Abstract
Objective:
Objective was to evaluate reproductive hormones levels in hypothyroid women and impact of treatment on their levels.
Materials and Methods:
A total of 59 women with untreated primary hypothyroidism were included in this prospective study. Venous blood was taken at baseline and after euthyroidism was achieved for measuring serum free thyroxine, free triiodothyronine (FT3), thyroid stimulating hormone (TSH), prolactin (PRL), follicular stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), testosterone (T), and thyroid peroxidase antibody. Thirty-nine healthy women with regular menstrual cycles without any hormonal disturbances served as controls. The statistical analysis was performed using the Statistical Package for the Social Sciences Version 20 ([SPSS] IBM Corporation, Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results:
On an average at diagnosis cases have more serum TSH (mean [M] = 77.85; standard error [SE] = 11.72), PRL (M = 39.65; SE = 4.13) and less serum E2 (M = 50.00; SE = 2.25) and T (M = 35.40; SE = 2.31) than after achieving euthyroidism (M = 1.74; SE = 0.73), (M = 16.04; SE = 0.84), (M = 76.25; SE = 2.60), and (M = 40.29; SE = 2.27), respectively. This difference was statistically significant t (58) = 6.48, P <0.05; t (58) = 6.49, P < 0.05; t (58) = 12.47; P < 0.05; and t (58) = 2.04, P < 0.05; respectively. Although average serum FSH (M = 12.14; SE = 0.40) and LH (M = 5.89; SE = 0.27) were lower in cases at diagnosis than after achieving euthyroidism (M = 12.70; SE = 0.40), (M = 6.22; SE = 0.25), respectively, but these differences were statistically insignificant t (58) = 1.61, P = 0.11; t (58) = 1.11, P = 0.27, respectively.
Conclusion:
The study has demonstrated low E2 and T levels in hypothyroid women which were increased after achieving euthyroidism. Although average serum FSH and LH were increased in hypothyroid women after achieving euthyroidism but this difference was statistically insignificant.
Keywords: Estradiol, follicular stimulating hormone, hypothyroidism
INTRODUCTION
Thyroid dysfunction is a common endocrine disorder. In the US National Health and Nutrition Examination Survey, the prevalence of hypothyroidism was 4.6%(0.3 overt and 4.3% subclinical) and the prevalence of hyperthyroidism 1.3%(0.5 overt and 0.7% subclinical) in people without known thyroid disease or a family history of thyroid disease.[1] A study from eight major Indian cities have shown the prevalence of hypothyroidism in the urban India is 10.95% in which 3.47% were previously undetected, and 7.48% were self-reported cases.[2] Thyroid dysfunction is usually acquired and may occur anytime in life. In the reproductive age women, thyroid autoimmunity is the most prevalent cause of thyroid dysfunction.[3,4] Thyroid hormones (TH) are secreted by follicular cells of the thyroid gland of which thyroxine (T4) is the major form and triiodothyronine (T3) is the predominant active form present in the circulation. At the tissue level, TH actions are regulated by a family of intracellular deiodinases (DIOs): Hepatic type 1 DIO mediates peripheral T4 to T3 conversion; DIO2 converts T4 to T3 in the hypothalamus and pituitary, thereby playing a central role in negative feedback regulation of the hypothalamic-pituitary-thyroid axis; in contrast DIO3 converts T4 to reverse T3 and T3 to T2, thereby limiting TH action.[5] Although nongenomic actions of TH are recognized but its major actions are mediated by binding to specific receptors which are called thyroid hormone receptors (TRs) in the nucleus of target cells.[6] There are two thyroid hormone receptor genes (TRa, TRb) present on chromosomes 17 and 3, respectively. Each thyroid hormone receptor genes gene undergoes alternate splicing to generate TRa1, TRa2, TRb1, and TRb2 isoforms, each with differing tissue distributions (e.g. TRa1 is predominantly expressed in the central nervous system, myocardium, colon, and skeletal muscle; TRb1 is mostly expressed in the liver and kidney; TRb2 plays a major role in negative feedback regulation at the level of the hypothalamus and pituitary).[7,8,9]
Recently, it was reported that TRs are also present in human ovarian surface epithelium and act on ovarian follicles and shows some slight localization in granulosa cells of ovarian follicles.[10] It has been reported that THs regulate a variety of biological processes including growth, cellular oxygen consumption, metabolism, embryonic development, tissue differentiation, and maturation.[11] In mammals, down-regulation of TRs lower the fertility and decrease follicle number.[12] Hypothyroidism has been associated with the altered ovarian function, menstrual irregularities, subfertility, and higher (recurrent) miscarriage rates, suggesting that thyroid hormone affects female reproductive axis.[13,14]
Hypothyroidism causes an increase in the levels of thyroid releasing hormone (TRH) which in turn stimulates secretion of thyroid stimulating hormone (TSH) and prolactin (PRL) and PRL inhibits the synthesis and secretion of gonadotrophins. Several studies have also confirmed abnormal menstrual patterns in overt hypothyroidism.[15] The association of hypothyroidism and infertility,[16] polycystic ovarian syndrome,[17] metabolic syndrome,[18] atherosclerosis,[19] heart disease,[20] and cognitive function[21] are well described in the literature but studies that have focused on association in between overt hypothyroidism and female reproductive hormone are sparse particularly from Indian subcontinent. Therefore, present study aimed to intensively evaluate the relationship in between overt hypothyroidism and female reproductive hormone and effect of thyroid replacement therapy.
