Abstract
The gradual decline in β-cell function is inevitable in type 2 diabetes mellitus and therefore, substantial proportions of patients require insulin subsequently, in order to achieve optimal glucose control. While weight gain, hypoglycemia, and fluid retention especially during dose intensification is a known limitation to insulin therapy, these adverse effects also reduce patient satisfaction and treatment adherence. It is also possible that the benefits of intensive control achieved by insulin therapy, perhaps get nullified by the weight gain and hypoglycemia. In addition, improvement in plasma glucose or glycated hemoglobin (HbA1c) itself is associated with weight gain. Notably, studies have already suggested that reduction in body weight by ~3–5%, may allow a significantly better glycemic control. Thus, a class of drugs, which can reduce HbA1c effectively, yet are weight neutral or preferably reduce body weight, could be the most sought out strategy as an add-on therapy to insulin. While sulfonylureas (SUs) are associated with weight gain and hypoglycemia, pioglitazone increases body weight and fluid retention. Moreover, SUs are not recommended once premix or prandial insulin is commenced. The addition of newer agents, such as glucagon-like peptide-1 receptor agonist to insulin certainly appears to be an effective tool in reducing both HbA1c and body weight as is evident across the studies; however, this approach incurs an additional injection as well as cost. Dipeptidyl peptidase-4 inhibitors (DPP-4I) and sodium-glucose co-transporter-2 inhibitors (SGLT-2I) are other exciting options, as an add-on to insulin therapy primarily because these are oral drugs and do not possess any intrinsic potential of hypoglycemia. Furthermore, these are either weight neutral or induce significant weight loss. This review article aims to comparatively analyze the safety and efficacy of DPP-4I and SGLT-2I, as an add-on therapy to insulin.
Keywords: Add-on to insulin, dipetidyl peptidase-4 inhibitors, genito-urinary infection, hypoglycemia, sodium-glucose co-transporter-2 inhibitors
INTRODUCTION
Type 2 diabetes mellitus (T2DM) being a progressive disease eventually leads to worsening of beta cell function. Consequently, the addition of insulin is largely necessary with the increasing duration of diabetes.[1,2] Intriguingly, there is limited recommendations as to how the other anti-diabetic agents (OAD) be used, once insulin is initiated. Studies have shown that addition of OADs such as sulfonylureas (SU), metformin, and pioglitazone to insulin therapy allows lesser requirement of insulin dose.[3] It is well-documented that addition of insulin sensitizers such as metformin as an add-on agents to insulin therapy is complementary and may counteract the weight gain associated with insulin.[4] However, utility of SU as an add-on agents to insulin therapy is less clear. The general recommendation is to continue SU with basal insulin and to reduce SU dosage, once the basal insulin titration is intensified. In addition, to discontinue SU, once prandial or premix insulin is commenced.[5] Nevertheless, SU causes weight gain and can potentiate the risk of hypoglycemia when used as an add-on to insulin.[3,6] Pioglitazone as an add-on to insulin has been associated with weight gain, edema, and increased risk of congestive heart failure.[6,7]
Glucagon-like peptide-1 receptor agonists (GLP-1RA) are other potentially effective add-on agent to the insulin that lowers glucose and body weight simultaneously.[8,9,10,11] However; this approach will incur an additional injection and cost. Single co-formulation of basal insulin with GLP-1RA such as IDegLira (insulin degludec with liraglutide) and LixiLan (insulin glargine with lixisenatide) can minimize the number of pricks.[12]
Newer class of OAD, such as dipeptidyl peptidase-4 inhibitors (DPP-4I) and sodium-glucose co-transporter-2 inhibitors (SGLT-2I) are other exciting option to improve glycemic control as add-on agents to insulin therapy. DPP-4I improves glycemic control, by enhancing concentrations of active incretin such as GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), both of which increase insulin secretion from the β-cells in a glucose-dependent manner. Moreover, there could perhaps be several other novel mechanisms by which DPP-4I improve glycemic control.[13] While DPP-4I significantly reduced glucagon during hyperglycemia, it does not compromise glucagon secretion during hypoglycemia and hence it has an ideal effect on glucagon dynamics. This GIP-mediated augmentation of glucagon secretion could be a plausible defense mechanism against hypoglycemia and makes this class a logical option of add-on agent to insulin therapy.[14,15] Moreover, DPP-4I are weight neutral, safe in elderly and patients with renal impairment. Furthermore, DPP-4I are cardiac neutral drug, as seen in different cardio-vascular outcome trials (CVOT) such as saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus (SAVOR-TIMI), Alogliptin after acute coronary syndrome in patients with type 2 diabetes (EXAMINE), and effect of sitagliptin on cardiovascular outcomes in type 2 diabetes (TECOS) trials. It is important to note that 41.6% patient in SAVOR-TIMI, 30.3% in EXAMINE and 23.5% of patients in TECOS trial were already receiving concomitant insulin therapy in these high-risk CV disease cohort.[16,17,18] This indirectly suggests no safety signals with DPP-4I and insulin combination therapy. Furthermore, as DPP-4I reduces postprandial glucose (PPG) predominantly, it will produce a complementary effect with basal insulin, in effectively managing overall glycemic control. Based on these characteristics, DPP-4I as an add-on to insulin therapy is expected to improve glycemic control without any apparent increase in hypoglycemia risk and weight gain.
SGLT-2I are newly approved OAD, which induces urinary glucose excretion by inhibiting renal glucose reabsorption and thereby reduces plasma glucose.[19] This β-cell independent mechanism of action of SGLT-2I protects them from hypoglycemia. Moreever, an increase in urinary glucose excretion leads to osmotic diuresis and additionally creates a negative calorie balance. Both calorie loss and osmotic diuresis induced by SGLT-2I has the potential to counter the insulin-induced weight gain and fluid retention. The lower rate of hypoglycemia which occurs due to the decrease in renal threshold for glucose or via insulin-independent mechanism also makes SGLT-2I a near-ideal partner to insulin. In addition, SGLT-2I can modulate other risk factors beyond glycemia including a reduction in blood pressure (BP), weight, visceral adiposity, hyperinsulinemia, arterial stiffness, albuminuria, uric acid levels, and oxidative stress.[20] Notably, a recently concluded CVOT with SGLT-2I empagliflozin (EMPA-REG), found a significant reduction in CV death and all-cause mortality. Interestingly, 48% of the patients in EMPA-REG outcome trial were concomitantly receiving insulin with empagliflozin and found similar benefit in CV outcomes.[21] These collective characteristics of weight loss, minimal hypoglycemia, BP reduction, osmotic diuresis, and unprecedented CV outcome makes SGLT-2I, a potentially useful add-on agent to insulin. However, it is worthwhile to mention that SGLT-2I are not indicated in moderate to severe renal impairment and precaution is required while using these agents in elderly patients on concomitant volume depleting therapies. The increase in glucagon and endogeneous glucose production with SGLT-2I can also offset its glycemic benefit and is a potentially concern. Furthermore, euglycemic ketoacidosis (EuDKA) has been reported with SGLT-2I, especially in long-standing insulinopenic T2DM, although its prevalence appears pretty low.[22,23] Moreover, urogenital infection which many diabetics and elderly patients are prone to, are commonly observed side effects with SGLT-2I, owing to its mechanism of action.
