Abstract
Context:
Accurate assessment of thyroid function during pregnancy is critical, for initiation of thyroid hormone therapy, as well as for adjustment of thyroid hormone dose in hypothyroid cases.
Aims:
We evaluated pregnant women who had no past history of thyroid disorders and studied their thyroid function in each trimester.
Settings and Design:
86 normal pregnant women in the first trimester of pregnancy were selected for setting reference intervals. All were healthy, euthyroid and negative for thyroid peroxidase antibody (TPOAb). These women were serially followed throughout pregnancy. 124 normal nonpregnant subjects were selected for comparison. Material and methods: Thyrotropin (TSH), free thyroxine (FT4), free triiodothyronine (FT3) and anti-TPO were measured using Roche Elecsys 1010 analyzer. Urinary iodine content was determined by simple microplate method. The 2.5th and 97.5th percentiles were calculated as the reference intervals for thyroid hormone levels during each trimester.
Statistical Analysis:
SPSS (version 14.0, SPSS Inc., Chicago, IL, USA) was used for data processing and analysis.
Results:
The reference intervals for the first, second and third trimesters for the following parameters: TSH 0.09-6.65, 0.51-6.66, 0.91-4.86 µIU/mL, FT4 9.81-18.53, 8.52-19.43, 7.39-18.28 pM/L and FT3 3.1-6.35, 2.39-5.12, 2.57-5.68 pM/L respectively. Thyroid hormone concentrations significantly differed during pregnancy at different stages of gestation. The pregnant women in the study had median urinary iodine concentration of 150-200 µg/l during each trimester.
Conclusions:
The trimester-specific reference intervals for thyroid tests during pregnancy have been established for pregnant Indian women serially followed during pregnancy using 2.5th and 97.5th percentiles.
Keywords: Anti-thyroid peroxidase, pregnant women, reference intervals, thyroid function tests, trimester
INTRODUCTION
Maternal and perinatal morbidities are well-documented complications of pregnancy in women with thyroid dysfunction, both clinical and subclinical. About 2–5% of pregnant women suffer from thyroid disorders, and timely intervention can be done if detected early.[1] It has been proven that maternal thyroid disorders influence the outcome of mother and fetus, during and also after pregnancy. Maternal hypothyroidism is the most frequent thyroid disorder in pregnancy and is associated with fetal loss, placental abruptions, preeclampsia, preterm delivery, and reduced intellectual function in the offspring.[2,3,4,5,6] In pregnancy, overt hypothyroidism is seen in 0.2% cases[4] and subclinical hypothyroidism in 2.3% cases.[7] Overt hyperthyroidism complicates pregnancy by fetal loss, fetal growth restriction, preeclampsia, and preterm delivery. Mild or subclinical hyperthyroidism is seen in 1.7%of pregnancies and is not associated with adverse outcomes.[8] Increasing attention has, therefore, focused on the diagnosis and treatment of maternal thyroid dysfunction during pregnancy.
Physiological alterations in the homeostatic control of thyroid hormones cause changes in thyroid function tests in pregnant women. These alterations in thyroid function tests are caused by an increase in thyroxine-binding globulin (TBG), thyroid stimulation by human chorionic gonadotropin and increased renal iodide clearance.[9,10] The rise in TBG can increase total thyroxine and total triiodothyronine levels to approximately 1.5 times the nonpregnant level by 16 weeks of gestation. Levels of free thyroxine (FT4) and free triiodothyronine (FT3) peak at 10–12 weeks of gestation and decline thereafter during second and third trimester to normal level. Because of physiological changes values of thyroid hormones during pregnancy differ from nonpregnant values. Values in pregnancy also vary between stages of gestation. Many factors such as ethnicity, age, manufacturer's methodology, iodine status, and calculation method may affect the establishment of reference intervals for thyroid function tests.[11,12] This necessitates the establishment of gestational age-specific and method-specific reference intervals for thyroid tests during pregnancy.
