 to 24 per cent among new cases of pulmonary TB and 16 to 47 per cent in case of already treated patients, during the past two decades18,19,20,21. In a review on drug resistance in tuberculosis, Dheda et al22 have observed that the prevalence of MDR-TB has increased globally in the past decade and is likely to continue to increase in many areas in resource-poor settings. A recent study from north India also confirmed an increase in MDR-TB during the past few years in our country23. The WHO Global Report 2014, estimated primary MDR-TB as 2.2 per cent and among previously treated patients as 15 per cent in our country during 20131. The limitation of our study was that the differentiation between primary and acquired resistance could not be done since the treatment history of patients was documented inadequately. The reasons for the higher rate of MDR-TB in our study compared to the WHO surveillance data may be due to the selection bias involved in the patients attending the tertiary care facility. Also, this being a retrospective study, alteration in diagnostic methods during the time period keeping with pace of newer advances, might have influenced our results. Still, our study showing a progression of MDR-TB during a 13 years time period in a tertiary care center and the difference in drug resistance in pulmonary versus extrapulmonary TB, are important in the background of scarcity of such data in Indian literature.
The surveillance data by European Centre for Disease Control and Prevention, report MDR-TB in 1.3 per cent of the extrapulmonary TB cases in comparison to 6.6 per cent of the pulmonary TB cases24. Our study confirmed the findings of two European studies that MDR-TB was less frequent among extrapulmonary TB cases than among pulmonary TB cases24,25. This may be due to the low number of previously treated extrapulmonary TB cases. Therefore, the risk of development of drug resistance also remains to be lower than for pulmonary TB cases as observed by other workers24,26. However, more studies with larger sample size need to be done to confirm.
An increase in the number of NTMs isolated in later years was seen in our study. This, being a retrospective study, the demographic details including HIV status of the patients were not completely available. To limit the reporting of only pathogenic isolates, NTM isolates were reported according to American Thoracic Society guidelines7 for respiratory isolates and after correlating with clinical, radiological and histopathological evidences and repeat isolation of the same organism wherever possible in case of extrapulmonary samples. NTMs are increasingly recognized to cause pulmonary and extrapulmonary infections27,28. In a study from Vellore, 3.9 per cent of all mycobacterial isolates were NTMs, with M. chelonae and M. fortuitum being the commonest29. In the present study also rapid growers represented by M. fortuitum and M. abscessus remained the most commonly isolated species. Majority of these isolates were sensitive to clarithromycin and amikacin. A study from north India reported 100 per cent sensitivity to clarithromycin and ciprofloxacin30. But in our study ciprofloxacin was found to have relatively good sensitivity for M. fortuitum, but not for M. chelonae and M. abscessus. A study from Vellore is also in agreement with our findings of good sensitivity to amikacin (99.2%) and poor sensitivity to ciprofloxacin (21%)29. The sensitivity of other NTMs was not attempted due to difficulties in interpretation of results and also in view of the increasing cost burden on the patients.
Increase in multidrug resistance from 4.7 to 19.8 per cent in the past 13 years, as observed in our study, needs to be noticed. In the present scenario of increasing prevalence of MDR-TB and lack of availability of many second line drugs, screening with culture and drug susceptibility testing should be recommended for all smear positive pulmonary patients. The WHO policy guidelines31 for the use of new rapid molecular based techniques for early detection of MDR-TB may help the high-burden countries in identifying and treating patients of MDR-TB quickly32. A study from India has reported that active household contact investigation is a powerful tool to detect and treat tuberculosis at an early stage to break the transmission cycle of the disease33. The existing policies need to be modified to actively find, rapidly diagnose and aggressively treat existing cases appropriately to eliminate transmission of disease.
References
- 1.Geneva, Switzerland: World Health Organization; 2014. Global Tuberculosis Report 2014 (WHO/HTM/TB/2014.8) [Google Scholar]
- 2.Gandhi NR, Nunn P, Dheda H, Schaaf HS, Zignol M, van Soolingen D, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Mohan A. Tuberculosis: From an incurable scourge to a curable disease - journey over a millennium. Indian J Med Res. 2013;137:455–93. [PMC free article] [PubMed] [Google Scholar]
- 4.Metchock BG, Nolte FS, Wallace RJ. Mycobacterium. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. Washington DC: American Society for Microbiology; 1999. pp. 399–437. [Google Scholar]
- 5.Hanscheid T, Monteiro C, Cristino M, Lito LM, Salgado MJ. Growth of Mycobacterium tuberculosis in conventional BacT/ALERT FA blood culture bottles allows reliable diagnosis of mycobacteremia. J Clin Microbiol. 2005;43:890–1. doi: 10.1128/JCM.43.2.890-891.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter E, Rusch-Gerdes S, Hillemann D. Evaluation of the GenoType Mycobacterium assay for identification of mycobacterial species from cultures. J Clin Microbiol. 2006;44:1769–75. doi: 10.1128/JCM.44.5.1769-1775.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 8.Kent PT, Kubica GP. Atlanta, Georgia: CDC; 1985. Public health mycobacteriology - a guide for the level 3 laboratory. [Google Scholar]