With this background information, this review will analyze the comparative safety and efficacy of DPP-4I or SGLT-2I as an add-on therapy to insulin.
REVIEW METHODS
The studies with DPP-4 inhibitors or SGLT-2 inhibitors as an add-on to insulin therapy were identified by conducting a literature search using an electronic database of PubMed, Scopus, and Cochrane review till September 2015. Several key MeSH words were used to retrieve efficacy and safety data of both DPP-4I and SGLT-2I as an add-on therapy to insulin. The eligibility/inclusion criteria for the studies included in this systematic review were patients more than 18 years of age with T2DM, receiving DPP-4I or SGLT-2I as an add-on to insulin, without any gender or ethnic origin bias. The studies had to report at least one outcome of interest.
RESULTS
Efficacy and safety data of dipeptidyl peptidase-4 inhibitor as an add-on to insulin therapy
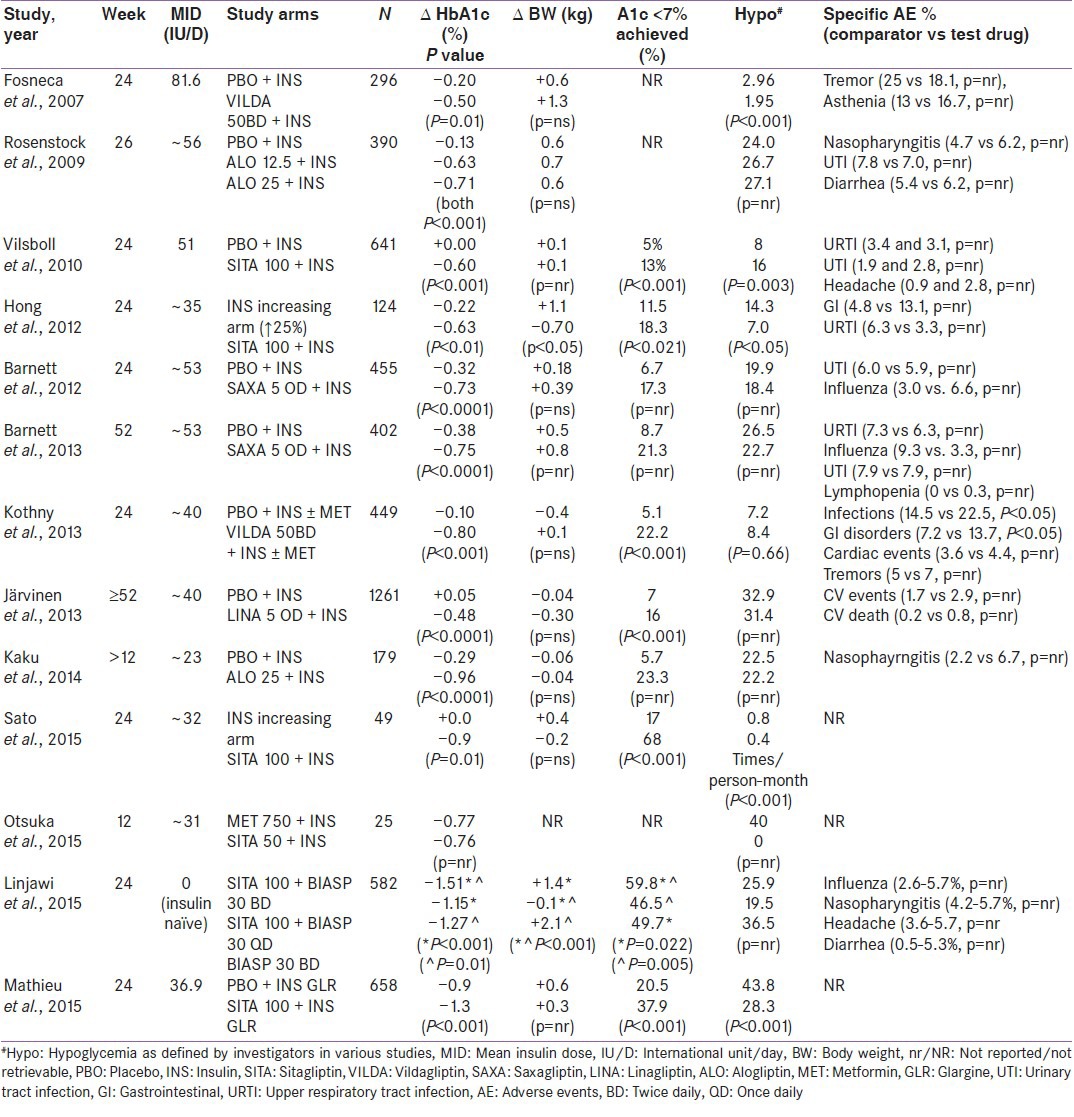
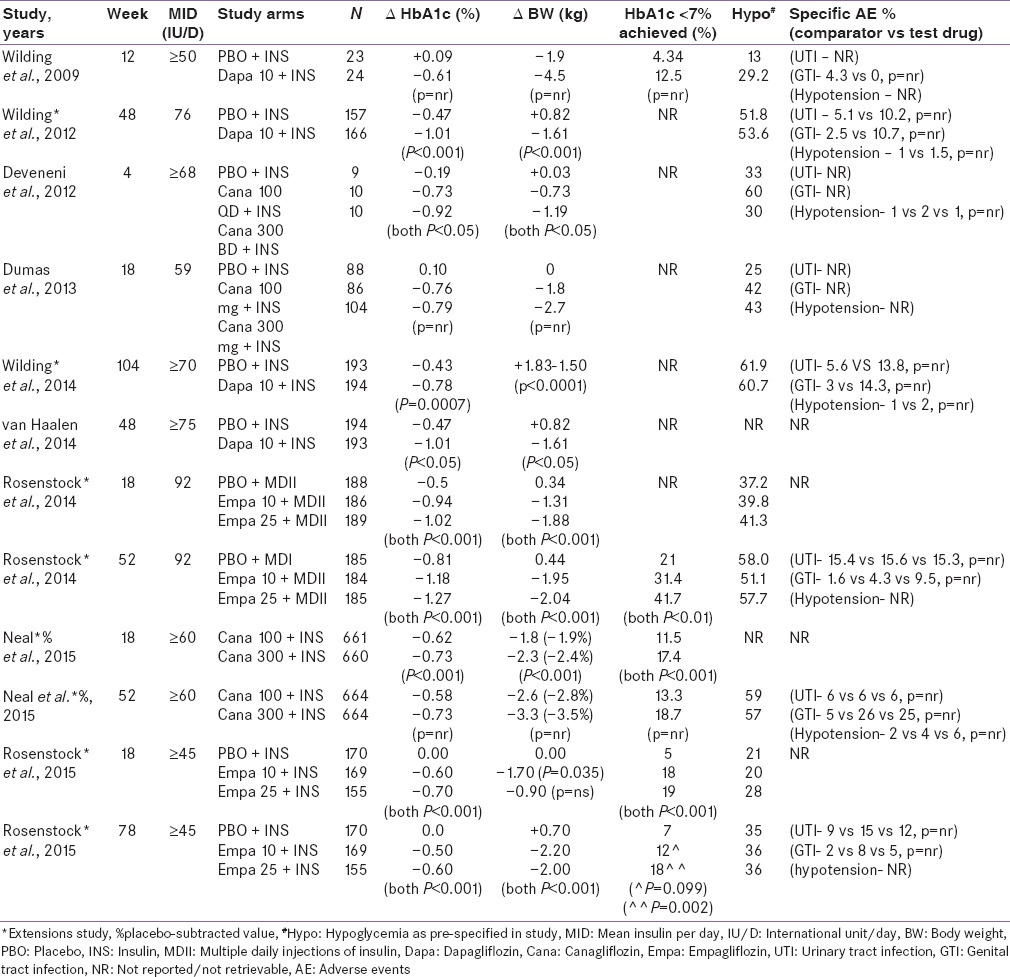
Efficacy and safety data of DPP-4I as an add-on to insulin therapy has been summarized in Table 1.[24,25,26,27,28,29,30,31,32,33,34,35,36]
Table 1.
Efficacy and safety of DPP-4 inhibitors as an add-on to insulin therapy
Sitagliptin with insulin
Vilsboll et al. in a randomized, double-blind, placebo-controlled, multi-centric, 24-week study (n = 641) demonstrated significant glycated hemoglobin (HbA1c) reduction with sitagliptin 100 mg add-on to insulin, compared to placebo (−0.60% vs. 0.0%; P < 0.001), keeping insulin dose stable in both arms. Both fasting plasma glucose (FPG) (−15 vs. −3.5 mg/dl; P < 0.001) and PPG (−36.1 vs. +5.2 mg/dl; P < 0.001) reduced significantly in sitagliptin arm, compared to placebo. Moreover, significantly higher proportion of patients achieved target HbA1c goal of <7% in sitagliptin arm (13 vs. 5%; P < 0.001), compared to placebo group, while no significant change in body weight was seen in either group. Notably, symptomatic hypoglycemia was significantly higher in sitagliptin group (16 vs. 8%; P = 0.003), compared to placebo. Other adverse events were small and similar in both arms.[24]
Hong et al., in an active-comparator, open-label, 24-weeks study (n = 124), randomized either by adding sitagliptin 100 mg or increasing the insulin dose by 25%, found a superior reduction of HbA1c in sitagliptin arm (−0.6 vs. −0.2%, difference −0.4%, P = 0.010). While the reduction of FPG was similar, reduction of PPG was significantly higher in sitagliptin group, compared to insulin increasing group (−74.5 vs. −21.7 mg/dl; P < 0.001). Moreover, a higher proportion of patients achieved the glycemic goal of HBA1C <7% compared to insulin increasing group (18.3 vs. 11.5%; P < 0.021). Interestingly, the reduction of body weight were significantly better in sitagliptin arm, compared to insulin increasing groups (−0.70 vs. +1.1 kg, Δ −1.8 kg; P < 0.05). Interestingly, hypoglycemia and severe hypoglycemia were significantly lower in sitagliptin add-on group (7.02 vs. 0.88 events per patient-year, respectively), compared to insulin dose-increasing group (14.29 and 2.81 events per patient-year, respectively; both P < 0.01). Overall AEs was similar in sitagliptin and insulin dose-increasing group (34.4 vs. 36.5%).[25]