Several studies from different regions of the world[13,14,15] are available, but only one study till now has presented the trimester-specific thyroid function values in Indian women.[16] Because of the current limited availability of reference intervals for thyroid function tests in pregnant Indian women, we decided to evaluate this in our study for more accurate and appropriate interpretation of thyroid hormone levels in pregnant women.
SUBJECTS AND METHODS
Settings
The longitudinal study was conducted by the Division of Endocrine Research at the Institute of Nuclear Medicine and Allied Sciences (INMAS), Delhi between June 2007 and June 2010. The subjects were enrolled from various hospitals in Delhi: Gokalpuri Urban Health Centre under Maulana Azad Medical College, St. Stephen's Hospital, and Hindu Rao Hospital. The Institutional Ethics Committee at INMAS approved the study protocol, and the subjects were recruited after prior informed written consent.
Subjects
A total of 553 healthy women (403 normal singleton pregnant women and 150 nonpregnant women) in the age group of 18–45 years consuming iodized salt were recruited. Pregnant women in the first trimester (0–13 weeks) of gestation were enrolled. Women with personal and/or family history of thyroid disorders, palpable goiter, and on medication altering thyroid function were excluded from the study. Obstetric history was elicited to know duration of gestation, gravida, parity, and number of abortions by the gynecologist on duty in the clinic we visited for recruitment of subjects. Physical examination included anthropometry, goiter grading, general, and systemic examination. Further women with the positive serum thyroid peroxidase (TPO) antibody (TPO Ab >34 IU/ml) were excluded on the basis of laboratory results. All the women were serially followed throughout pregnancy. The women who missed any of the visits during pregnancy were also excluded from the analysis. The Flow chart of subject enrollment in study is depicted below.
Flow chart.
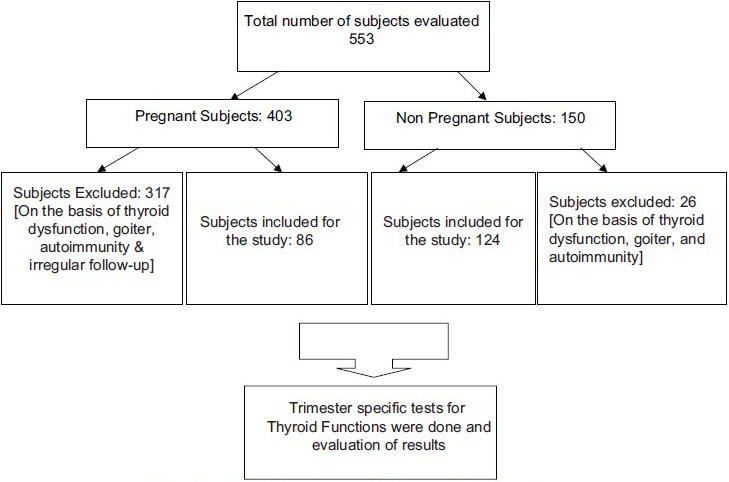
Flow chart of the subjects enrolled for the study
The values of serum FT3, FT4, and thyroid stimulating hormone (TSH) from this pregnant women group (n = 86) and nonpregnant women (n = 124) who were considered as “normal subjects” were used to calculate thyroid function test reference intervals.
The study has been approved by the Institutional Ethics Committee of INMAS, Delhi, India on the 5th March 2004.
Laboratory methods
Serum was immediately stored at −80°C until analysis. Serum TSH, FT3, FT4, and anti-TPO were estimated by the electrochemiluminescence (ECL) technique using commercially available kits from Roche Diagnostics (Mannheim, Germany) with Elecsys 1010 analyzer. The analytical sensitivity and total precision values for FT3, FT4, and TSH assays were 0.4 picomoles per liter (pM/L) and 2.2%, 0.3 pM/L and 2.7%, and 0.005 micro international units per milliliter (µIU/ml) and 2.2%, respectively. The laboratory reference ranges were FT3 (3.7–7.2 pM/L), FT4 (12–23 pM/L), and TSH (0.27–4.2 µ IU/ml). The intra-assay coefficients of variation (CV) for the assays were 1.7%, 1.4%, and 1.9%, respectively. The corresponding values for intra-assay CV, total precision and analytical sensitivity for anti-TPO were 5.6%, 7.6 and <5.01 IU/ml. Anti-TPO Ab was considered elevated if levels were >34 IU/ml.