MATERIALS AND METHODS
A total of 113 women with untreated primary hypothyroidism among those attending Outpatient Department of Endocrinology Clinic of LLRM Medical College, Meerut, Western Uttar Pradesh between August 2011 and June 2015 were recruited for this prospective study. As people living in rural areas around the Meerut were devoid of medical services, of those some patients presented with a full-blown clinical picture of hypothyroidism. Pregnancy occurred in three patients during the study, and these patients were excluded from the final analyses. The study was approved by the Institutional Committee of Ethical practice of our institution, and all of the study participants provided written informed consent. Only reproductive age group patients were included in the study who had no history of previous ovarian surgery and had not received any medication that could affect adrenal hormone metabolism. Only 1st time diagnosed patient of primary hypothyroidism were included in the study. Patients who had received thyroid hormone replacement therapy prior to the presentation were excluded from the study. Primary hypothyroidism was diagnosed on the basis of low serum free thyroxine (FT4) (<0.7 ng/dL) and elevated TSH (>5 μIU/mL) levels together with the presence of signs and symptoms of hypothyroidism. Any patient with the presence of secondary hypothyroidism, congenital adrenal hyperplasia (CAH), Cushing syndrome, androgen-secreting ovarian or adrenal tumor and polycystic ovary syndrome (PCOS) were excluded from the study. Exclusion of PCOS was based on Rotterdam criteria.[22] CAH was excluded with fasting 8 am 17-hydroxyprogesterone in the follicular phase and if was inconclusive then an intravenous adrenocorticotropic hormone stimulation test was done. Thirty-nine healthy women with regular menstrual cycles without any hormonal disturbances served as controls. Then, patients were divided into two groups: (1) Controls (with normal menstrual cycle, normal hormonal profile, and without any endocrinologic diseases) (2) primary hypothyroid patients. Data on the demographic characteristics (e.g., age, education, and occupation), lifestyle characteristics (e.g., smoking, alcohol consumption, and physical activity), health status, and medical history were collected using a standardized proforma. The anthropometric measurements were performed by trained personnel using a standardized protocol.
Venous blood was taken at baseline and after euthyroidism was achieved for measuring serum FT4, free triiodothyronine (FT3), TSH, PRL, follicular stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), testosterone (T), and thyroid peroxidase antibody (anti-TPO). Serum for FSH, LH, E2 and T were taken in the fasting state at 8 am in the early follicular phase (in women with regular menstrual cycles) or on an appropriate day for amenorrheic patient. Patients with hypothyroidism were treated with sufficient doses of L-thyroxine in the form of a tablet (Thyronorm, abbott). After achieving a euthyroid state, serum hormones levels were re-evaluated. Subjected to the age of the patient, severity of the hypothyroidism and compliance, the mean time to achieve euthyroidism was 5.7 months in the study. Finally, 74 patients completed the follow-up out of which 59 were oligomenorrhic which were included in the study. The underlying cause of hypothyroidism was autoimmune thyroiditis (n = 56), postpartum hypothyroidism (n = 2) or treatment with radioactive iodine (n = 1). Most of the patients had autoimmune thyroiditis and were, for the 1st time in their lives, found to be hypothyroid. Therefore, the mean duration of hypothyroidism in the study population could not be determined in retrospect.