Sato et al. in a prospective, controlled, active-comparator, open-label, 24-week Japanese study (n = 49) compared sitagliptin 50–100 mg to intensifying insulin arm and found significantly better HbA1c reduction in sitagliptin arm (−0.9 vs. 0%, difference −9.0%; P = 0.01). Moreover, a greater proportion of patients in sitagliptin group achieved the glycemic goal of HbA1c <7% (68% vs. 17%; P < 0.001), compared to intensify insulin arm. Furthermore, 20% dose reduction of insulin was required in sitagliptin arm to avoid hypoglycemia, although no difference in body weight noted. Interestingly, significantly less hypoglycemic events reported in sitagliptin group (0.4 ± 1.3 vs. 0.8 ± 1.3 times/person-month, difference −0.3 times/person-month; P < 0.001), compared to insulin-intensification arm.[26]
Mathieu et al. in a multicentric, randomized, double-blind, placebo-controlled clinical trial (n = 660) also compared sitagliptin 100 mg or placebo as add-on to ongoing glargine insulin titrated accordingly. Significant reduction in HbA1c observed in sitagliptin group compared to placebo (−0.9%) group (−1.3 vs. 0.9%, difference −0.4%; P < 0.001). Sitagliptin group required a significantly lesser increment in the daily dose of insulin, compared to placebo (between group difference was −4.7 IU; P = 0.009). In addition, FPG reduction was higher in sitagliptin (−3.1 vs. −2.5 mmol/L, difference −0.6 mmol/L; P = 0.001) compared to placebo group. Moreover, higher percentage reached the HbA1c goal of <7.0% in sitagliptin arm (38 vs. 21%, difference 17.3%; P < 0.001) compared to placebo although both group had slight increase in body weight. Although both arm had a similar adverse event, less hypoglycemia observed in sitagliptin arm versus placebo (Δ −15.5%; 95% CI: −22.7 to −8.2).[29]
Vildagliptin plus insulin
Fonseca et al. in a multicentric, double-blind, randomized, placebo-controlled, parallel group 24-week study (n = 296), randomized either vildagliptin (50 mg twice daily) or placebo to patients who were uncontrolled on insulin therapy. The study found a significant HbA1c reduction in vildagliptin group (−0.5 vs. −0.2%, difference −0.3%; P = 0.01), compared to placebo although no difference in FPG and body weight noted. Although adverse events were similar in both group (81.3 vs. 82.9%), the confirmed and severe hypoglycemia were significantly lower in the vildagliptin (1.95 and 0 events per patient-year, respectively), compared to placebo arm (2.96 and 0.10 events per patient-year, respectively; P < 0.001 and P = 0.032 respectively).[30]
Kothny et al., in a multicentric, double-blind, placebo-controlled, parallel group, 24-weeks clinical trial (n = 449), studied the effect of vildagliptin or placebo as add-on to stable insulin therapy with or without background metformin. HbA1c reduction was significantly better in vildagliptin arm (−0.8 vs. −0.1, difference −0.7%; P < 0.001), compared to placebo. FPG reduction was also better in vildagliptin arm (−0.8 vs. −0.2 mmol/L, difference −0.6 mmol/L; P = 0.05) compared to placebo. Moreover, substantially higher proportion of patients achieved HbA1c goal of <7%, in vildagliptin group (22.2 vs. 5.1%; P < 0.001), compared to placebo. While higher incidence of adverse events noted in vildagliptin group compared to placebo group (57.7 vs. 47.5%; P < 0.05), hypoglycemic events were similar in both group (8.4 vs. 7.2%; P = 0.66).[31]
Saxagliptin plus insulin