Overt hypothyroidism was defined as the subject having biochemical values of low FT4 and high TSH as per laboratory reference range, whereas subjects with normal FT4 and high TSH were diagnosed as subclinical hypothyroidism.
Overt hyperthyroidism was defined as high values of FT3 and/or FT4 with a low value of TSH as per laboratory reference range, whereas normal FT3 and FT4 with low TSH was considered as subclinical hyperthyroidism.
Iodine content in urine was estimated using simple microplate method based on Sandell–Kolthoff reaction.[17]
Statistical analysis
SPSS(version 14.0, SPSS Inc., Chicago, IL, USA) was used for data processing and analysis. The average concentration of iodine in urine was calculated as median because of skewed data distribution. Mean with standard deviation, median, and 2.5th and 97.5th percentiles (i.e., a central 95% interval) were calculated for serum FT3 and FT4 at each trimester in comparison with nonpregnancy. TSH did not follow a normal distribution and has to be normalized using log-transformation and summarized as geometric mean (95% confidence interval). For data comparisons between pregnancy and nonpregnancy, and among three trimesters One-Way ANOVA with further Bonferroni post-hoc analysis was applied. A two-tailed P < 0.05 was considered statistically significant.
Ethical approval
The study has been approved by the Institutional Ethics Committee of INMAS, Delhi, India on the 5th March 2004.
RESULTS
The total study population consisted of 403 pregnant women. Two hundred and two subjects were primiparous, and history of abortion was present in 98 (24.31%) subjects. Clinical examination revealed goiter in 22.58% and anti-TPO Ab were elevated in 40 (9.93%) of the study subjects. Using the kit reference values, the incidence of overt hypothyroidism and overt hyperthyroidism was found to be 8.2% (33/403) and 0.25% (1/403), respectively. One hundred and forty-two subjects of total 403 subjects had complete three trimester's thyroid function tests values. Out of this 23 subjects had some thyroid dysfunction and had to be excluded from the study. After the exclusion of subjects that were anti-TPO positive had a goiter and were not euthyroid, total of 86 subjects were left with serial thyroid hormone and urinary iodine test values.
Among the 150 subjects in nonpregnant group, clinical examination revealed goiter in 19 (12.6%) and anti-TPO were elevated in 8 (5.3%). The incidence of overt hypothyroidism was 2.6%.
Normal study population
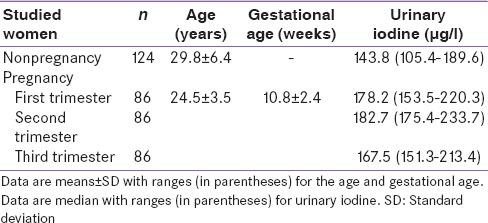
After applying the exclusion criteria, 317 women in the pregnant group and 26 women in the nonpregnant group were excluded from the study. The remaining subjects constituted the normal study population. The age, gestational age, and iodine nutritional status of the normal study population are summarized in Table 1. Pregnant women had a median urinary iodine concentration of 178.2 µg/l, 182.8 µg/l, and 167.5 µg/l during the first, second, and third trimester, respectively. All levels fell within the range (150–250 µg/l) recommended by WHO/UNICEF/ICCIDD, 2007 for pregnant women.[18] Urinary iodine levels of pregnant women were higher than those of nonpregnant women (143.8 µg/l).
Table 1.
