Abstract
Context:
There is limited literature on the dietary fat intake of rural Indian populations, particularly in relation to the risk of metabolic syndrome (MS).
Aim:
This study aims to assess the dietary fat intake and analyze its association with the risk of selected components of the MS among rural population in the state of Tamil Nadu.
Settings and Design:
Adults (n = 27012) ≥20 years of age were recruited from the rural component of the Chennai Urban Rural Epidemiological Study, a cross-sectional study conducted in 42 villages in Kanchipuram District of Tamil Nadu.
Subjects and Methods:
Using a validated food frequency questionnaire, data were obtained on the fat intake among 6907 adults. Anthropometric and clinical measures were collected using standard methods. The components of the MS assessed were abdominal obesity, hypertension, and impaired fasting glucose. All analyses were performed using SPSS software (version 20).
Results:
Prevalence of abdominal obesity, hypertension, and impaired fasting glucose were significantly higher in the highest quintile of fat intake (33%, P < 0.001; 39%, P = 0.04, and 23.3%, P = 0.003, respectively). Highest intake of fat was also significantly associated with risk of abdominal obesity (P < 0.001), hypertension (P = 0.04), and impaired fasting glucose (P = 0.01). Sunflower oil as the main cooking oil was significantly associated with a higher risk of these components of the MS (P for trend <0.001) compared to traditional oils and palmolein.
Conclusions:
Higher dietary fat was significantly associated with risk of components of the MS and use of sunflower oil as main cooking oil increased metabolic risk in rural South Indians.
Keywords: Dietary fat, metabolic syndrome, rural South Indians, vegetable oil
INTRODUCTION
In many developing countries, there is a rapid dietary transition with increased domestic production and import of oilseeds and vegetable oils. Consequently, there have been marked increases in edible oil consumption and energy density of the diet, in both urban and rural areas.[1,2] Notably, total fat intake has increased considerably over the last three decades (1973–2005) in rural populations in India.[3] Similarly, the National Sample Survey Organization (NSSO) survey results indicate that the fat intake of rural populations has increased from 31.4 g/d/capita in 1993–1994 to 41.6 g/d/capita in 2011–2012 (NSSO, 2014).[4]
The term metabolic syndrome (MS) is used to refer to a constellation of metabolic abnormalities characterized by abdominal obesity, dyslipidemia, elevated blood pressure, impaired fasting glucose, and insulin resistance.[5] Adoption of unhealthy diets and declining physical activity are major contributors to the rising prevalence of MS in developing countries. A high prevalence of MS has been reported in urban India, which has been linked to physical inactivity,[6] high intake of refined grain,[7] and the type of cooking oil used.[8] High prevalence of MS (30.2%) has also been reported in rural Andhra Pradesh[9] and in rural Tamil Nadu (10.5%).[10] Current data indicate that both the quantity and quality of dietary fats can affect the lipid profile and are directly related to obesity, diabetes, and MS.[11,12]
In India, vegetable oils used in cooking represent 80% of the visible fat consumed, besides, fat intake is income-dependent and a single oil is generally chosen for cooking, especially in rural areas.[13] Consequent to the nutrition transition, there has been a shift from traditional oils such as groundnut oil to other oils such as palmolein and sunflower oil and increased consumption of ghee.[14] However, replacing the traditional vegetable oils with sunflower oil has not restrained the rising prevalence of atherosclerosis and diabetes in India.[15] Recent findings reveal that the use of sunflower oil has adverse effects on the lipid profile.[8,16] Furthermore, greater prevalence of MS among urban South Indians using sunflower oil has been reported.[8] As there is little data from rural India on this important subject, we investigated the intake of dietary fats and their association with components of the MS in a rural South Indian population in the state of Tamil Nadu.
SUBJECTS AND METHODS
Adults (n = 27012) ≥20 years of age were recruited from the rural component of the Chennai Urban Rural Epidemiological Study, conducted in 42 villages in Kanchipuram District of Tamil Nadu. Every third participant (n = 9004) was recruited for detailed dietary assessment and of these, 7331 completed the detailed dietary assessment questionnaire (response rate 81.4%). Participants with self-reported history of diabetes (n = 363), unrealistic reported energy intake (<500 kcal/d and >3500 kcal/d for female and <800 kcal/d and 4000 kcal/d for male)[17] (n = 61), and those with missing anthropometric data (n = 87) were excluded. Thus, a total of 6907 participants were finally included in the study. The study protocol was approved by the Institutional Ethics Committee and written informed consent was obtained from all the participants.
