Abstract
Background & objectives:
The southern part of India has witnessed an increase in scrub typhus (ST) during the past ten years. ST outbreaks occurred during winter months but at intervals of one to three years. With only a few reports of ST in Puducherry, this study was undertaken to look for the persistence of ST cases in Puducherry and Tamil Nadu in the winter months.
Methods:
During relatively cooler months of September, 2012 to March, 2013, a total of 45 patients with fever and clinical suspicion of ST and who provided both acute and convalescent blood samples were included. Total WBC, platelet counts, serum creatinine, liver enzymes levels and a rapid immunochromatographic test (RICT) for ST were first done. Paired serum samples were analysed by two specific tests - ST IgM and IgG ELISA- and a non-specific, but widely used Weil-Felix (WF) test.
Results:
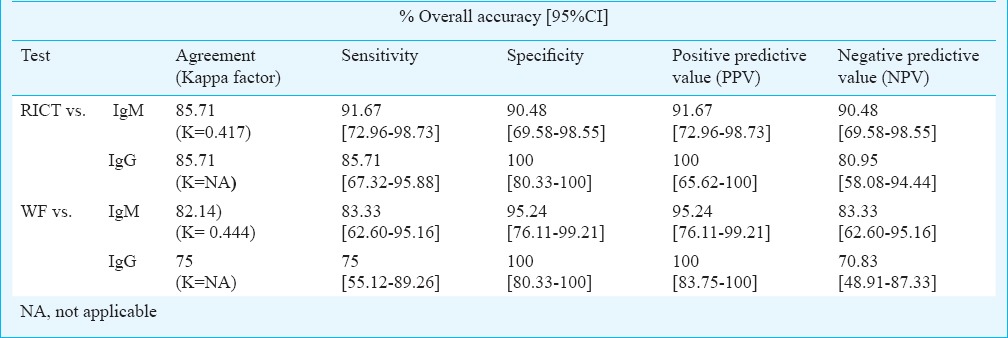
Of the 45 patients, 21 adults and seven children were confirmed as ST based on clinical and laboratory findings, and positivity in specific serological test(s). Setting ST IgM and IgG ELISA as reference, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for RICT were 91.67, 85.71 per cent; 90.48, 100 per cent; 91.67, 100 per cent and 90.48, 80.95 per cent, respectively. Similarly, for WF the values were 83.33, 75 per cent; 95.24, 100 per cent; 95.24, 100 per cent and 83.33, 70.83 per cent, respectively.
Interpretation & conclusions:
ST continues to persist in the cooler months in Puducherry and neighbouring Tamil Nadu with fever and myalgia as prominent features. None of the tests evaluated in this study was found to be ideal, but ST IgM/IgG ELISA was useful for batch testing and the non-specific WF test can be used in resource poor settings.
Keywords: ELISA, Rickettsia tsutsugamushi, scrub typhus, tsutsugamushi fever, Weil-Felix test
Scrub typhus (ST) caused by Orientia tsutsugamushi, originally reported in the Himalayan foothills and South-East Asia, has now spread to different parts of India, involving several States and Union Territories, particularly in southern India1,2,3,4,5,6,7,8,9,10. It has also been reported from north and northeastern parts of the country11,12,13,14,15, as also scattered reports from western India16. During the past six years Puducherry has recorded a cluster of 50 cases of ST during 2006-20085, followed by ST meningitis in 2011-20126 and presumptive ST outbreaks in children and adults during the same period7,8. As a follow up, investigation was carried out in the relatively cooler months of September, 2012 - March, 2013 to look for the continued persistence of this disease in Puducherry and the neighbouring State Tamil Nadu. The objectives of this study were to analyse the clinical findings in patients with presumptive ST, interpretation of haemogram, biochemical tests and ST specific serological tests such as rapid immunochromatographic test (RICT), ST IgM/ST IgG ELISA and the non-specific Weil Felix (WF) test in paired serum samples. In view of non-availability of gold standard immunofluorescent antibody (IFA) kits in India until recently and due to their technical complexity, ST IgM/ST IgG ELISA were taken as gold standard.