Characteristics of studied women
Reference intervals for thyroid testing at each trimester of pregnancy
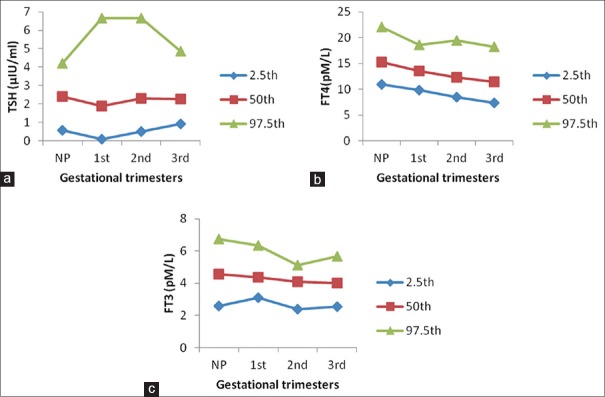
Trimester-specific reference intervals for thyroid hormones, based on the data of this study, are shown in Table 2. Manufacturer's nonpregnant adult reference intervals are also included for comparison. The 2.5th, 50th, and 97.5th percentiles for each hormone measured and for each trimester are shown in Figure 1a–c and demonstrate the profile and trend for reference intervals during pregnancy compared with nonpregnancy.
Table 2.
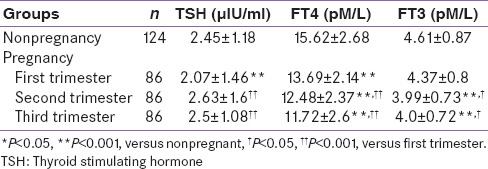
Trimester-specific reference intervals for thyroid function tests of pregnancy in comparison with nonpregnancy
Figure 1.
The profiles and trends of reference interval during three trimesters for thyroid stimulating hormone (a), free thyroxine (b), and free triiodothyronine (c) derived from 86 normal pregnant women with a comparison of 124 normal nonpregnant women. For all graphs, the bottom line indicates the lower reference limit (2.5th percentile), the middle line is the median level (50th percentile), and the top line is the upper reference limit (the 97.5th percentile). On horizontal axis, NP: Nonpregnancy; first, pregnancy at the first trimester; second, pregnancy at the second trimester; third, pregnancy at the third trimester
The TSH reference interval showed that lower TSH reference limit of 0.09 µ IU/ml occurred in the first trimester, which is significantly lower than the nonpregnant study population (0.58 µ IU/ml). The lower TSH reference limit in the second trimester almost approached the nonpregnancy level. The upper TSH reference limit remained stable in the first and second trimester (approximately 6.6 µ IU/ml) and then decreased. The lower reference intervals of FT4 decreased, whereas upper reference interval remained almost same throughout pregnancy. The reference intervals of FT3 decreased from first to the second trimester and then increased in the third trimester.
Trimester changes in thyroid hormone concentration during pregnancy
The average concentration of each hormone during each trimester is shown in Table 3. When comparing each hormone between pregnancy and nonpregnancy and among gestational stages, TSH concentration showed statistically significant decline in first trimester (P < 0.001), followed by a rise which almost reached the nonpregnancy level in the second trimester and third trimester. The mean concentration of FT4 gradually decreased throughout pregnancy, and it was significantly lower in second and third trimester as compared to the first trimester. The lowest levels of FT4 were recorded during the third trimester (24.97% below nonpregnancy levels). The mean concentrations of FT3 decreased significantly by 13.45% below the nonpregnancy level in the second trimester and then remained unchanged during the third trimester.
Table 3.
Thyroid hormone concentrations in pregnant versus nonpregnant study population
DISCUSSION
The findings of this study are in confirmation with those of other authors that changes in thyroid hormone levels during pregnancy differed between stages of gestation and compared with nonpregnancy levels. These findings support the use of gestational age-specific reference intervals for evaluating thyroid status in pregnant women.