Anthropometric and clinical measures including height, weight, and waist measurements were collected using standard methods.[18] Blood pressure was recorded in the sitting position in the right arm to the nearest 1 mmHg using the electronic Omron™ blood pressure monitor (Omron Corporation, Kyoto, Japan). Two readings were taken 5 min apart, and the mean of the two was taken as the final blood pressure reading. Fasting capillary blood glucose was assessed using a hand-held glucose monitor (One Touch® Ultra®, LifeScan, Milpitas, CA, USA).[19] Based on the International Diabetes Federation[20] consensus definition of the MS, individual components of MS are: (1) Central obesity waist circumference (WC) ≥80 cm for female, ≥90 cm for male (Asia Pacific cut-off). (2) Impaired fasting glucose (defined as fasting plasma glucose ≥5.6 mmol/l (≥100 mg/dl) and (3) Elevated blood pressure (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg). Detailed dietary assessment was done using a validated semi quantitative food frequency questionnaire, developed for the rural population. The average daily food and nutrient intake was computed using the in-house EpiNu database. According to the Indian Council of Medical Research (ICMR) dietary guidelines,[21] participants were classified as sedentary, moderate, and heavy workers, based on their occupation. As the number of participants engaged in heavy work was small, they were included in the moderately active group.
Statistical analysis
All analyses were performed using SPSS software (version 20; SPSS Inc., Chicago, IL, USA). Values not normally distributed were represented as median and inter quartile range and tested using Kruskal–Wallis test. Chi-square test was used for proportions. Logistic regression analysis was used to study the association among dietary fats, cooking oils, and the risk for MS. The odds ratio (OR) and 95% confidence intervals (CI) for components of the MS were estimated taking the individuals in the lowest quintile as the reference group, after adjusting for potential confounders. All tests of significance were 2-tailed, and P < 0.05 was considered significant.
RESULTS
The general characteristics, as well as the distribution of study participants with regard to obesity, smoking, and physical activity (sedentary or moderate, as defined by ICMR) and selected components of MS across quintile (Q) categories of fat are shown in Table 1. The median fat intake of the participants ranged from 18.1 (4.1) g/d in the lowest quintile to 48.6 (14.4) g/d in the highest quintile. Compared with participants in the lower quintiles of fat intake, those in the upper quintiles of fat intake were younger and had significantly higher body mass index (BMI) and WC (P < 0.001). Highest quintile of fat intake group had more males (51.4%), more smokers (8.3%), and alcohol consumers (18.8%). In addition, this group also showed greater physical inactivity (42.1%, P < 0.001). Although majority of the participants had normal BMI and WC, higher fat intake was associated with higher BMI (20.1 [4.8]–21.2 [5.1], P < 0.001) and WC (69.3 [14]–74.1 [15], P < 0.001). Higher intake of fat was associated with higher levels of systolic (121 [22]–123 [20] mmHg, P < 0.001) and diastolic blood pressure (77 [15]–79 [14] mmHg, P < 0.001). Median fasting glucose was also significantly higher in the highest quintile of fat intake (89 [16]–90 [17] mg/dl, P = 0.01). Prevalence of abdominal obesity (19.6–33%, P < 0.001), hypertension (36.6–39%, P = 0.04), and impaired fasting glucose (18.4–23.3%, P = 0.003) were significantly higher in the highest quintiles of fat intake as compared to the lowest quintiles.
Table 1.
General characteristics of study participants as per quintiles of total fat intake (n=6907)
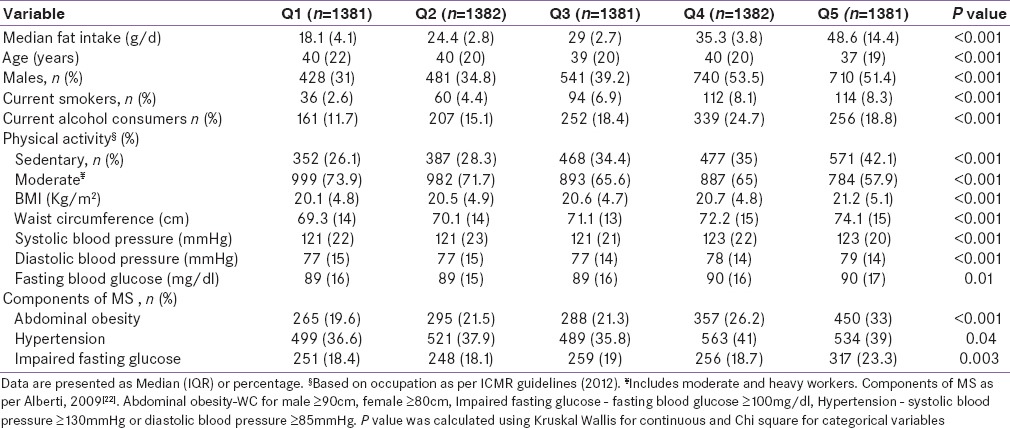
The nutrient and food intake of the participants as per quintiles of fat intake is presented in Table 2. Participants in the highest quintile of fat intake had a significantly higher intake of total calories and higher percentage of fat calories (P < 0.001). Consumption of refined cereals, legumes, fruit and vegetables, animal foods, added sugar and salt, and edible oil increased significantly (P < 0.001) with increasing quintiles of fat intake.