- 9.Bemer P, Bodmer T, Munzinger J, Perrin M, Vincent V, Drugeon H. Multicenter evaluation of the MB/BACT system for susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol. 2004;42:1030–4. doi: 10.1128/JCM.42.3.1030-1034.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffner SE, Klintz L, Olsson-Liljequist B, Bolmstrom A. Evaluation of Etest for rapid susceptibility testing of Mycobacterium chelonae and M. fortuitum. J Clin Microbiol. 1994;32:1846–9. doi: 10.1128/jcm.32.8.1846-1849.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DB, Mowry TA. A practical odyssey. 7th ed. USA: Brooks/Cole; 2012. Exponential and logarithmic function in mathematics; pp. 731–806. [Google Scholar]
- 12.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]
- 13.Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis. 2008;8:8. doi: 10.1186/1471-2334-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan DS, Choy MY, Wang S, Sng LH. An evaluation of the recovery of mycobacteria from urine specimens using the automated Mycobacteria Growth Indicator Tube system (BACTEC MGIT 960) J Med Microbiol. 2008;57:1220–2. doi: 10.1099/jmm.0.2008/001685-0. [DOI] [PubMed] [Google Scholar]
- 15.Hillemann D, Richter E, Ru¨ sch-Gerdes S. Use of the BACTEC Mycobacteria Growth Indicator Tube 960 automated system for recovery of mycobacteria from 9,558 extrapulmonary specimens, including urine samples. J Clin Microbiol. 2006;44:4014–7. doi: 10.1128/JCM.00829-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilby JM, Marques MB, Jaye DL, Tabereaux PB, Reddy VB, Waites KB. The yield of bone marrow biopsy and culture compared with blood culture in the evaluation of HIV-infected patients for mycobacterial and fungal infections. Am J Med. 1998;104:123–8. doi: 10.1016/s0002-9343(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 17.Duong S, Dezube BJ, Desai G, Eichelberger K, Qian Q, Kirby JE. Limited utility of bone marrow culture: a ten-year retrospective analysis. Labmedicine. 2009;40:37–8. [Google Scholar]
- 18.Sharma SK, Kaushik G, Jha B, George N, Arora SK, Gupta D, et al. Prevalence of multidrug-resistant tuberculosis among newly diagnosed cases of sputum-positive pulmonary tuberculosis. Int J Med Res. 2011;133:308–11. [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma SK, Mohan A. Multidrug-resistant tuberculosis. Indian J Med Res. 2004;120:354–76. [PubMed] [Google Scholar]
- 20.Hanif M, Malik S, Dhingra VK. Acquired drug resistance pattern in tuberculosis cases at the State Tuberculosis Centre, Delhi, India. Int J Tuberc Lung Dis. 2009;13:74–8. [PubMed] [Google Scholar]
- 21.D’souza DT, Mistry NF, Vira TS, Dholakia Y, Hoffner S, Pasvol G, et al. High levels of multidrug resistant tuberculosis in new and treatment-failure patients from the Revised National Tuberculosis Control Programme in an urban metropolis (Mumbai) in Western India. BMC Public Health. 2009;9:211. doi: 10.1186/1471-2458-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dheda K, Warren RM, Zumla A, Grobusch MP. Extensively drug-resistant tuberculosis: epidemiology and management challenges. Infect Dis Clin North Am. 2010;24:705–25. doi: 10.1016/j.idc.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Maurya AK, Singh AK, Kumar M, Umrao J, Kant S, Nag VL, et al. Changing patterns and trends of multidrug-resistant tuberculosis at referral centre in Northern India: a 4-year experience. Indian J Med Microbiol. 2013;31:40–6. doi: 10.4103/0255-0857.108720. [DOI] [PubMed] [Google Scholar]
- 24.Sandgren A, Hollo V, van der Werf MJ. Extrapulmonary tuberculosis in the European Union and European Economic Area, 2002 to 2011. [accessed on February 18, 2014];Euro Surveill. 2013 18 pii: 20431. Available from: http://www.eurosurveillance.org . [PubMed] [Google Scholar]
- 25.Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999-2006. Thorax. 2009;64:1090–5. doi: 10.1136/thx.2009.118133. [DOI] [PubMed] [Google Scholar]
- 26.Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49:1350–7. doi: 10.1086/605559. [DOI] [PubMed] [Google Scholar]
- 27.Katoch VM. Infections due to non-tuberculous mycobacteria (NTM) Indian J Med Res. 2004;120:290–304. [PubMed] [Google Scholar]
- 28.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49:e124–9. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 29.Jesudason MV, Gladstone P. Non tuberculous mycobacteria isolated from clinical specimens at a tertiary care hospital in South India. Indian J Med Microbiol. 2005;23:172–5. doi: 10.4103/0255-0857.16589. [DOI] [PubMed] [Google Scholar]
- 30.Sankar MM, Gopinath K, Singla R, Singh S. In-vitro antimycobacterial durg susceptibility testing of non-tubercular mycobacteria by tetrazolium microplate assay. Ann Clin Microbiol Antimicrob. 2008;7:15. doi: 10.1186/1476-0711-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geneva, Switzerland: WHO; 2010. [accessed on April 9, 2014]. World Health Organization (WHO). Framework for implementing new tuberculosis diagnostics. Available from: http://www.stoptb.org/retooling . [Google Scholar]
- 32.Raveendran R, Wattal C, Oberoi JK, Goel N, Datta S, Prasad KJ. Utility of GenoType MTBDRplus assay in rapid diagnosis of multidrug resistant tuberculosis at a tertiary care centre in India. Indian J Med Microbiol. 2012;30:58–63. doi: 10.4103/0255-0857.93034. [DOI] [PubMed] [Google Scholar]
- 33.Singh J, Sankar MM, Kumar S, Gopinath K, Singh N, Mani K, et al. Incidence and prevalence of tuberculosis among household contacts of pulmonary tuberculosis patients in a peri-urban population of South Delhi, India. PLoS One. 2013;8:e69730. doi: 10.1371/journal.pone.0069730. [DOI] [PMC free article] [PubMed] [Google Scholar]