Ethnic background could play a part in the prevalence of thyroid disease, as well as the establishment of reference intervals for TSH and FT4. Boucai and Surks reported that reference intervals of TSH and FT4 were significantly influenced by race and age and suggested that age and race-specific TSH reference intervals should be employed to provide accurate intervals for a specific population. This would minimize the misclassification of patients with thyroid disease.[19,20] Yan et al., compared reference intervals for TSH and FT4 between different authors and different manufacturer methods used for analysis and found that the major discrepancy exists in the upper not in the lower TSH reference limits.[21] Our data showed relatively higher cut-off values for the upper TSH reference limits in comparison to other studies.[12,14,21,22,23,24,25] This may be attributed to ethnic variance. A high prevalence of raised TSH was found in Whites as compared to Asians and Blacks when second trimester thyroid function was done.[12] Another study showed that Asian women have lower TSH than that of Caucasian women during the second trimester of pregnancy.[26] However, no difference was found by Price et al., 2001 in the thyroid hormone changes between Asian and Caucasian pregnant women.[27]
Several studies have been conducted on pregnant subjects among which some are cross-sectional while others are longitudinal in the same study group. They also vary on the assay method used for the estimation of thyroid hormones.
Analysis of our longitudinal data on mean TSH showed that it significantly increased in the second and third trimester as compared to first, whereas mean FT4 and FT3 significantly decreased with advancing pregnancy. Similar findings were reported by a recent study conducted by Mehran et al. where mean TSH rose significantly during second and third trimester as compared to the first.[28] However, Marwaha et al. reported no significant difference in values of FT3 and TSH between the trimesters though same assay method was used for the evaluation of thyroid hormones.[16] This difference may be due to the fact that we serially followed the women during gestation. Another study from India used radioimmunoassay to evaluate pregnant women and showed that TSH increased progressively with each trimester while serum T3 and T4 values increased from first to the second trimester, but declined from second to the third trimester.[29]
A study on group of pregnant women using immunoassay and mass spectrometry (MS) to measure FT4 and TSH reported that mean FT4 decreased by about 15% from the first to second trimester and then remained stable during the remainder of pregnancy. On the other hand, TSH concentration increased from first to second and then remained stable throughout pregnancy.[15]
Another longitudinal study carried out on paired samples from first and second trimesters of 20 Asian pregnant women to establish trimester-specific reference range of thyroid hormones used immunoassay technique.[27] They found that TSH increased from first to second while FT4 decreased with progress in pregnancy. FT3 did not change between first and second trimesters.
In a cross-sectional study on pregnant Chinese women, authors found an obvious decline in median TSH concentration during the first trimester compared with nonpregnancy (P < 0.01) followed by a rise in the second trimester. The mean concentrations of FT4 and FT3 gradually decreased throughout pregnancy with the lowest levels present during the third trimester (21.2% and 14.4% below nonpregnancy levels, respectively).[21]
A cross-sectional study of 522 pregnant subjects from Japan using ECL kits showed significant decrease in both FT3 and FT4 and increase in TSH with advancing pregnancy.[30]
Yan et al., compared the TSH reference intervals for nonpregnant adults provided by different manufacturers and concluded that manufacturer's methodology can significantly influence the setting of reference intervals, and such methodology should be standardized to enable comparison of data between countries. Moreover, each laboratory should determine reference intervals for the population it serves.[21]
Reference intervals for FT4 and TSH varied according to different manufacturer methods and between authors even when the same manufacturer method was applied.[21] The difference in trimester-specific reference intervals also depends on the calculation method (i.e., a central 95% interval with the 2.5th and 97.5th percentile values or fifth and 95th percentile value or fifth, and 98th percentile values) used. As compared to another Indian study,[16] our reference interval has higher upper TSH limit while the lower FT4 reference value was lesser. This may be attributed to the fact that we used 2.5th and 97.5th percentile for calculation instead of fifth and 95th percentile.