Table 2.
Nutrient and food intake of participants as per Quintiles of total fat intake (n=6907)
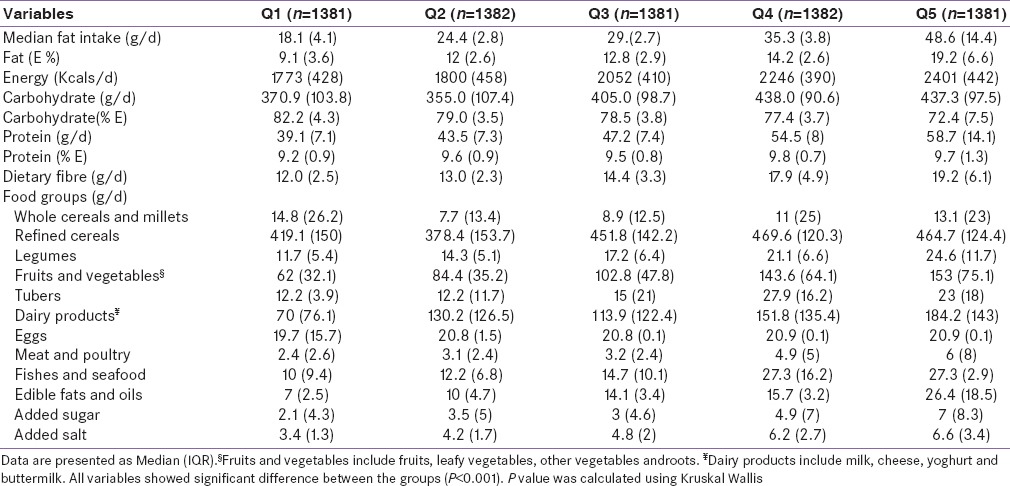
Multivariate adjusted ORs for risk of components of the MS across quintiles of fat intake are presented in Table 3. After adjustment for potential confounders such as age, sex, smoking, alcohol, physical activity, energy, protein, dietary fiber, refined cereal, eggs, fruits and vegetables, fishes, and seafood intakes, there was a significant increase in the risk of abdominal obesity (OR 1.61, CI 1.23–2.13), hypertension (OR 1.39, CI 1.06–1.81), and impaired fasting glucose (OR 1.46, CI 1.07–2.0) with increasing fat intake.
Table 3.
Multivariate-adjusted odds ratios (OR (95%CI))ψ for selected components of MS across quintiles of fat intake (g/d)
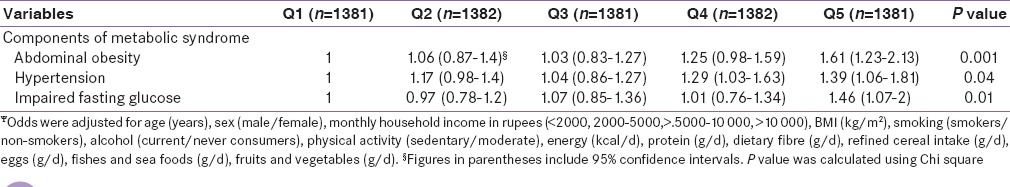
Since vegetable oils formed the major component of the visible fats and oils, we further evaluated the main type of cooking oil used by the participants and its association with components of the MS. The major cooking oils used were traditional oils such as groundnut and gingelly oil (41%), followed by palmolein (40.4%). Sunflower oil was used as the main cooking oil by 17.2% of the participants (data not shown).
Table 4 shows the comparison of dietary intakes and prevalence of MS components of the participants according to the type of cooking oil used by them. Participants from the sunflower oil group were younger and had a significantly higher BMI. The median energy (2100 [546] kcal/d), fiber intake (15.3 [5.9] g/d), and fat intake (14.7 [7.1] g/d) were significantly higher in the sunflower oil group (P < 0.001). Participants from the sunflower oil group also had higher total polyunsaturated fatty acid (PUFA) (5.7 [3.1] %E, P < 0.001), omega 6 (n-6) intake (5.5 [3.1] %E, P < 0.001), and higher n-6/n-3 ratio (35.5 [17.4], P < 0.001) as compared to those who used traditional oils and palmolein. Intake of refined cereals was the highest in the participants from palmolein group while intake of all other foods except eggs was higher in the sunflower oil group. Prevalence of all the 3 components of MS was highest in the sunflower oil group (P < 0.001). On analyzing the risk of components of MS according to the type of cooking oil used, with traditional oils as the reference group [Figure 1], participants who used sunflower oil as the main cooking oil were found to have significantly higher risk of MS components - abdominal obesity (OR 1.61 [95% CI 1.2–2.1]), elevated blood pressure (OR 1.34 [95% CI 1–1.7]), and impaired fasting blood sugar (OR 1.49 [95% CI 1.1–2]) P for trend <0.001) as compared to those who used palmolein or traditional oils.