Serum FT4, FT3, TSH, PRL, E2, T, and anti-TPO all were measured with chemiluminescence method by abbott architect i1000SR in endocrinology laboratory of the hospital.
Statistical analysis
For numerical variables, descriptive statistics was performed, and the results were expressed as a mean ± standard deviation. Pearson correlation was used for normally distributed variables. Pretreatment comparisons between controls and primary hypothyroid patients were performed by the unpaired t-test. The pretreatment and posttreatment data of patients with hypothyroidism were compared using the paired t-test. The Statistical analysis was performed using the Statistical Package for the Social Sciences Version 20 ([SPSS] IBM Corporation, Armonk, NY, USA). P < 0.05 was considered statistically significant.
RESULTS
As Table 1 shows baseline characteristic of study population, a total 74 cases and 39 control subjects of the same demographic profile were included in the study. All parameters Age, body mass index (BMI), systolic blood pressure, diastolic blood pressure, FT4, FT3, TSH, FSH, LH, PRL, E2, and T are highly variable in all participants. In the cases mean age, BMI, TSH, FSH, LH, PRL, E2, and T were 27.88 ± 5.39, 24.91 ± 3.13, 77.85 ± 90.09, 12.14 ± 3.12, 5.89 ± 2.12, 39.65 ± 31.78, 50.00 ± 17.33, and 35.40 ± 17.8, respectively. In controls mean age, BMI, TSH, FSH, LH, PRL, E2, and T were 26.79 ± 4.00, 25.04 ± 3.07, 2.24 ± 0.80, 13.19 ± 2.93, 6.25 ± 2.69, 7.58 ± 4.00, 81.48 ± 34.52, and 43.57 ± 18.67, respectively.
Table 1.
Baseline characteristic
Comparison in between serum levels of reproductive hormones of cases and controls by independent t-test
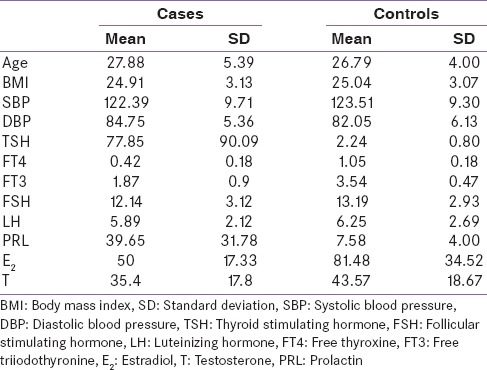
Using independent t-test as shown in Table 2, we analyzed the difference between serum levels reproductive hormones of cases and controls. On an average cases have more serum TSH (mean [M] = 77.85; standard error [SE] = 11.72) and PRL (M = 39.65; SE = 4.13) than controls (M = 2.24; SE = 0.12), (M = 7.58; SE = 0.64), respectively. This difference was statistically significant t (96) = 5.23, P < 0.05; t (96) = 6.25, P < 0.05.
Table 2.
Comparison in between serum levels of reproductive hormones of cases and controls by independent t-test
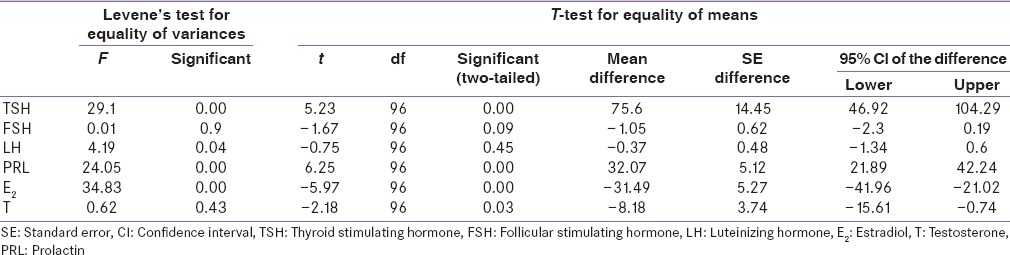
However, serum E2 (M = 50.00; SE = 2.25) and T (M = 35.40; SE = 2.31) was less in cases than controls (M = 81.48; SE = 5.52), (M = 43.57; SE = 2.99), respectively. This differences were statistically significant t (96) = 5.97, P < 0.05; t (96) = 2.18, P < 0.05, respectively.