Material & Methods
This study was carried out at Mahatma Gandhi Medical College and Research Institute, Puducherry, India, catering to majority of rural population from Puducherry and neighbouring districts of Tamil Nadu. The research proposal was approved by Institutional Human Ethical Committee. The patients were from two health care institutions: a private sector medical college hospital and a Government general hospital, both located at a distance of 18 km apart from each other. Since the outbreaks of ST in south India were recorded in cooler months at intervals of one to three years, this study looked for continuing presence of ST in the relatively cooler months of September, 2012 to March, 2013. Written informed consent was obtained from all patients prior to collection of blood samples. Only 45 consecutive patients suspected to have ST and who voluntarily provided both acute and convalescent blood samples during the above period were included in this study. Inclusion criteria were high grade fever with or without chills and rigour; fever with rash/eschar/hepatosplenomegaly/jaundice/lymphadenopathy/thrombocytopenia; fever with constitutional symptoms like malaise, myalgia, nausea, vomiting; fever with capillary leak syndrome (Pleural effusion, ascitis, pedal oedema); and fever with bleeding diathesis (petechia, purpura)/fever with shock. Exclusion criteria were known cases of immunocompromised patients like AIDS/lymphomas; malignancy secondaries; bleeding disorders and fever of more than four weeks duration (pulmonary tuberculosis, etc.).
Whenever felt necessary, additional tests were carried out like blood culture, urine culture, Widal test, serological tests for leptospirosis and dengue and peripheral blood film examination for malarial parasites and malarial antigen detection in blood. The paired serum samples were collected at an interval of 14 to 21 days. On receipt, acute blood samples were initially subjected to haemogram (total WBC and platelet counts) and biochemical tests [serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP)]. A commercial rapid immunochromatography screening test for ST (SD Bioline Tsutsugamushi kit, Standard Diagnostics, Korea) based on lateral flow technique was performed17,18,19. The serum samples were aliquoted and kept frozen at -20◦ C for carrying out ST IgM, ST IgG ELISA (Scrub Typhus Detect IgM/IgG ELISA System, InBios International, USA) and WF later. OXK agglutination in the WF test20 was carried out with coloured OXK antigen (Plamatec, USA). Six doubling dilutions ranging from 1:20 to 1:640 to exclude prozone phenomenon were done. Further titrations were carried out as per the need. ST IgM and IgG ELISA tests21 were performed following the manufacturer's instructions. Both ELISA plates were coated with ten recombinant antigens of O. tsutsugamushi. Patients’ serum samples were initially diluted with 1:100 with the sample dilution buffer provided in the kit and other reagents were added as outlined in the procedure. OD (optical density) readings were taken at 450 nm in iMark Microplate Reader (Bio-Rad, Japan). Twenty samples were collected from healthy volunteers from an ST endemic area (Kurinjipadi taluk, Cuddalore district, Tamil Nadu) and used in the calculation of the cut-off value in both ELISA tests. Cut-off values were calculated as follows:
Cut-off value = Average of the normal human serum samples (NHS) + three times SD of NHS.
The samples with OD values above the cut-off were considered positive and those below the cut-off were taken as negative. Borderline samples were tested in triplicate.
Criteria for presumptive and confirmatory diagnosis of ST based on interpretation of serological tests available in India, were as follows:
-
(i)
ST IgM ELISA positivity in acute (A) and/or convalescent (C) serum-confirms ST.
-
(ii)
ST IgG ELISA positivity in A+C/C (seroconversion)-confirms ST.
-
(iii)
ST RICT alone positive - presumptive ST.
-
(iv)
a. WF alone positive with four-fold or more increase/fall in OXK titre with the minimum initial titre of 1:40 - presumptive ST.
b. OXK titre ≥ 320 in A and/or C without any four-fold increase/decrease - suspect ST and correlate clinically.
Complications in ST positive patients were diagnosed based on the following criteria16: (i) Liver dysfunction/hepatitis: patients with serum bilirubin >3 mg per cent and/or elevation of serum transaminase of >2 times the upper limit of the normal; (ii) Meningeal involvement/meningitis: patients with altered sensorium and signs of meningeal irritation along with elevated CSF proteins and neutrophilia in CSF; (iii) Congestive cardiac failure: the inability/failure of the heart to adequately meet the need of organs and tissues for oxygen and nutrients; and (iv) ARDS (acute repiratory distress syndrome): acute onset of non-cardiogenic pulmonary oedema manifesting with bilateral alveolar or interstitial infiltrates on chest radiograph and partial pressure arterial oxygen and fraction of inspired oxygen (PaO2/FIO2) <200 mmHg on arterial blood gas.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated22 considering ST IgM and IgG ELISA as gold standard. For other parameters (Spearman's correlation and Kappa) statistical analysis was performed using IBM SPSS Statistics 17 for Windows (SPSS Inc; Chicago, USA). Chi square test with Yates correction was performed for categorical data.