Table 4.
Dietary intakes and prevalence of MS components of the participants according to the type of cooking oil used (n=6783)¥
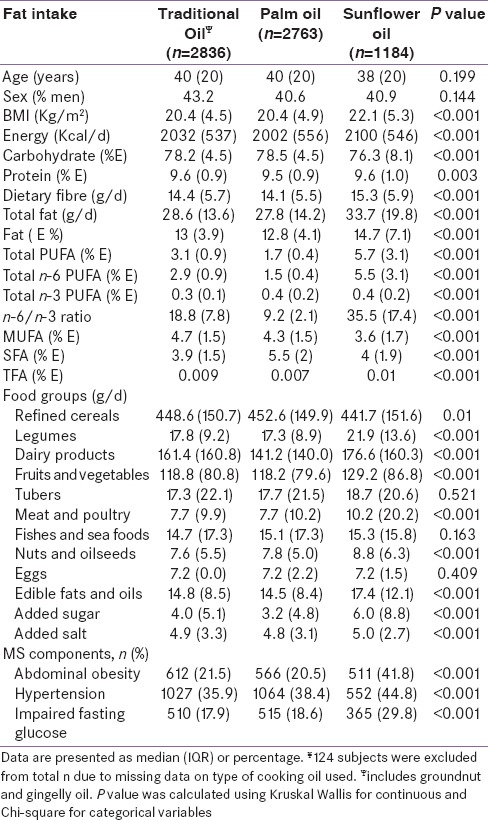
Figure 1.
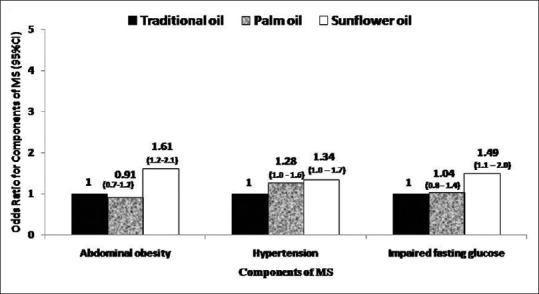
Multivariate adjusted odds ratiosΨ for components of metabolic syndrome with major cooking oil choices of rural population (n = 6783). ΨOdds were adjusted for age (years), sex (male/female), smoking (smokers/nonsmokers), alcohol (never and current consumers), monthly household income in rupees (<2000, 2000–5000, >5000–10,000, >10,000), education (illiterate/literate) physical activity (sedentary/moderate), body mass index (kg/m2, energy (kcal/d), carbohydrate (%E), protein (%E), dietary fiber (g/d), fats and oils (g/d), total saturated fatty acid (%E), total monounsaturated fat (%E), nuts and oils seeds (g/d), added salt (g/d), and added sugar (g/d). *P for trend <0.001 for all 3 components; calculated by using the odds ratio of each category as a continuous variable in the regression model
DISCUSSION
We evaluated the association of dietary fat intake with the components of MS in a rural population of Kanchipuram District, South India. Prevalence and risk of components of MS were higher in participants with a higher fat intake. Use of sunflower oil as the main cooking oil was also associated with higher prevalence and risk for the chosen components of the MS. Fat intake of the participants ranged from 18.1 g/d to 48.6 g/d (9.1–19.2% E), which is well within the ICMR recommended allowance of 20–30% E.[21] Reports from socioeconomic surveys in India state that the fat intake of the rural population ranges from 9.5–18.5% E,[3] which are comparable to our findings. The saturated fatty acid (SFA) intake of this population (3.9 [1.5]–4 [1.9] %E) was also within the Food and Agriculture Organization (FAO) recommendation of <10% energy[22] and the intake of trans fats was negligible.
The present study showed a significant rise in metabolic risk with increasing fat intake and fat calories, with participants in the upper quintiles of fat intake having significantly higher BMI and WC. Previous studies have indicated that high dietary fat intake is associated with the development of obesity and a significant effect of fat calories on BMI has been reported earlier.[23] As obesity is a major risk factor for type 2 diabetes, hypertension and coronary heart disease,[24] a reduction in fat intake, especially among overweight participants would be beneficial.