Barnett et al., in a randomized, double-blind, multicenter, 24-week study (n = 455), studies the efficacy of saxagliptin 5 mg versus placebo as add-on therapy to insulin with or without metformin background therapy keeping insulin dose fixed during entire study period. HbA1c was significantly reduced better in saxagliptin arm (−0.73 vs. −0.32%, difference −0.41%, P < 0.0001), compared to placebo. While the reduction of FPG were insignificant, PPG reduction was significantly higher in vildagliptin arm (−27.2 vs. −4.2 mg/dl; P = 0.0016), compared to placebo. Moreover, higher percentage of patients achieved glycemic goal of <7% in saxagliptin arm (17.3% vs. 6.7%, P value not reported), compared to placebo, although no difference in body weight observed.[32] Twenty eight-week extension of same study (n = 402), when insulin dosage modification were allowed, found similar results. HbA1c was significantly reduced in saxagliptin arm (−0.75 vs. −0.38%, difference −0.37%; P < 0.0001), compared to placebo group at week 52. Similarly, the proportion of patients who achieved glycemic goal of <7% were higher in saxagliptin group compared to placebo group (21.3% vs. 8.7%, difference 12.6%; P value not reported) although no difference in body weight observed. Hypoglycemia and other adverse events were similar in both arms at week 24 and 52.[33]
Linagliptin plus insulin
Järvinen et al., in a randomized, placebo-controlled, 52-weeks trial (n = 1261), studied the efficacy of linagliptin 5 mg versus placebo as an add-on therapy to insulin with or without metformin and or pioglitazone therapy. While the insulin dose was kept fixed for first 24-weeks, however, it was allowed to titrate thereafter. HbA1c reduction was significantly better in linagliptin arm (−0.58 vs. 0.07%, difference −0.65%; P < 0.0001), compared to placebo. Similarly, HbA1c lowering was superior with linagliptin (−0.48 vs. 0.05%, difference −0.53%; P < 0.0001), compared to placebo at week 52. Moreover, proportion of patients who achieved glycemic goal of <7%, were also significantly higher in linagliptin arm (16% vs. 7%; P < 0.001), compared to placebo, although no significant change in body weight noted. All other adverse events were similar, including hypoglycemia.[34]
Alogliptin plus insulin
Rosenstock et al., in a randomized, double-blind, placebo-controlled, 26-week study (n = 390), evaluated the efficacy and safety of alogliptin add-on to stable insulin therapy. HbA1c reduction was significantly higher with alogliptin 12.5 mg, 25 mg compared to placebo (−0.63, −0.71% vs. −0.13%; both P < 0.001). Moreover, the proportion of patient required hyperglycemic rescue was significantly lesser in alogliptin 12.5 and 25 mg compared to placebo (21, 20 and vs. 40%; both P < 0.001) arm. No difference in hypoglycemia, weight change, and other adverse events noted.[35]
Kaku et al., in a randomized, double-blind, 12-week Japanese study (n = 179), studied the efficacy and safety of alogliptin 25 mg added to insulin verses placebo. HbA1c reduction was significantly higher in alogliptin arm (−0.96 vs. −0.29%; difference −0.66%; P < 0.0001), compared to placebo. Moreover, higher proportions of patients achieved HbA1c <7% in alogliptin group compared to placebo (23.3 vs. 5.7%) although P value not reported. No difference in hypoglycemia or other adverse events noted in this study.[36]
Efficacy and safety data of sodium-glucose co-transporter-2 inhibitors inhibitor as an add-on to insulin therapy
Efficacy and safety data of SGLT-2I as an add-on to insulin therapy has been summarized in Table 2.[37,38,39,40,41,42,43,44,45]
Table 2.