The rigor applied for selection of normal subjects may account for variations in reference intervals by different authors, despite the use of the same methodology for testing. The reference population should be as exclusive as possible. The U.S. National Academy of Clinical Biochemistry recommends that “trimester-specific reference intervals should be used when reporting thyroid function test values in pregnant women.”[31]
CONCLUSION
Prior to the introduction of screening for thyroid dysfunction in early pregnancy, it is necessary to establish trimester-specific reference intervals for TSH, FT4, and FT3 based on ethnic background, methods of analysis, iodine status, selection criteria of normal subjects, and calculation method. The reference intervals determined in this study for each trimester of pregnancy are recommended for evaluation of pregnant Indian women. These study findings are a step toward establishing trimester-specific normative data of thyroid function tests in ethnic Indian pregnant population.
Limitations of the study
Thyroid ultrasonography was not be performed in this study as we focused mainly on the biochemical parameters
Estimation of anti-thyroglobulin was not performed in this study, however, anti-TPO was done on all these subjects, and it is a better marker of thyroid autoimmunity
This study has no data available for these subjects in pre-and post-pregnancy period because of the lack of facilities for follow-up.
Acknowledgments
The authors would like to extend their special thanks to the Department of Community Medicine, MAMC, doctors and entire staff of Urban Health Centre, Gokulpuri, Department of Gynaecology and Obstetrics and Department of Endocrinology, St. Stephens Hospital. Further we would like to thank Director, INMAS for his constant support and guidance for carrying out the study. Thanks are due to each of our subjects for their consent and time.
The study was funded by a research grant from the INMAS, Defence Research and Development, Ministry of Defence, New Delhi, India under the Science and Technology Project (INM 305).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Glinoer D, Soto F, Bourdoux P. Pregnancy in patients with mild thyroid abnormalities: Maternal and neonatal repercussions. J Clin Endocrinol Metab. 1991;73:421–7. doi: 10.1210/jcem-73-2-421. [DOI] [PubMed] [Google Scholar]
- 2.Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12:63–8. doi: 10.1089/105072502753451986. [DOI] [PubMed] [Google Scholar]
- 3.Allan WC, Haddow JE, Palomaki GE. Maternal thyroid deficiency and pregnancy complications: Implications for population screening. J Med Screen. 2000;7:127–30. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 4.Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol. 2006;108:1283–92. doi: 10.1097/01.AOG.0000244103.91597.c5. [DOI] [PubMed] [Google Scholar]
- 5.Morreale de Escobar G, Obregón MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–87. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 6.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 7.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 8.Casey BM, Dashe JS, Wells CE, McIntire DD, Leveno KJ, Cunningham FG. Subclinical hyperthyroidism and pregnancy outcomes. Obstet Gynecol. 2006;107(2 Pt 1):337–41. doi: 10.1097/01.AOG.0000197991.64246.9a. [DOI] [PubMed] [Google Scholar]
- 9.Glinoer D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 10.Rinaldi MD, Stagnaro-Green AS. Thyroid disease and pregnancy: Degrees of knowledge. Thyroid. 2007;17:747–53. doi: 10.1089/thy.2007.0080. [DOI] [PubMed] [Google Scholar]
- 11.Roti E, Gardini E, Minelli R, Bianconi L, Flisi M. Thyroid function evaluation by different commercially available free thyroid hormone measurement kits in term pregnant women and their newborns. J Endocrinol Invest. 1991;14:1–9. doi: 10.1007/BF03350244. [DOI] [PubMed] [Google Scholar]
- 12.La’ulu SL, Roberts WL. Second-trimester reference intervals for thyroid tests: The role of ethnicity. Clin Chem. 2007;53:1658–64. doi: 10.1373/clinchem.2007.089680. [DOI] [PubMed] [Google Scholar]