There was a significant increase in the risk of hypertension among participants in the upper quintiles of fat intake. Few studies have examined the effect of total fat on hypertension. Among Finnish adults, a significant decrease in both systolic and diastolic blood pressure by reducing fat intake in the diet has been reported.[25] In rural Nigerians, total fat intake was associated with increase in diastolic blood pressure.[26] Another intervention study showed that a low-fat, high fiber diet, in combination with exercise significantly lowered both systolic and diastolic pressure.[27] Other authors have reported that diets rich in fruits, vegetables, and low-fat dairy foods and reduced saturated and total fat can substantially lower blood pressure.[28,29] Therefore, decreasing total fat intake, along with the above dietary modifications could effectively lower the risk of hypertension in this population.
The present study showed a significant rise in the prevalence of impaired fasting glucose with increasing fat intake. In an another study, fat intake has been positively associated with both recently diagnosed and undiagnosed type 2 diabetes.[30] Earlier studies have shown that the intake of fat, especially SFAs, was associated with the development of impaired glucose tolerance and diabetes.[31] Similarly, a Dutch study revealed that patients with newly diagnosed diabetes had a higher intake of total fat (40.9% E).[32] However, another study failed to show significant association between total dietary fat and incidence of diabetes, but inverse association between vegetable fat intake and incident type 2 diabetes was reported.[33] In a recent intervention study among individuals with type 1 diabetes, dietary fat was shown to affect glycemic control and increase glucose levels and insulin requirements.[34] On the whole, the present findings indicate that apart from quantity, the quality of fat also has significant effects on the components of the MS.
High fat intake has been associated with greater risk of MS.[11,35,36] Though the latest United States Department of Agriculture Dietary Guidelines[37] emphasize on optimizing types of dietary fat rather than decreasing total fat intake, our study showed the association between dietary fat intake and components of MS even at the modest median fat intake (13.2%E). However, our results also indicate a significant difference in the quality of fat consumed by the participants according to the cooking oil used. Participants who used sunflower oil had a higher n-6 intake and n-6: n-3 ratio, whereas participants who used traditional oil had a higher monounsaturated fat (MUFA) intake (4.7 [1.5] % E]. Dietary MUFA decreases the risk of MS by favorably altering blood lipids, blood pressure, and insulin sensitivity.[38] Therefore, traditional oils such as groundnut oil with higher MUFA content are healthier options.[8] Palmolein is the liquid fraction of palm oil[39] and widely used in India. As palmolein contains higher percentage (42%) of SFA,[12] intake of SFA was highest among participants who used palmolein.
In recent years, there has been a shift from traditional oils such as groundnut and gingelly oil to other oils such as sunflower oil in rural areas, possibly due to advertising,[40] changing lifestyles, increased availability, changes in cost of edible oils, and income levels of rural households.[41] While sunflower oil was initially mooted as healthy (mainly to replace the SFA in the diet), subsequent findings have brought out the adverse metabolic effects of this oil, owing to its high n-6 PUFA content and n-6/n-3 ratio.[8,16] In the present study also, participants who used sunflower oil had higher intake of n-6 PUFA (5.5 [3.1] %E) and higher n-6/n-3 ratio (35.5) and concurrently, higher prevalence of components of MS. Animal studies have also shown that dietary supplementation with n-3 PUFA improves insulin sensitivity,[42] while n-6 PUFAs contribute to insulin resistance.[43] The exact mechanism by which dietary fatty acids affect insulin sensitivity is not clear. However, the effect of dietary fatty acids on insulin sensitivity may be partly mediated through the fatty acid composition of cell membranes, which could influence insulin action by altering insulin receptor binding and influencing ion permeability and cell signaling.[44] Metabolites of n-6 PUFA are pro-inflammatory[45] and may cause impaired insulin action, leading progressively to insulin resistance.[46] Therefore, high n-6 PUFA and high n-6/n-3 ratio can contribute to the increased incidence of diabetes mellitus, MS, and coronary heart disease. On the other hand, n-3PUFA increases insulin sensitivity,[47] glucose utilization, decreases insulin resistance and risk of type 2 diabetes,[48] and also inhibit the production of inflammatory cytokines such as interleukin (IL)-6, IL-1β, and tumor necrosis factor-α,[49] thereby lowering the risk of MS. However, multiple mechanisms could be involved in these effects; particularly, there appears to be a modulation of gene expression by fatty acids, which influences obesity and insulin sensitivity.[50] PUFA are said to suppress lipogenic gene expression, whereas SFA and MUFA have little effect on lipogenic gene expression.[51] Notably, studies from Western populations have shown that adipose tissue gene expression was associated with fatty acid intake, substantiating the effect of fatty acids on adipose tissue gene expression.[52] Such studies on Asian populations would clarify the role of genes in modifying the adverse effects of fatty acids.