Efficacy and safety of SGLT-2 inhibitors as an add-on to insulin therapy
Dapagliflozin plus insulin
Wilding et al., in a randomized, double-blind, placebo-controlled, multi-centric, proof-of-concept, 12-week study (n = 75), evaluated dapagliflozin 10 mg as add-on to insulin versus placebo keeping insulin dose fixed. The study found −0.61% reduction in HbA1c with dapagliflozin compared to placebo (increase in HbA1c by 0.09%), despite 50% baseline reduction in the total daily insulin dosage in both arms. Interestingly, 65.2% of patients had a HbA1c reduction of ≥0.5% in dapagliflozin arm, compared to 15.8% in the placebo group at week 12. Placebo-subtracted body weight reduction was −2.6 kg and systolic BP (SBP) reduction was −10 mm of Hg in dapagliflozin arm, although P value was not reported. Surprisingly, no case of urinary tract infection (UTI) or genital tract infection (GTI) reported in dapagliflozin arm, compared to 4.3% cases in the placebo arm. Although rates of hypoglycemia was higher in dapagliflozin arm (29 vs. 13%, P value not reported), compared to placebo. This could have happened perhaps due to the fixed insulin regime, not being allowed to lower the dose of insulin in dapagliflozin arm.[37]
In another larger (n = 808), longer (48-weeks), randomized, placebo-controlled and multi-centric study, Wilding et al. studied the effect of dapagliflozin 10 mg as add-on to insulin, without reducing the baseline insulin dose. This 48-weeks study (24-weeks randomized, with further 24-weeks extension period) found that addition of dapagliflozin 10 mg (N = 178), significantly reduced the HbA1c (−1.0% vs. −0.47%, difference −0.54%; both P < 0.001), compared to placebo. Notably, the adjusted proportion of patients who required at least 10% lesser dose from the baseline was significantly higher in dapagliflozin 10 mg arm (18.6% vs. 10.5% in control, P = 0.024), compared to control. Overall, a significantly lower dose of insulin required in dapagliflozin 10 mg arm (−11.2 IU, P < 0.001), compared to placebo. Significant reduction in body weight also observed in dapagliflozin arm (−1.6 vs. 0.8 kg, difference −2.43 kg; both P < 0.001), compared to placebo. Notably, reported events of UTI were 2-times and GTI 5-times higher in dapagliflozin-treated arm, although no P value reported.[38]
In the 56-weeks extension (total 104 weeks) of the same study of Wilding et al. significant reduction in HbA1c (difference −0.35%, P = 0.0007) and body weight (difference −3.3 kg, P < 0.0001) were observed with dapagliflozin versus placebo. Similarly, proportion of patient requiring mean daily insulin dose reduction of ≥10% was significantly higher in dapagliflozin arm (17 vs. 7%, difference 10%, P = 0.0022), compared to control. Furthermore, the mean difference of change in total insulin daily dose was significantly lower in dapagliflozin 10 mg arm (−0.8 vs. 18.3 IU, difference −19.2 IU, P < 0.0001), compared to control. Significant reduction in body weight observed in dapagliflozin arm (−1.5 vs. 1.8 kg, difference −3.3 kg, P < 0.0001) compared to control. While no difference in hypoglycemia observed in this study, hypotension was higher in dapagliflozin arm. Both UTI and GTI were 2-to 5-times higher in dapagliflozin arm (although P value not reported).[39]
In both of these study, UTI and GTI events were more commonly observed in women, mainly as a single episode, categorized as mild to moderate, and mostly occurred in the first 24 weeks of treatment. The reported cases were based primarily on symptoms (not by culture studies) and managed easily with standard treatment.[38,39]
In a Dutch healthcare setting, van Haalen et al. found significant reduction of HbA1c (−1.01 vs. −0.47%; P < 0.05) and body weight (−1.61 vs. −0.82 kg; P < 0.05) in dapagliflozin versus placebo at week 48. A significant placebo-subtracted reduction of body weight (−2.4 kg, P < 0.05) also observed with dapagliflozin. Moreover, dapagliflozin in combination with insulin was found to be cost-effective using Cardiff diabetes model.[40]
Canagliflozin plus insulin
Deveneni et al., in a phase 1b, 4-week dose-finding study (n = 29), found significant reduction in HbA1C with canagliflozin 100 mg QD and 300 mg BD, as an add-on therapy to insulin versus placebo (−0.73, −0.92% vs. −0.19%; both P < 0.05), although hypoglycemic event rate were higher in canagliflozin arm.[41]
Dumas et al., in an exploratory analysis (n = 278) of Canagliflozin Cardiovascular Assessment Study (CANVAS), found that addition of canagliflozin 100 and 300 mg add-on to basal insulin markedly reduced HbA1c (−0.76, −0.79% vs. 0.10%, P value not reported), compared to placebo, although the hypoglycemic event rates were higher in canagliflozin arm.[42]