Results
Of the 45 patients included in the study, 28 were confirmed as ST by their specific serological response. These included 21 adults and seven children. Male and female patients’ ratio was 1:1. The youngest patient was one year old and the oldest was an 89 yr old woman. Mean age was 31.36 + 21.44 yr.
The physical signs and symptoms of 28 ST patients are presented in Table I. The most common clinical symptoms was fever of seven days and more duration (100%). Other salient features included: chills and rigour (71.43%), myalgia (64.29%), headache (64.29%), vomiting (50%), nausea (46.43%), abdominal pain (42.86%), cough (39.29%), malaise (39.29%), retro-orbital pain (17.86%), expectoration (17.86%) and capillary leak (14.29%). The common clinical signs of patients were lymphadenopathy (seen in children only), (42.86%), hepatomegaly (39.29%) and splenomegaly (35.71%). Eschar, one of the important signs of ST was seen only in six patients (21.43%). Compared to adults, though children had higher percentage of hepatomegaly (71.43%), splenomegaly (57.14%) and lymphadenopathy (42.86%), the difference was significant only for lymphadenopathy (P<0.05). However, the number of ST positive children was only seven compared to 21 adults. Some of our ST patients had mixed infection with dengue (2)/leptospirosis (2)/and vivax malaria (1).
Table I.
Clinical presentation of scrub typhus cases (n=28)
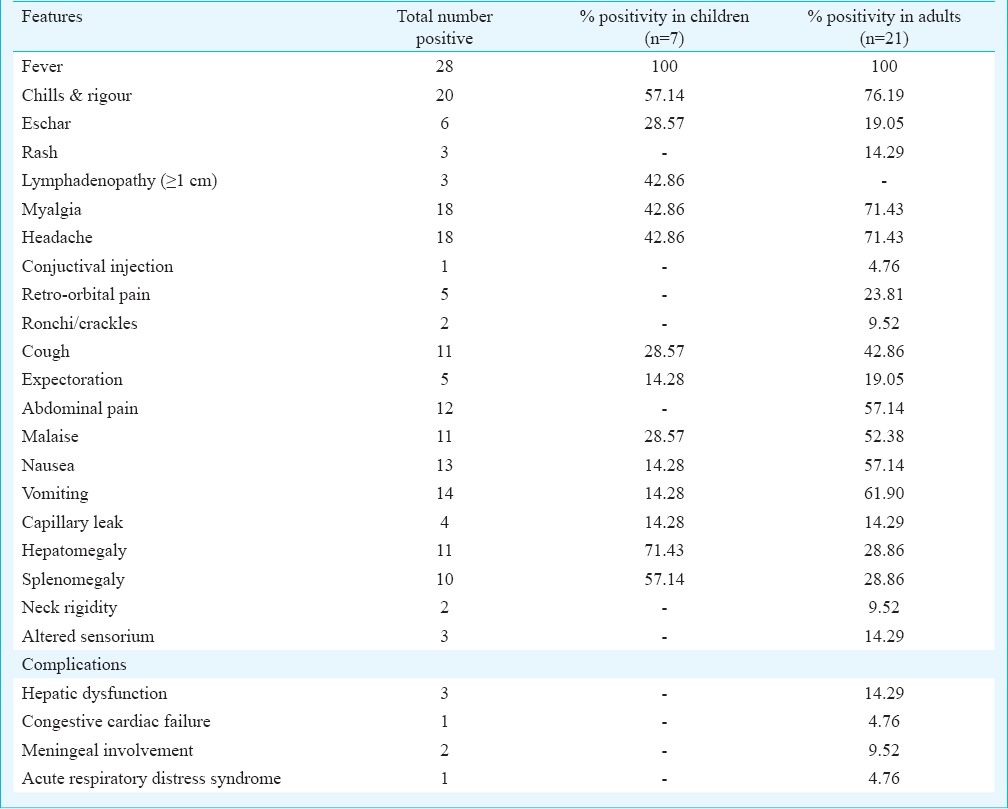
Thrombocytopenia (<150 000/mm3) was observed in one child and 12 adults. Of the 25 patients analyzed for total WBC count, five of the seven children and four of the 18 adults had leucocytosis (>11,000/mm3). Of the 28 patients, 15 were analysed for liver enzymes and significant rise (>twice normal) was recorded in one child and 14 adults. This child had higher values of all three liver enzymes: AST, ALT and ALP. Among the 14 adult patients, nine had elevated AST levels, seven had increased ALT and three had higher levels of ALP. Only one adult showed elevated serum creatinine level (>1.4 mg%) (Table II). The only child for whom serum creatinine was done showed normal value.