Among urban South Indians, the use of refined grains plus sunflower oil was reported to have a synergistic effect to increase the risk of MS.[8] Notably, the intake of refined cereal was very high in this population (419.1 [150]–464.7 [124.4] g/d). Therefore, in the present study also, the increasing risk of components of the MS could be attributed to the synergistic effect of high refined cereal and fat intake.
In contrast, palm oil was originally considered as unhealthy due to its higher content of SFA. However, subsequent studies revealed that in spite of its high SFA and low PUFA content, palm oil did not adversely alter the plasma lipid profile.[53,54,55] Among Malaysian aborigines, palm oil consumption reduced systolic blood pressure significantly.[56]
According to FAO recommendations,[22] there is no rationale for a specific n-6: n-3 ratio, if n-6 and n-3 PUFA intakes are within the recommendations. Nevertheless, the basic parameters to judge an oil as healthy are – the fatty acid profile, n-6: n-3 ratio, and presence of natural antioxidants.[57,58] As there is no single oil which meets these requirements adequately, a combination of cooking oils would be beneficial.[8,13]
A major limitation of our study is that we do not have data on the lipid profile of the participants. However, the effect of dietary fats on blood lipids is well-known and hence we chose to study the other components of the MS. In addition, we have considered only occupation-based physical activity; therefore, the possibility of confounding by physical activity in various domains could not be ruled out. Another limitation was that, being a large, population-based study, we could estimate only fasting capillary glucose using glucometer rather than venous glucose. However, our study has several strengths first; we have captured the dietary intakes using a validated food frequency questionnaire. Second, this study is the first to assess dietary fat intake and its relation to MS in a rural South Indian state with larger sample size. Third, our findings are on par with nationwide surveys and the results can be extrapolated to the whole of rural India.
Conclusion
Our data suggest that total fat intake is a risk factor for components of MS in a rural South Indian population. Our findings also confirm the association between sunflower oil and components of MS. In view of the significant risk of MS even at modest levels of fat intake, a comprehensive lifestyle modification with decreased fat and refined cereal intake would be beneficial. In addition, use of a combination of cooking oils would be effective in lowering the risk of MS. However, more robust evidence from randomized control trials on the health effects of various cooking oils would help policy makers to formulate recommendations on quality and quantity of fat intake.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Popkin BM, Gordon-Larsen P. The nutrition transition: Worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28(Suppl 3):S2–9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 2.Popkin BM. An overview on the nutrition transition and its health implications: The Bellagio meeting. Public Health Nutr. 2002;5:93–103. doi: 10.1079/phn2001280. [DOI] [PubMed] [Google Scholar]
- 3.Misra A, Singhal N, Sivakumar B, Bhagat N, Jaiswal A, Khurana L. Nutrition transition in India: Secular trends in dietary intake and their relationship to diet-related non-communicable diseases. J Diabetes. 2011;3:278–92. doi: 10.1111/j.1753-0407.2011.00139.x. [DOI] [PubMed] [Google Scholar]
- 4.National Sample Survey Organization, Ministry of Statistics and Program Implementation, Government of India. Nutritional Intake in India (July 2011-June 2012). Report of the NSS 68th Round. 2014. Oct, [Last accessed on 2015 Mar 04]. Available from: http://www.mospi.gov.in/nsso .
- 5.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 6.Mohan V, Gokulakrishnan K, Deepa R, Shanthirani CS, Datta M. Association of physical inactivity with components of metabolic syndrome and coronary artery disease – The Chennai Urban Population Study (CUPS no 15) Diabet Med. 2005;22:1206–11. doi: 10.1111/j.1464-5491.2005.01616.x. [DOI] [PubMed] [Google Scholar]
- 7.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57) Metabolism. 2009;58:675–81. doi: 10.1016/j.metabol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Lakshmipriya N, Gayathri R, Praseena K, Vijayalakshmi P, Geetha G, Sudha V, et al. Type of vegetable oils used in cooking and risk of metabolic syndrome among Asian Indians. Int J Food Sci Nutr. 2013;64:131–9. doi: 10.3109/09637486.2012.728197. [DOI] [PubMed] [Google Scholar]