Neal et al., in a randomized, placebo-controlled, two-point outcome (week 18 and 52), insulin substudy (N = 2072) of CANVAS, studied the efficacy and safety of canagliflozin as an add-on to insulin. Placebo-subtracted HbA1c (−0.62 and −0.73%; P < 0.001) and weight reduction (−1.9 and −2.4%, P < 0.001) was significantly higher with canagliflozin 100 and 300 mg, respectively, at week 18. Similarly HbA1c was lower with canagliflozin 100 and 300 mg at week 52, respectively (−0.58 and −0.73%), although P value was not reported. Moreover, 13.3% and 18.7% of patients achieved the HbA1c target of <7% with canagliflozin 100 and 300 mg at week 52, respectively. In addition, canagliflozin showed a dose-dependent reduction of SBP compared to placebo at both time points, although P value not reported. No difference in hypoglycemia observed versus placebo arm. Hypotension was higher in canagliflozin arm. While UTI rates were similar in all arms, the GTI rates were 5-times higher in canagliflozin 100 and 300 mg (26% and 25%, respectively), compared to placebo (5%). Nevertheless, GTI were managed with topical or oral antifungal medications.[43]
Empagliflozin plus insulin
Rosenstock et al., in a randomized, placebo-controlled, parallel group, two-point (week 18 and 52) outcome study (n = 563), studied the effect of empagliflozin 10 mg and 25 mg to placebo, as an add-on to multiple daily injections of insulin. Insulin dose was kept stable for first 18-week, then titrated to meet target from weeks 19 to 40, followed by further stable dose from weeks 41 to 52. Study found significant reduction in HbA1c with empagliflozin 10 mg (Δ −0.44, P < 0.001) and 25 mg (Δ −0.52, P < 0.001) at week 18, respectively, versus placebo. Similarly, HbA1c significantly reduced with empagliflozin 10 mg (Δ −0.38, P < 0.001) and 25 mg (Δ −0.46, P < 0.001) at week 52, versus placebo. The daily total insulin dose were significantly lower in empagliflozin 10 mg (Δ −8.8 IU/day, P = 0.004) and 25 mg (Δ −11.2, P < 0.001), versus placebo at week 52. Moreover, there was a significant reduction in body weight (Δ −1.6 kg and Δ −2.2 kg, both P < 0.001) with empagliflozin 10 and 25 mg, respectively, versus placebo. Furthermore, empagliflozin 10 mg reduced SBP significantly (Δ −2.4 mm Hg, P = 0.03) at week 18 versus placebo, although this reduction were not statistically significant at week 52 with any dose of empagliflozin. Hypoglycemia was similar in all arms at both point of time. While UTI were similar in all arms, GTI was 2-to 6-times higher in empagliflozin arm, at week 52.[44]
In a two-point (week 18 and 76) outcome study (n = 494), Rosenstock et al. evaluated the addition of empagliflozin 10 mg and 25 mg to basal insulin versus placebo. Insulin dose was kept constant for the first 18-week, then was allowed to be up-or down-titrated for next 60-weeks. HbA1c reduction was significantly lower with empagliflozin 10 mg and 25 mg at week 18 (Δ −0.6 and −0.7%, all P < 0.001) and at week 78 (−0.50 and −0.60%; all P < 0.001) respectively versus placebo. Moreover, higher proportion of patients could achieve the target HbA1c of <7% in empagliflozin 10 and 25 mg week 18 (18 and 19% vs. 5%; both P < 0.001), compared to placebo. However, only empagliflozin 25 mg could achieve significant proportion of patient reaching HbA1c target of <7% (27%, P = 0.002) at week 78, versus placebo. The rate of hypoglycemic episodes appeared higher during the first 18-weeks with a fixed insulin dose regime, especially with empagliflozin 25 mg; however, it was similar to placebo at week 78. While both UTI and GTI were 1.5-to 4-times higher in empagliflozin arm, these events were higher in women, generally mild and managed easily with standard care of therapy.[45]
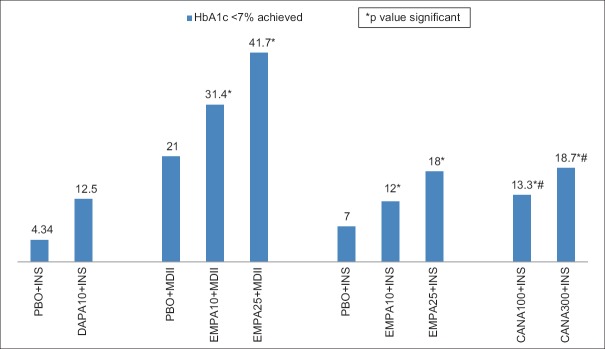
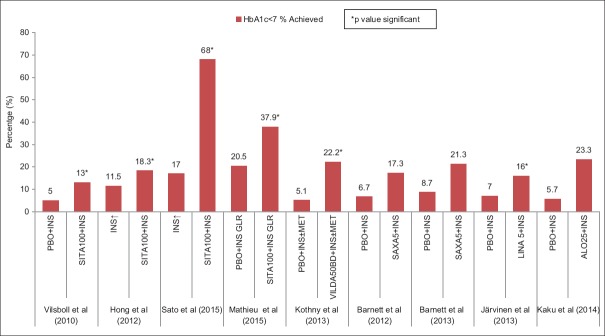
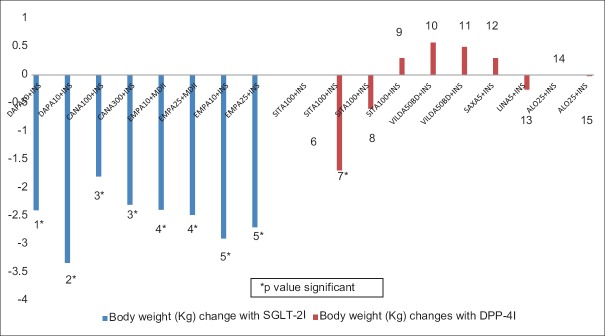
Comparative placebo-subtracted HbA1c lowering with DPP-4I or SGLT-2I as an add-on to insulin therapy has been depicted in Figure 1. The proportion of patients achieving target HbA1c of <7% with SGLT-2I and DPP4-I, as an add-on to insulin therapy has been summarized in Figures 2 and 3, respectively. A comparative placebo-subtracted change in body weight with SGLT-2I and DPP-4I as an add-on to insulin has been depicted in Figure 4.
Figure 1.
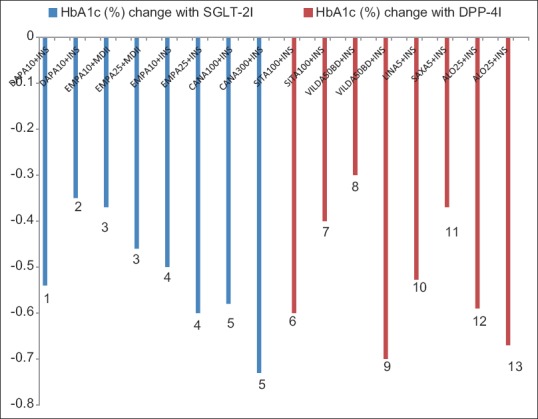
Placebo-subtracted glycated hemoglobin reduction with sodium-glucose co-transporter-2 inhibitors plus insulin (blue-panel) and dipeptidyl peptidase-4 inhibitors plus insulin (red-panel), all P significant (1: Wilding et al., 2012. 2: Wilding et al., 2014. 3: Rosenstock et al., 2014. 4: Rosenstock et al., 2015. 5: Neal et al., 2015. 6: Vilsboll et al., 2010. 7: Mathieu et al., 2015. 8: Fonseca et al., 2007. 9: Kothny et al., 2013. 10: Jarvinen et al., 2013. 11: Barnett et al., 2013. 12: Rosenstock et al., 2009. 13: Kaku et al., 2014)
Figure 2.