Table II.
Results of laboratory tests in scrub typhus patients (n=28)
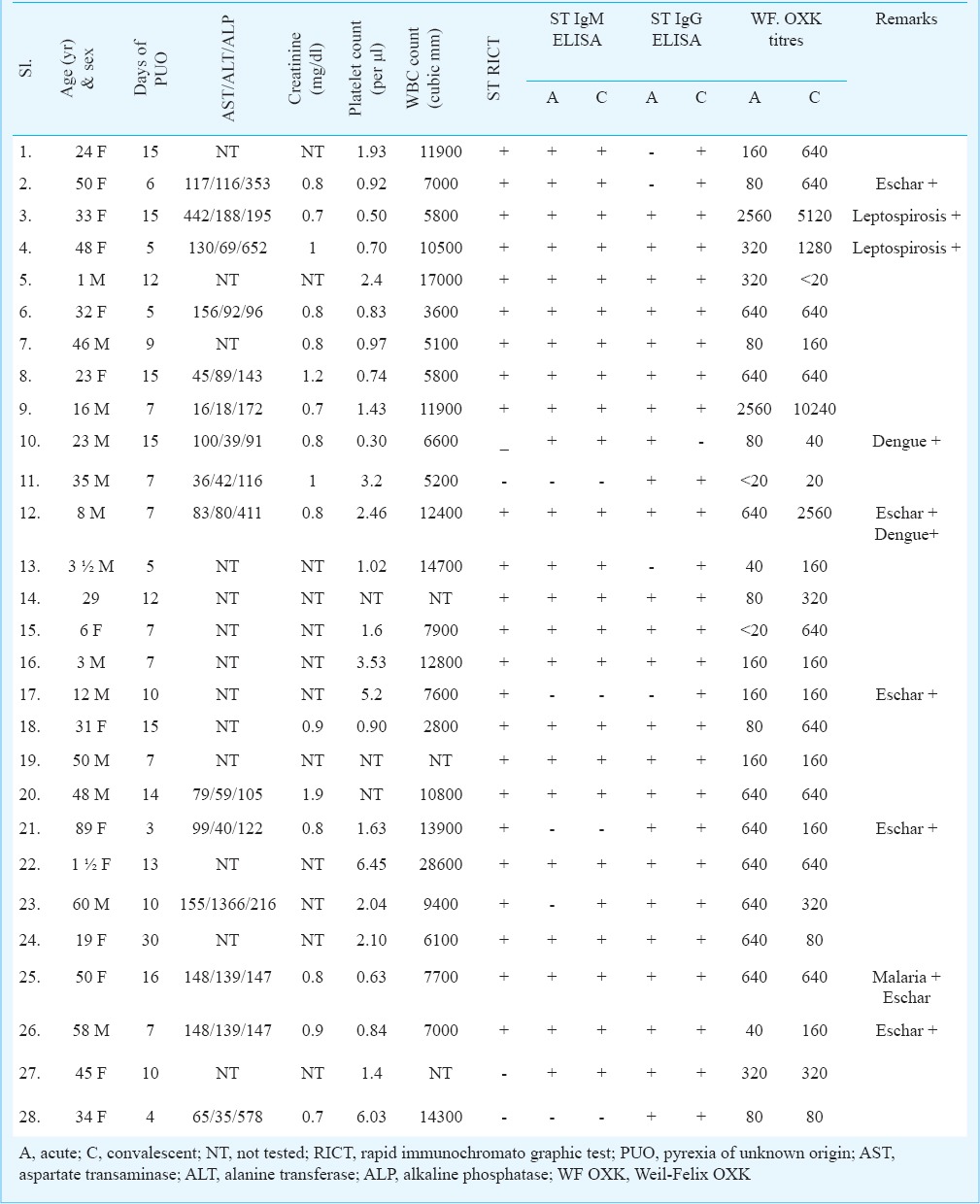
ST RICT: Among 28 patients, 24 were positive in ST RICT and also ST IgM and ST IgG ELISA. The remaining four were considered as false negative in RICT.