- 9.Chow CK, Naidu S, Raju K, Raju R, Joshi R, Sullivan D, et al. Significant lipid, adiposity and metabolic abnormalities amongst 4535 Indians from a developing region of rural Andhra Pradesh. Atherosclerosis. 2008;196:943–52. doi: 10.1016/j.atherosclerosis.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Misra R, Misra A, Kamalamma N, Vikram NK, Gupta S, Sharma S, et al. Difference in prevalence of diabetes, obesity, metabolic syndrome and associated cardiovascular risk factors in a rural area of Tamil Nadu and an urban area of Delhi. Int J Diabetes Dev Ctries. 2011;31:82–90. [Google Scholar]
- 11.Freire RD, Cardoso MA, Gimeno SG, Ferreira SR. Dietary fat is associated with metabolic syndrome in Japanese Brazilians. Diabetes Care. 2005;28:1779–85. doi: 10.2337/diacare.28.7.1779. [DOI] [PubMed] [Google Scholar]
- 12.Misra A, Singhal N, Khurana L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: Role of dietary fats and oils. J Am Coll Nutr. 2010;29(3 Suppl):289S–301S. doi: 10.1080/07315724.2010.10719844. [DOI] [PubMed] [Google Scholar]
- 13.Ghafoorunissa G. Requirements of dietary fats to meet nutritional needs & prevent the risk of atherosclerosis – An Indian perspective. Indian J Med Res. 1998;108:191–202. [PubMed] [Google Scholar]
- 14.Singh PN, Arthur KN, Orlich MJ, James W, Purty A, Job JS, et al. Global epidemiology of obesity, vegetarian dietary patterns, and noncommunicable disease in Asian Indians. Am J Clin Nutr. 2014;100(Suppl 1):359S–64S. doi: 10.3945/ajcn.113.071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sircar S, Kansra U. Choice of cooking oils – Myths and realities. J Indian Med Assoc. 1998;96:304–7. [PubMed] [Google Scholar]
- 16.Vijayalaxmi MP, Kasturiba B, Naik RK, Malagi U. Influence of fats and oils intake on the lipid profile of adults belonging to different income groups. Karnataka J Agric Sci. 2010;20:112–4. [Google Scholar]
- 17.Willett WC. New York: Oxford University Press; 1998. Nutritional Epidemiology. [Google Scholar]
- 18.Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, et al. The Chennai Urban Rural Epidemiology Study (CURES) – Study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–70. [PubMed] [Google Scholar]
- 19.Mohan V, Deepa M, Pradeepa R, Prathiba V, Datta M, Sethuraman R, et al. Prevention of diabetes in rural India with a telemedicine intervention. J Diabetes Sci Technol. 2012;6:1355–64. doi: 10.1177/193229681200600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.2nd ed. Hyderabad: National Institute of Nutrition; 2012. National Institute of Nutrition. Dietary Guidelines for Indians – A Manual. [Google Scholar]
- 22.Report of an Expert Consultation. Geneva: 2010. FAO-WHO. Fats and Fatty Acids in Human Nutrition. Rome: FAO Food and Nutrition Paper # 91; p. 17. [PubMed] [Google Scholar]
- 23.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–73. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 24.Pi-Sunyer FX. The obesity epidemic: Pathophysiology and consequences of obesity. Obes Res. 2002;10(Suppl 2):97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 25.Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartianinen E, Pietinen P, et al. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet. 1983;1:1–5. doi: 10.1016/s0140-6736(83)91556-8. [DOI] [PubMed] [Google Scholar]
- 26.Glew RH, Conn CA, Vanderjagt TA, Calvin CD, Obadofin MO, Crossey M, et al. Risk factors for cardiovascular disease and diet of urban and rural dwellers in northern Nigeria. J Health Popul Nutr. 2004;22:357–69. [PubMed] [Google Scholar]
- 27.Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002;106:2530–2. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- 28.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 29.Hermansen K. Diet, blood pressure and hypertension. Br J Nutr. 2000;83(Suppl 1):S113–9. doi: 10.1017/s0007114500001045. [DOI] [PubMed] [Google Scholar]
- 30.Thanopoulou AC, Karamanos BG, Angelico FV, Assaad-Khalil SH, Barbato AF, Del Ben MP, et al. Dietary fat intake as risk factor for the development of diabetes: Multinational, multicenter study of the Mediterranean Group for the Study of Diabetes (MGSD) Diabetes Care. 2003;26:302–7. doi: 10.2337/diacare.26.2.302. [DOI] [PubMed] [Google Scholar]
- 31.Feskens EJ, Virtanen SM, Räsänen L, Tuomilehto J, Stengård J, Pekkanen J, et al. Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care. 1995;18:1104–12. doi: 10.2337/diacare.18.8.1104. [DOI] [PubMed] [Google Scholar]