Although serum FSH (M = 12.14; SE = 0.40) and LH (M = 5.89; SE = 0.27) were lower in cases as compare to controls (M = 13.19; SE = 0.46), (M = 6.25; SE = 0.43), respectively, but these differences were statistically insignificant t (96) = 1.67, P = 0.09; t (96) = 0.75, P = 0.45, respectively.
Comparison between serum level of reproductive hormones of cases before and after achieving euthyroidism by dependent t-test
Using independent t-test as shown in Table 3 we analyzed the difference between serum levels reproductive hormones of cases at diagnosis and after achieving euthyroidism. On an average at diagnosis cases have more serum TSH (M = 77.85; SE = 11.72) and PRL (M = 39.65; SE = 4.13) than after achieving euthyroidism (M = 1.74; SE = 0.73), (M = 16.04; SE = 0.84), respectively. This difference was statistically significant t (58) = 6.48, P < 0.05; t (58) = 6.49, P < 0.05.
Table 3.
Comparison between serum level of reproductive hormones of cases before and after achieving euthyroidism by dependent t-test
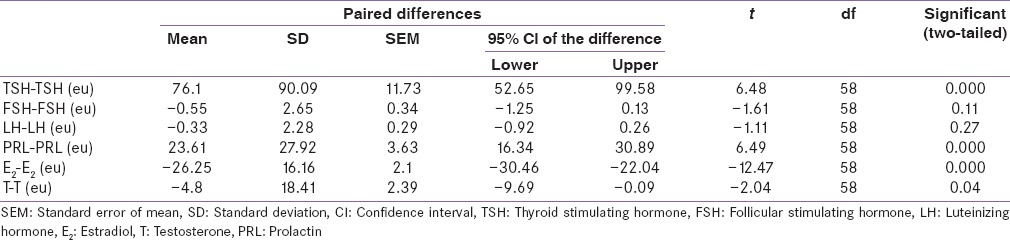
However, serum E2 (M = 50.00; SE = 2.25) and T (M = 35.40; SE = 2.31) was less in cases at diagnosis than after achieving euthyroidism (M = 76.25; SE = 2.60), (M = 40.29; SE = 2.27), respectively. These differences were statistically significant t (58) = 12.47, P < 0.05; t (58) = 2.04, P < 0.05, respectively.
Although average serum FSH (M = 12.14; SE = 0.40) and LH (M = 5.89; SE = 0.27) were lower in cases at diagnosis than after achieving euthyroidism (M = 12.70; SE = 0.40), (M = 6.22; SE = 0.25) respectively but these differences were statistically insignificant t (58) = 1.61, P = 0.11; t (58) = 1.11, P = 0.27, respectively.