- 13.Dhatt GS, Jayasundaram R, Wareth LA, Nagelkerke N, Jayasundaram K, Darwish EA, et al. Thyrotrophin and free thyroxine trimester-specific reference intervals in a mixed ethnic pregnant population in the United Arab Emirates. Clin Chim Acta. 2006;370:147–51. doi: 10.1016/j.cca.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Panesar NS, Li CY, Rogers MS. Reference intervals for thyroid hormones in pregnant Chinese women. Ann Clin Biochem. 2001;38(Pt 4):329–32. doi: 10.1258/0004563011900830. [DOI] [PubMed] [Google Scholar]
- 15.Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: Trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008;115:602–6. doi: 10.1111/j.1471-0528.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi T, Yamaki M, Pandav CS, Karmarkar MG, Irie M. Simple microplate method for determination of urinary iodine. Clin Chem. 2000;46:529–36. [PubMed] [Google Scholar]
- 18.3rd ed. Geneva: WHO; 2007. WHO/UNICEF/ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination. [Google Scholar]
- 19.Boucai L, Surks MI. Reference limits of serum TSH and free T4 are significantly influenced by race and age in an urban outpatient medical practice. Clin Endocrinol (Oxf) 2009;70:788–93. doi: 10.1111/j.1365-2265.2008.03390.x. [DOI] [PubMed] [Google Scholar]
- 20.Surks MI, Boucai L. Age-and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab. 2010;95:496–502. doi: 10.1210/jc.2009-1845. [DOI] [PubMed] [Google Scholar]
- 21.Yan YQ, Dong ZL, Dong L, Wang FR, Yang XM, Jin XY, et al. Trimester-and method-specific reference intervals for thyroid tests in pregnant Chinese women: Methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf) 2011;74:262–9. doi: 10.1111/j.1365-2265.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 22.Moleti M, Lo Presti VP, Campolo MC, Mattina F, Galletti M, Mandolfino M, et al. Iodine prophylaxis using iodized salt and risk of maternal thyroid failure in conditions of mild iodine deficiency. J Clin Endocrinol Metab. 2008;93:2616–21. doi: 10.1210/jc.2008-0352. [DOI] [PubMed] [Google Scholar]
- 23.Lambert-Messerlian G, McClain M, Haddow JE, Palomaki GE, Canick JA, Cleary-Goldman J, et al. First-and second-trimester thyroid hormone reference data in pregnant women: A FaSTER (First-and Second-Trimester Evaluation of Risk for aneuploidy) Research Consortium study. Am J Obstet Gynecol. 2008;199:62.e1–6. doi: 10.1016/j.ajog.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, et al. Evaluation of maternal thyroid function during pregnancy: The importance of using gestational age-specific reference intervals. Eur J Endocrinol. 2007;157:509–14. doi: 10.1530/EJE-07-0249. [DOI] [PubMed] [Google Scholar]
- 25.Gong Y, Hoffman BR. Free thyroxine reference interval in each trimester of pregnancy determined with the Roche Modular E-170 electrochemiluminescent immunoassay. Clin Biochem. 2008;41:902–6. doi: 10.1016/j.clinbiochem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Price A, Davies R, Heller SR, Milford-Ward A, Weetman AP. Asian women are at increased risk of gestational thyrotoxicosis. J Clin Endocrinol Metab. 1996;81:1160–3. doi: 10.1210/jcem.81.3.8772593. [DOI] [PubMed] [Google Scholar]
- 27.Price A, Obel O, Cresswell J, Catch I, Rutter S, Barik S, et al. Comparison of thyroid function in pregnant and non-pregnant Asian and western Caucasian women. Clin Chim Acta. 2001;308:91–8. doi: 10.1016/s0009-8981(01)00470-3. [DOI] [PubMed] [Google Scholar]
- 28.Mehran L, Amouzegar A, Delshad H, Askari S, Hedayati M, Amirshekari G, et al. Trimester-specific reference ranges for thyroid hormones in Iranian pregnant women. J Thyroid Res 2013. 2013:651517. doi: 10.1155/2013/651517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Gupta N, Nath T, Sharma JB, Sharma S. Thyroid function tests in pregnancy. Indian J Med Sci. 2003;57:252–8. [PubMed] [Google Scholar]
- 30.Kurioka H, Takahashi K, Miyazaki K. Maternal thyroid function during pregnancy and puerperal period. Endocr J. 2005;52:587–91. doi: 10.1507/endocrj.52.587. [DOI] [PubMed] [Google Scholar]
- 31.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]