ST IgM ELISA: Twenty three patients had IgM antibody in both acute as well as convalescent serum samples. Seroconversion (in the convalescent sample) was observed in one patient only. Four patients were negative for IgM antibody in paired samples.
ST IgG ELISA: Twenty three patients had IgG antibody in both acute and convalescent samples. Seroconversion was seen in four. In one patient, IgG antibody was seen in acute sample only. Of the 28 patients, 24 were positive for both IgM and IgG antibodies in acute and/or convalescent serum samples.
WF: Twenty one patients were positive in WF test. The OXK titres ranged from 1:40 to 1:10240 (Table II). Four-fold or more increase in OXK titre was observed in 10 acute samples with titres from 40 to 2560. Fall in titre (four-fold or more) was observed in three cases. In the remaining eight, significant levels of OXK titres ranged from 320 to 5120, without four-fold rise/fall.
Among the 17 ST negative patients lymphadenopathy, hepatomegaly and splenomegaly were observed in five, three and one patients, respectively. Retro-orbital pain, capillary leak and delirium were observed in one patient each. Significantly elevated liver enzymes (AST/ALT/ALP) were recorded in two of the seven patients tested. Increased creatinine level was seen in one patient. Thrombocytopenia was observed in four of the 13 patients tested. All the 17 patients were negative for ST RICT, ST IgM, ST IgG, and WF. There was no seroconversion in either IgM or IgG ELISA. OXK titres ranged from 20 to 80 without any four-fold increase/fall in paired serum samples. Of these 17 patients, one was positive for leptospirosis (IgM) and another one for vivax malaria.
Taking ST IgM ELISA as reference, the sensitivity, specificity, PPV and NPV for RICT were 91.67, 90.48, 91.67 and 90.48 per cent, respectively. These values were also calculated using ST IgG ELISA as reference (Table III).
Table III.
Comparison between RICT, WF (OXK), IgM and IgG ELISA (n=28)
Discussion
Presence of eschar, considered as a significant sign of ST was seen in only six patients, which is rather low. Similar observation has been made in a few studies3,4,16,23, while a moderate to higher percentage was reported by others from India and abroad5,11,15,24. However, eschars can go unnoticed in dark skinned people.
Thrombocytopenia is an important feature of ST, dengue, malaria and leptospirosis. This was presented by about half of our patients. Leucocytosis was seen in 36 per cent patients. Significantly elevated levels of one or more liver enzymes which is commonly observed in ST, were also observed. The haematological and biochemical parameters were comparable with other reports from India and abroad3,4,5,15,24.
ST RICT detects IgM, IgG and IgA antibodies to O. tsutsugamushi serotypes- Gilliam, Karp and Kato. The performance of this kit varies from very low25 to moderate and good7,8,17,18. In our study, samples of 24 patients showed concordance in three ST specific serological tests: RICT, ST IgM and ST IgG ELISA. RICT failed to detect four cases. Thus, ST RICT kits need further evaluation in different field conditions so as to warrant their recommendation as a dependable screening test in rural and urban laboratories.
For serological confirmation of rickettsial diseases, indirect fluorescent antibody (IFA) test is the gold standard21,25. However, this test is technically very demanding, subjective, needs an experienced observer and not commonly available in India. ELISA on the contrary, is simple, easy to interpret, can be performed in laboratories. ELISA is fairly comparable with IFA in terms of sensitivity and specificity21. InBios ST ELISA kits have been validated in India and found reliable by different Indian researchers3,4,7,8,16,26.