DISCUSSION
Thyroid dysfunction can cause disturbances in the ovarian cycle and also ovulation, but, the molecular link between these two disorders still largely unrevealed. Hypothyroidism causes decreased rates of metabolic clearance of androstenedione and estrone in women and unveils an increase in peripheral aromatization.[23] Hypothyroid women also exhibit decreased 5 α/β ratio of androgen metabolites, and also show an increase in excretion of 2-oxygenated estrogens.[24] In hypothyroidism plasma binding activity of SHBG is decreased, which results in decreased plasma concentrations of both total testosterone and E2, but their unbound fractions are increased.[25] Altered metabolism of these gonadal steroids disappears when a euthyroid state is restored. The gonadotropins (Gn) level usually remains normal in hypothyroidism.[26]
Ovarian follicles, from the pool of resting primordial follicles either continue to grow from preantral to antral follicles due to survival signals, such as gonadotrophins and growth factors, or degenerate and die by the process of follicular atresia. Expressions of hormones and growth factors have been shown to regulate the destiny of the ovarian follicle. In humans, disorders of the thyroid gland are responsible for a dysregulation of the hypothalamus, pituitary, gonadal axis, and hypothyroidism is associated with oligomenorrhea.[16] The follicular fluid composition might be the important regulator for developing oocytes and may play a substantial role in oocyte quality. Both T3 and T4 are found in the follicular fluid of humans, and a positive correlation was demonstrated between serum T4 and follicular fluid T4 levels.[27] The presence of thyroid hormone receptors in human oocytes may explain TH response on the ovaries. Both isoforms of TRs messenger RNA (mRNA) are expressed in the human oocyte. Therefore, thyroid hormone may directly affect the oocyte.[28] Although the presence of TRa and TRb mRNA in granulosa cells has been reported earlier, Zheng et al. have demonstrated that TRa1 is expressed and localized in oocytes, granulosa cells and theca cells during follicular development.[28,29]
The results of the present study provided a novel understanding of the involvement of thyroid hormones and female reproductive hormones. In this study, we examined the alteration in the levels of reproductive hormones in reproductive age group hypothyroid female and the effect of thyroid hormone replacement therapy on their levels was also examined. We demonstrated that normal levels of pituitary Gn in overt hypothyroidism. We confirmed the hypothyroidism by measuring serum levels of TSH, FT4, and FT3. We further reported that hypothyroidism diminished serum E2 concentrations in reproductive age group female. To the best of our knowledge, this is the first study of hypothyroidism and diminished E2 level in India.
Serum level of FSH and LH are significantly low in cases of overt hypothyroid women when done between day 2 and 5 of the cycle.[30] Studies have demonstrated that serum estradiol was also reduced significantly in the hypothyroid state when compared to the control.[31] In other study Hapon et al. have shown hypothyroidism does not influence the classical preovulatory patterns of LH and FSH secretion in rats.[32] Studies have demonstrated a positive correlation in between TSH and PRL in hypothyroid women.[33] In several study, it was shown that T4 administration in hypothyroidism normalizes PRL and LH levels, increased folliculogenesis and estradiol secretion, reverses menstrual abnormalities and increases spontaneous fertility.[34] T3 is considered a biological amplifier of the stimulatory action of gonadotrophins on granulosa cell function.
CONCLUSION
Our study demonstrated that hypothyroidism in women causes menstrual irregularities, mostly oligomenorrhea. Hypothyroidism is associated with hyperprolactinemia, and it decreases the serum levels of E2, T, and Gn which increase after achieving euthyroidism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank The Principal, LLRM Medical College for supporting endocrinology laboratory for doing all investigations free of cost. No other potential conflicts of interests relevant to this article were reported.
REFERENCES
- 1.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T (4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647–52. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 4.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T (4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 5.Bianco AC, Kim BW. Deiodinases: Implications of the local control of thyroid hormone action. J Clin Invest. 2006;116:2571–9. doi: 10.1172/JCI29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osmak-Tizon L, Poussier M, Cottin Y, Rochette L. Non-genomic actions of thyroid hormones: Molecular aspects. Arch Cardiovasc Dis. 2014;107:207–11. doi: 10.1016/j.acvd.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Gurnell M, Visser T, Beck-Peccoz P. Resistance to thyroid hormone. In: Jameson JL, De Groot LJ, editors. Endocrinology. 6th ed. Philadelphia, PA: Saunders Elsevier; 2010. pp. 1745–59. [Google Scholar]