Glycemic goal of glycated hemoglobin <7% achieved (%) in various studies with sodium-glucose co-transporter-2 inhibitors as an add-on to insulin therapy [Wilding et al., 2009 (24-weeks), Rosenstock et al. (52-weeks), Rosenstock et al. (78-weeks), Neal et al., (52-weeks, #placebo-subtracted)]. * P value significant
Figure 3.
Glycemic goal of glycated hemoglobin <7% achieved (%) in various studies with dipeptidyl peptidase-4 inhibitors as an add-on to insulin therapy (*P value significant)
Figure 4.
Change in placebo-subtracted body weight (Kg) with sodium-glucose co-transporter-2 inhibitors plus insulin (blue panel) and dipeptidyl peptidase-4 inhibitors plus insulin (red panel)(1: Wilding et al.[48-weeks]. 2: Wilding et al.[104-weeks]. 3: Neal et al.[18-weeks]. 4: Rosenstock et al.[52-weeks]. 5: Rosenstock et al.[78-weeks]. 6: Vilsboll et al.[24-weeks]. 7: Hong et al.(24-weeks). 8: Sato et al.[24-weeks]. 9: Mathieu et al.[24-weeks]. 10: Fonseca et al. [24-weeks]. 11: Kothny et al.[weeks-24]. 12: Barnett et al.[52-weeks]. 13: Jarvinen et al.[52-weeks]. 14: Rosenstock et al.[24-weeks]. 15: Kaku et al. [24-weeks] *P value significant)
CONCLUSION
Metformin being insulin sensitizers undoubtedly remains the best partner to insulin therapy. Moreover, it can potentially counter the insulin-induced weight gain and do not augment hypoglycemia. Indeed, metformin should be used in all T2DM patients, unless it is not tolerated or contraindicated. Pioglitazone is even more effective insulin sensitizer; however as an add-on agent to insulin therapy does carry the potential of weight gain, fluid retention, and precipitation of heart failure.
While the addition of SU to basal insulin therapy is a logical choice considering its cost, time-tested use, ability to reduce post-prandial plasma glucose, and, therefore, overall glycemic control, however, augmentation of hypoglycemia and weight gain is a potential concern. Moreover, SU would be preferably discontinued, once prandial or premix insulin is commenced. GLP-1 RA as an add-on to insulin therapy is an effective combination and has the potential to counter the insulin-induced weight gain without potentiating hypoglycemia. However, this regime incurs additional injection and cost.
DPP-4I, as an add-on to insulin therapy, have shown a significant reduction in HbA1c, without any apparent increase in hypoglycemia and body weight. In addition, DPP-4I with insulin required~10–20% reduction in total daily dose of insulin and significantly higher proportion of patients achieved the target HbA1c of <7%. Similarly, SGLT-2I as an add-on to insulin therapy have shown a significant reduction in HbA1c and body weight, without any apparent increase in hypoglycemia. Furthermore, the addition of SGLT-2I required~10% reduction in total daily dose of insulin and significant higher proportion of patients achieved the target HbA1c of <7%. It should be noted that gross reduction in insulin dosage with concomitant SGLT-2I therapy has been reported to be associated with EuDKA.
Taken together, DPP-4I or SGLT-2I both appears as a near-ideal partner to insulin. Both DPP-4I and SGLT-2I have an advantage of oral route, in contrast to GLP-1 RA. However, significant weight loss with all SGLT-2I consistently seen across the studies, and CV benefits observed with empagliflozin; SGLT-2I appears as an ideal partner to insulin in renal uncompromised T2DM, in authors’ opinion. Nevertheless, DPP-4I would be preferred choice as an add-on to insulin in elderly and renal compromised T2DM patient. In addition, reduction in insulin dose while on concomitant SGLT-2I therapy should carefully be done to avoid Eu DKA, especially in insulinopenic T2DM. Future studies would be even more interesting to find the outcomes of DPP-4I plus SGLT-2I combination as an add-on to insulin therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors would like to thank Dr. Kalpesh Prakashchandra Jain and Dr. Jaydeep Mazumdar in collecting data while preparing this manuscript.
REFERENCES
- 1.Zangeneh F, Arora PS, Dyck PJ, Bekris L, Lernmark A, Achenbach SJ, et al. Effects of duration of type 2 diabetes mellitus on insulin secretion. Endocr Pract. 2006;12:388–93. doi: 10.4158/EP.12.4.388. [DOI] [PubMed] [Google Scholar]
- 2.Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. U.K. Prospective Diabetes Study Group. Sulfonylurea inadequacy: Efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. prospective diabetes study (UKPDS 57) Diabetes Care. 2002;25:330–6. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- 3.Yki-Järvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care. 2001;24:758–67. doi: 10.2337/diacare.24.4.758. [DOI] [PubMed] [Google Scholar]
- 4.Yki-Järvinen H, Ryysy L, Nikkilä K, Tulokas T, Vanamo R, Heikkilä M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1999;130:389–96. doi: 10.7326/0003-4819-130-5-199903020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, et al. American association of clinical endocrinologists and American college of endocrinology – Clinical practice guidelines for developing a diabetes mellitus comprehensive care plan-2015. Endocr Pract. 2015;21(Suppl 1):1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McFarland MS, Knight TN, Brown A, Thomas J. The continuation of oral medications with the initiation of insulin therapy in type 2 diabetes: A review of the evidence. South Med J. 2010;103:58–65. doi: 10.1097/SMJ.0b013e3181c35776. [DOI] [PubMed] [Google Scholar]
- 7.Clar C, Royle P, Waugh N. Adding pioglitazone to insulin containing regimens in type 2 diabetes: Systematic review and meta-analysis. PLoS One. 2009;4:e6112. doi: 10.1371/journal.pone.0006112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: A randomized, controlled trial. Ann Intern Med. 2011;154:103–12. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 9.Seino Y, Min KW, Niemoeller E, Takami A. EFC GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia) Diabetes Obes Metab. 2012;14:910–7. doi: 10.1111/j.1463-1326.2012.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: A 24-week, randomized, placebo-controlled study (GetGoal-Duo 1) Diabetes Care. 2013;36:2497–503. doi: 10.2337/dc12-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenstock J, Fonseca VA, Gross JL, Ratner RE, Ahrén B, Chow FC, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: A comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice-daily prandial insulin lispro. Diabetes Care. 2014;37:2317–25. doi: 10.2337/dc14-0001. [DOI] [PubMed] [Google Scholar]