Drawback of ST IgM/IgG ELISA is that these are only qualitative tests and are suitable for testing in batches only. Positivity/negativity depends upon the duration of illness at the time of blood collection. Although WF is a non specific test, but it is still being widely used in India and other resource-poor countries because of its affordability, availability, ease of performance and interpretation in any laboratory. An OXK titre of ≥ 1: 80 was reported to correlate well with the gold standard IFA test4. We applied the criteria of a single OXK titre of ≥1:320 or a four-fold increase in titre of paired serum samples with a minimum acute phase titre of 1:40 as significant for ST. In our study, WF correlated with ST IgM/IgG ELISA in 21 cases [Spearman correlation, P=0.01]. OXK agglutinins appear early in the acute phase of ST. In these 28 cases, WF positivity was a supportive finding of ST, and not a sole confirmatory criterion.
WF test is considered the last choice where facilities for other specific tests do not exist. This is because WF is a non-specific test based on the principle of heterophil agglutination and there is a possibility for false positivity21,25,27,28.
The patients were initially started on third generation cephalosporins. Doxycycline (or azithromycin for some children) was added to the regime, once RICT test results became positive and supported by clinical correlation. In a majority of patients, the defervescence was observed within one to two days after administering doxycycline. All patients responded well to the treatment and there was no mortality.
In conclusion, our study has shown that ST outbreaks continued in the cooler months in Puducherry and neighbouring Tamil Nadu. Fever and myalgia were the most prominent features of the ST. A thorough physical examination for eschar is useful. Elevated liver enzymes are important laboratory parameters for ST. Doxycycline treatment worked well for highly suspected cases. ST IgM/IgG ELISA suited for batch testing and retrospective study. ST RICT needs more trials. WF may be continued in resource-poor settings and interpreted with clinical correlation.
Acknowledgment
The authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for funding this ad-hoc research project. Authors are also grateful to the Chairman, Vice-Chancellor, and Dean of MGMC & RI for providing the facilities.
Footnotes
Conflicts of Interest: None.
References
- 1.Batra HV. Spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res. 2007;126:101–3. [PubMed] [Google Scholar]
- 2.Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E. Serological evidence for wide distribution of spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res. 2007;126:128–30. [PubMed] [Google Scholar]
- 3.Mathai E, Rolain JM, Verghese GM, Abraham OC, Mathai D, Mathai M, et al. Outbreak of scrub typhus in southern India during the cooler months. Ann NY Acad Sci. 2003;990:359–64. doi: 10.1111/j.1749-6632.2003.tb07391.x. [DOI] [PubMed] [Google Scholar]
- 4.Isaac R, Varghese GM, Mathai E, Manjula J, Joseph I. Scrub typhus: prevalence and diagnostic issues in rural southern India. Clin Infect Dis. 2004;39:1395–6. doi: 10.1086/424748. [DOI] [PubMed] [Google Scholar]
- 5.Vivekanandan M, Mani A, Priya YS, Singh AP, Jayakumar S, Purty S. Outbreak of scrub typhus in Pondicherry. J Assoc Physicians India. 2010;58:24–8. [PubMed] [Google Scholar]
- 6.Viswanathan S, Muthu V, Iqbal N, Remalayam B, George T. Scrub typhus meningitis in South India - 0 a retrospective study. PLoS One. 2013;8:e66595. doi: 10.1371/journal.pone.0066595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephen S, Kandhakumari G, Vinithra SM, Pradeep J, Venkatesh C, Namachivayam V. Outbreak of pediatric scrub typhus in South India - a preliminary report. J Pediatr Infect Dis. 2013;8:125–9. [Google Scholar]
- 8.Stephen S, Kandhakumari G, Pradeep J, Vinithra SM, Siva PK, Hanifah M, et al. Scrub typhus in south India: a re-emerging infectious disease. Jpn J Infect Dis. 2013;66:552–4. doi: 10.7883/yoken.66.552. [DOI] [PubMed] [Google Scholar]