- 8.Huang YH, Tsai MM, Lin KH. Thyroid hormone dependent regulation of target genes and their physiological significance. Chang Gung Med J. 2008;31:325–34. [PubMed] [Google Scholar]
- 9.Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, et al. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod Biomed. 2009;18:337–47. doi: 10.1016/s1472-6483(10)60091-0. [DOI] [PubMed] [Google Scholar]
- 11.Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function. J Endocrinol. 2008;199:351–65. doi: 10.1677/JOE-08-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 13.Krassas GE, Papadopoulou F, Tziomalos K, Zeginiadou T, Pontikides N. Hypothyroidism has an adverse effect on human spermatogenesis: A prospective, controlled study. Thyroid. 2008;18:1255–9. doi: 10.1089/thy.2008.0257. [DOI] [PubMed] [Google Scholar]
- 14.van den Boogaard E, Vissenberg R, Land JA, van Wely M, van der Post JA, Goddijn M, et al. Significance of (sub) clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: A systematic review. Hum Reprod Update. 2011;17:605–19. doi: 10.1093/humupd/dmr024. [DOI] [PubMed] [Google Scholar]
- 15.Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Paunkovic J, Paunkovic N, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf) 1999;50:655–9. doi: 10.1046/j.1365-2265.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 16.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–55. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 17.Singla R, Gupta Y, Khemani M, Aggarwal S. Thyroid disorders and polycystic ovary syndrome: An emerging relationship. Indian J Endocrinol Metab. 2015;19:25–9. doi: 10.4103/2230-8210.146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogbera AO, Kuku S, Dada O. The metabolic syndrome in thyroid disease: A report from Nigeria. Indian J Endocrinol Metab. 2012;16:417–22. doi: 10.4103/2230-8210.95688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–44. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 20.Farwell A, Hennessey JV, Wartofsky L. Hypothyroidism and heart disease. J Clin Endocrinol Metab. 2013;98:39A–40A. [Google Scholar]
- 21.Samuels MH. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 2014;21:377–83. doi: 10.1097/MED.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Redmond GP. Thyroid dysfunction and women's reproductive health. Thyroid. 2004;14(Suppl 1):S5–15. doi: 10.1089/105072504323024543. [DOI] [PubMed] [Google Scholar]
- 24.Krassas GE, Pontikides NE. Werner and Ingbar's the Thyroid. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2013. The male and female reproductive system in hypothyroidism; pp. 585–6. [Google Scholar]
- 25.Hampl R, Kancheva R, Hill M, Bicíková M, Vondra K. Interpretation of sex hormone-binding globulin levels in thyroid disorders. Thyroid. 2003;13:755–60. doi: 10.1089/105072503768499644. [DOI] [PubMed] [Google Scholar]
- 26.Brent GA, Devis TF. Hypothyroidism and thyroiditis. In: Melmed S, editor. Williams Textbook of Endocrinology. 11th ed. Philadelphia: WB Saunders; 2011. pp. 412–3. [Google Scholar]
- 27.Cedíková M, Babuška V, Rajdl D, Zech NH, Kališ V, Králícková M. Comparison of prolactin, free T3 and free T4 levels in the follicular fluid of infertile women and healthy fertile oocyte donors. Ceska Gynekol. 2012;77:471–6. [PubMed] [Google Scholar]
- 28.Zhang SS, Carrillo AJ, Darling DS. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol Hum Reprod. 1997;3:555–62. doi: 10.1093/molehr/3.7.555. [DOI] [PubMed] [Google Scholar]
- 29.Zheng K, Sulieman FJ, Li J, Wei Q, Xu M, Shi F. Nitric oxide and thyroid hormone receptor alpha 1 contribute to ovarian follicular development in immature hyper-and hypo-thyroid rats. Reprod Biol. 2015;15:27–33. doi: 10.1016/j.repbio.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Acharya N, Acharya S, Shukla S, Inamdar SA, Khatri M, Mahajan SN. Gonadotropin levels in hypothyroid women of reproductive age group. J Obstet Gynaecol India. 2011;61:550–3. doi: 10.1007/s13224-011-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ajayi AF, Akhigbe RE, Ajayi LO. Hypothalamic-pituitary-ovarian axis in thyroid dysfunction. West Indian Med J. 2013;62:835–8. doi: 10.7727/wimj.2013.038. [DOI] [PubMed] [Google Scholar]
- 32.Hapon MB, Gamarra-Luques C, Jahn GA. Short term hypothyroidism affects ovarian function in the cycling rat. Reprod Biol Endocrinol. 2010;8:14. doi: 10.1186/1477-7827-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binita G, Suprava P, Mainak C, Koner BC, Alpana S. Correlation of prolactin and thyroid hormone concentration with menstrual patterns in infertile women. J Reprod Infertil. 2009;10:207–12. [PMC free article] [PubMed] [Google Scholar]
- 34.Atis G, Dalkilinc A, Altuntas Y, Atis A, Caskurlu T, Ergenekon E. Sexual dysfunction in women with clinical hypothyroidism and subclinical hypothyroidism. J Sex Med. 2010;7:2583–90. doi: 10.1111/j.1743-6109.2010.01815.x. [DOI] [PubMed] [Google Scholar]