- 12.Morales J, Merker L. Minimizing hypoglycemia and weight gain with intensive glucose control: Potential benefits of a new combination therapy (IDeg Lira) Adv Ther. 2015;32:391–403. doi: 10.1007/s12325-015-0208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh AK. Dipeptidyl peptidase-4 inhibitors: Novel mechanism of actions. Indian J Endocrinol Metab. 2014;18:753–9. doi: 10.4103/2230-8210.141319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farngren J, Persson M, Schweizer A, Foley JE, Ahrén B. Vildagliptin reduces glucagon during hyperglycemia and sustains glucagon counterregulation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab. 2012;97:3799–806. doi: 10.1210/jc.2012-2332. [DOI] [PubMed] [Google Scholar]
- 15.Farngren J, Persson M, Schweizer A, Foley JE, Ahrén B. Glucagon dynamics during hypoglycaemia and food-re-challenge following treatment with vildagliptin in insulin-treated patients with type 2 diabetes. Diabetes Obes Metab. 2014;16:812–8. doi: 10.1111/dom.12284. [DOI] [PubMed] [Google Scholar]
- 16.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. NEngl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 17.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. NEngl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 18.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. NEngl J Med. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 19.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 20.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. NEngl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 22.Kalra S, Gupta Y, Patil S. Sodium-glucose cotransporter-2 inhibition and the insulin: Glucagon ratio: Unexplored dimensions. Indian J Endocrinol Metab. 2015;19:426–9. doi: 10.4103/2230-8210.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh AK. Sodium glucose co-transporter-2 inhibitors and euglycemic ketoacidosis: Wisdom of hindsight. Indian J Endocrinol Metab. 2015;19:722–30. doi: 10.4103/2230-8210.167554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilsbøll T, Rosenstock J, Yki-Järvinen H, Cefalu WT, Chen Y, Luo E, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–77. doi: 10.1111/j.1463-1326.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 25.Hong ES, Khang AR, Yoon JW, Kang SM, Choi SH, Park KS, et al. Comparison between sitagliptin as add-on therapy to insulin and insulin dose-increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes Obes Metab. 2012;14:795–802. doi: 10.1111/j.1463-1326.2012.01600.x. [DOI] [PubMed] [Google Scholar]
- 26.Sato S, Saisho Y, Kou K, Meguro S, Tanaka M, Irie J, et al. Efficacy and safety of sitagliptin added to insulin in Japanese patients with type 2 diabetes: The EDIT randomized trial. PLoS One. 2015;10:e0121988. doi: 10.1371/journal.pone.0121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otsuka Y, Yamaguchi S, Furukawa A, Kosuda M, Nakazaki M, Ishihara H. Addition of sitagliptin or metformin to insulin monotherapy improves blood glucose control via different effects on insulin and glucagon secretion in hyperglycemic Japanese patients with type 2 diabetes. Endocr J. 2015;62:133–43. doi: 10.1507/endocrj.EJ14-0148. [DOI] [PubMed] [Google Scholar]
- 28.Linjawi S, Sothiratnam R, Sari R, Andersen H, Hiort LC, RaoP The study of once-and twice-daily biphasic insulin aspart30 (BIAsp 30) with sitagliptin, and twice-daily BIAsp 30 without sitagliptin, in patients with type 2 diabetes uncontrolled on sitagliptin and metformin-The Sit2Mix trial. Prim Care Diabetes. 2015;9:370–6. doi: 10.1016/j.pcd.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Mathieu C, Shankar RR, Lorber D, Umpierrez G, Wu F, Xu L, et al. Arandomized clinical trial to evaluate the efficacy and safety of co-administration of sitagliptin with intensively titrated insulin glargine. Diabetes Ther. 2015;6:127–42. doi: 10.1007/s13300-015-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–55. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 31.Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:252–7. doi: 10.1111/dom.12020. [DOI] [PubMed] [Google Scholar]
- 32.Barnett AH, Charbonnel B, Donovan M, Fleming D, Chen R. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin. Curr Med Res Opin. 2012;28:513–23. doi: 10.1185/03007995.2012.665046. [DOI] [PubMed] [Google Scholar]
- 33.Barnett AH, Charbonnel B, Li J, Donovan M, Fleming D, Iqbal N. Saxagliptin add-on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52-week safety and efficacy. Clin Drug Investig. 2013;33:707–17. doi: 10.1007/s40261-013-0107-8. [DOI] [PubMed] [Google Scholar]
- 34.Yki-Järvinen H, Rosenstock J, Durán-Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: A ≥52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–81. doi: 10.2337/dc12-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA(1C) without causing weight gain or increased hypoglycaemia. Diabetes Obes Metab. 2009;11:1145–52. doi: 10.1111/j.1463-1326.2009.01124.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaku K, Mori M, Kanoo T, Katou M, Seino Y. Efficacy and safety of alogliptin added to insulin in Japanese patients with type 2 diabetes: A randomized, double-blind, 12-week, placebo-controlled trial followed by an open-label, long-term extension phase. Expert Opin Pharmacother. 2014;15:2121–30. doi: 10.1517/14656566.2014.956722. [DOI] [PubMed] [Google Scholar]
- 37.Wilding JP, Norwood P, T’joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: Applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–62. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: A randomized trial. Ann Intern Med. 2012;156:405–15. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 39.Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: Efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–36. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 40.van Haalen HG, Pompen M, Bergenheim K, McEwan P, Townsend R, Roudaut M. Cost effectiveness of adding dapagliflozin to insulin for the treatment of type 2 diabetes mellitus in the Netherlands. Clin Drug Investig. 2014;34:135–46. doi: 10.1007/s40261-013-0155-0. [DOI] [PubMed] [Google Scholar]
- 41.Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, et al. Canagliflozin improves glycaemic control over28days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–45. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 42.Dumas R, Davies M, Rosenstock J, Desai M, Alba M, Capuano G, et al. Effects of canagliflozin added on to basal insulin +/-other antihyperglycemic agents in type 2 diabetes. Can J Diabetes. 2013;37:S13–84. Abstract 73. [Google Scholar]
- 43.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–11. doi: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–23. doi: 10.2337/dc13-3055. [DOI] [PubMed] [Google Scholar]
- 45.Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. EMPA-REG BASALTM Trial Investigators. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: A 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–48. doi: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]