- 32.Van de Laar FA, van de Lisdonk EH, Lucassen PL, Tigchelaar JM, Meyboom S, Mulder J, et al. Fat intake in patients newly diagnosed with type 2 diabetes: A 4-year follow-up study in general practice. Br J Gen Pract. 2004;54:177–82. [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer KA, Kushi LH, Jacobs Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care. 2001;24:1528–35. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- 34.Wolpert HA, Atakov-Castillo A, Smith SA, Steil GM. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: Implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care. 2013;36:810–6. doi: 10.2337/dc12-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Pang Z, Li K. Dietary fat, sedentary behaviors and the prevalence of the metabolic syndrome among Qingdao adults. Nutr Metab Cardiovasc Dis. 2009;19:27–34. doi: 10.1016/j.numecd.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Melanson EL, Astrup A, Donahoo WT. The relationship between dietary fat and fatty acid intake and body weight, diabetes, and the metabolic syndrome. Ann Nutr Metab. 2009;55:229–43. doi: 10.1159/000229004. [DOI] [PubMed] [Google Scholar]
- 37.Washington (DC): USDA and US Department of Health and Human Services; 2015. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. [Google Scholar]
- 38.Gillingham LG, Harris-Janz S, Jones PJ. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 2011;46:209–28. doi: 10.1007/s11745-010-3524-y. [DOI] [PubMed] [Google Scholar]
- 39.Kusum R, Bommayya H. Palm oil and rice bran oil: Current status and future prospects. Int J Plant Physiol Biochem. 2011;3:125–32. [Google Scholar]
- 40.Ghafoorunissa G. Dietary lipids and heart disease – The Indian context. Natl Med J India. 1994;7:270–6. [PubMed] [Google Scholar]
- 41.Govindaraj GN, Suryaprakash S, Sivaramane N, Sundaramoorthy C, Murali P. Dynamics of Household Edible Oil Consumption in Rural and Urban Tamil Nadu (India). In 2012 Conference, August 18-24, 2012. Foz do Iguacu, Brazil No. 126318. International Association of Agricultural Economists. 2012 [Google Scholar]
- 42.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237:885–8. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 43.Jucker BM, Cline GW, Barucci N, Shulman GI. Differential effects of safflower oil versus fish oil feeding on insulin-stimulated glycogen synthesis, glycolysis, and pyruvate dehydrogenase flux in skeletal muscle: A 13C nuclear magnetic resonance study. Diabetes. 1999;48:134–40. doi: 10.2337/diabetes.48.1.134. [DOI] [PubMed] [Google Scholar]
- 44.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–56. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J Nutr Metab 2012. 2012:539426. doi: 10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry EM. Are diets high in omega-6 polyunsaturated fatty acids unhealthy? Eur Heart J. 2001;3(Suppl D):D37–41. [Google Scholar]
- 47.Farsi PF, Djazayery A, Eshraghian MR, Koohdani F, Saboor-Yaraghi AA, Derakhshanian H, et al. Effects of supplementation with omega-3 on insulin sensitivity and non-esterified free fatty acid (NEFA) in type 2 diabetic patients. Arq Bras Endocrinol Metabol. 2014;58:335–40. doi: 10.1590/0004-2730000002861. [DOI] [PubMed] [Google Scholar]
- 48.Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr. 2006;83(6 Suppl):1499S–504S. doi: 10.1093/ajcn/83.6.1499S. [DOI] [PubMed] [Google Scholar]
- 49.Zhao G, Terry D, Keith RE, Martin PJ, Sheila GG, Penny MW. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:385–91. doi: 10.1093/ajcn/85.2.385. [DOI] [PubMed] [Google Scholar]
- 50.Haag M, Dippenaar NG. Dietary fats, fatty acids and insulin resistance: Short review of a multifaceted connection. Med Sci Monit Basic Res. 2005;11:RA359–67. [PubMed] [Google Scholar]
- 51.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48:44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–68. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 53.Hornstra G, van Houwelingen AC, Kester AD, Sundram K. A palm oil-enriched diet lowers serum lipoprotein(a) in normocholesterolemic volunteers. Atherosclerosis. 1991;90:91–3. doi: 10.1016/0021-9150(91)90247-z. [DOI] [PubMed] [Google Scholar]
- 54.Sundram K, Hornstra G, von Houwelingen AC, Kester AD. Replacement of dietary fat with palm oil: Effect on human serum lipids, lipoproteins and apolipoproteins. Br J Nutr. 1992;68:677–92. doi: 10.1079/bjn19920125. [DOI] [PubMed] [Google Scholar]
- 55.Ghafoorunissa G. Nutrition and health implications of palm oil in Indian diets. Indian J Med Res. 1995;102:233–40. [PubMed] [Google Scholar]
- 56.Alias IZ, Jr, Mdisa Z, Abdulkadir K, Ali O. The effect of increased consumption of edible palm oil on the nutritional status, lipid profiles and lipid peroxidation among Malaysian aboriginestc. Malays J Nutr. 2002;8:137–56. [PubMed] [Google Scholar]
- 57.Johnson S, Saikia N, Mathur HB, Agarwal HC. Fatty acids profile of edible oils and fats in India. Cent Sci Environ. 2009;1:3–31. [Google Scholar]
- 58.Mishra S, Manchanda SC. Cooking oils for heart health. J Prev Cardiol. 2012;1:123–31. [Google Scholar]