- 9.Boorugu H, Dinaker M, Roy ND, Jude JA. Reporting a case of scrub typhus from Andhra Pradesh. J Assoc Physicians India. 2010;58:519–20. [PubMed] [Google Scholar]
- 10.Sundhindra BK, Vijayakumar S, Kutty KA, Tholpadi SR, Rajan RS, Mathai E, et al. Rickettsial spotted fever in Kerala. Natl Med J India. 2004;17:51–2. [PubMed] [Google Scholar]
- 11.Singh SI, Devi KP, Tilotama R, Ningombam S, Gopalkrishna Y, Singh TB, et al. An outbreak of scrub typhus in Bishnupur district of Manipur, India, 2007. Trop Doct. 2010;40:169–70. doi: 10.1258/td.2010.090468. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad S, Srivastava S, Verma SK, Puri P, Shirazi N. Scrub typhus in Uttarakhand, India: a common rickettsial disease in an uncommon geographical region. Trop Doct. 2010;40:188–90. doi: 10.1258/td.2010.090447. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhry D, Garg A, Singh I, Tandon C, Saini R. Rickettsial diseases in Haryana: not an uncommon entity. J Assoc Physicians India. 2009;57:334–7. [PubMed] [Google Scholar]
- 14.Sharma A, Mahajan S, Gupta ML, Kanga A, Sharma V. Investigation of an outbreak of scrub typhus in the himalayan region of India. Jpn J Infect Dis. 2005;58:208–10. [PubMed] [Google Scholar]
- 15.Digra SK, Saini GS, Singh V, Sharma SD, Kaul R. Scrub typhus in children: Jammu experience. JK Sci. 2010;12:95–7. [Google Scholar]
- 16.Narvencar KP, Rodrigues S, Nevrekar RP, Dias L, Dias A, Vaz M, et al. Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. 2012;136:1020–4. [PMC free article] [PubMed] [Google Scholar]
- 17.Ching WM, Rowland D, Zhang Z, Bourgeois AL, Kelly D, Dasch GA, et al. Early diagnosis of scrub typhus with a rapid flow assay using recombinant major outer membrane protein antigen (r56) of Orientia tsutsugamushi. Clin Diagn Lab Immunol. 2001;8:409–14. doi: 10.1128/CDLI.8.2.409-414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watt G, Strickman D, Kantipong P, Jongsakul K, Paxton H. Performance of a dot blot immunoassay for the rapid diagnosis of scrub typhus in a longitudinal case series. J Infect Dis. 1998;177:800–2. doi: 10.1086/517813. [DOI] [PubMed] [Google Scholar]
- 19.Weddle JR, Chan TC, Thompson K, Paxton H, Kelly DJ, Dasch G, et al. Effectiveness of a dot-blot immunoassay of anti-Rickettsia tsutsugamushi antibodies for serologic analysis of scrub typhus. Am J Trop Med Hyg. 1995;53:43–6. [PubMed] [Google Scholar]
- 20.Bassett DC, Ho AK, Tam JS, Lam LY, Cheng AF. The laboratory diagnosis of rickettsial diseases in Hong Kong. J Trop Med Hyg. 1992;95:327–30. [PubMed] [Google Scholar]
- 21.Coleman RE, Sangkasuwan V, Suwanabun N, Eamsila C, Mungviriya S, Devine P, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg. 2002;67:497–503. doi: 10.4269/ajtmh.2002.67.497. [DOI] [PubMed] [Google Scholar]
- 22.Parikh R, Mathai A, Parikh S, Chandra Sekhar G, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008;56:45–50. doi: 10.4103/0301-4738.37595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silpapojakul K. Scrub typhus in the western pacific region. Ann Acad Med Singapore. 1997;26:794–800. [PubMed] [Google Scholar]
- 24.Chanta C, Chanta S. Clinical study of 20 children with scrub typhus at Chiang Rai regional hospital. J Med Assoc Thai. 2005;88:1867–72. [PubMed] [Google Scholar]
- 25.La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715–27. doi: 10.1128/jcm.35.11.2715-2727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakash JA, Abraham OC, Mathai E. Evaluation of tests for serological diagnosis of scrub typhus. Trop Doct. 2006;36:212–3. doi: 10.1258/004947506778604715. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan SK, Kashyap R, Kanga A, Sharma V, Prasher BS, Pal LS. Relevance of Weil-Felix test in diagnosis of scrub typhus in India. J Assoc Physicians India. 2006;54:619–21. [PubMed] [Google Scholar]
- 28.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–70. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]


