Abstract
Introduction:
Choline fenofibrate is a newly developed choline salt of fenofibric acid, which is more hydrophilic than fenofibrate. This study was initiated to evaluate the safety and efficacy of choline fenofibrate in comparison to micronized fenofibrate among Indian patients of mixed dyslipidemia.
Methods:
A multicenter, open-label, randomized, active controlled, comparative, parallel group study was conducted at around 10 centers spread all across the country. Mixed dyslipidemia patients (serum triglycerides [TG] levels between 150 and 500 mg/dl), aged 18–70 years and taking stable statin dose for 8 weeks were randomized to choline fenofibrate 135 mg delayed release tablets and micronized fenofibrate 160 mg tablets once daily for 12 weeks. The primary endpoint of the study was percentage change in serum TG level at the end of 12 weeks.
Results:
A total of 226 patients were enrolled in this study, of which 116 patients were administered choline fenofibrate and 110 patients were administered micronized fenofibrate. At the end of 12 weeks, there was a significant reduction in TG level (34.24% in choline fenofibrate group and 38.13% reduction in micronized fenofibrate group). However, the difference between group was not statistically different (P = 0.471). Similarly, there was a significant increase in high-density lipoprotein cholesterol at the end of 12 weeks (10% increase in choline fenofibrate group and 9% increase in micronized fenofibrate group); but the difference between the group was not statistically significant (P = 0.598). Both the treatment was safe and well tolerated.
Conclusion:
Choline fenofibrate delayed release 135 mg is as safe and effective as 160 mg of micronized fenofibrate in Indian patients with mixed dyslipidemia.
Keywords: Choline fenofibrate, micronized fenofibrate, mixed dyslipidemia
INTRODUCTION
Mixed or combined dyslipidemia is described by elevated levels of triglycerides (TG) and reduced high-density lipoprotein cholesterol (HDL-C), with or without elevated levels of low-density lipoprotein cholesterol (LDL-C). Mixed dyslipidemia is typically linked with an increase of small, dense LDL particles, and an elevated apolipoprotein B. All these changes predispose to an increased risk of cardiovascular disease.[1] Mixed dyslipidemia is the most frequent lipid disorder found in patients with type 2 diabetes.[2]
Initial treatment options for mixed dyslipidemia include lifestyle modification and statin therapy. Nevertheless, statin monotherapy is often insufficient to normalize multiple lipid parameters.[3] The HDL-C and TG levels are strong predictor of cardiovascular risk even in patients treated with a statin and has achieved an optimum LDL-C levels.[4,5] The addition of fibrates to statin therapy offers the prospective for overall lipid control in patients with mixed dyslipidemia. Several results of short-term studies in various patient populations support this hypothesis.[6,7,8,9]
Fenofibrate, which belongs to the class of fibrates, has a comparatively low potential for pharmacokinetic interaction as well as lower rates of rhabdomyolysis with statins when compared to gemfibrozil.[10] Choline fenofibrate is a newly developed choline salt of fenofibric acid and is more hydrophilic than fenofibrate. It does not require first pass hepatic metabolism to become active, as it dissociates to free fenofibric acid within the gastrointestinal tract and rapidly absorbed throughout the gastrointestinal tract.[11] Of the various fenofibrate formulations available, choline fenofibrate is the only formulation of fenofibrate which is approved by the US FDA for co-administration with statins.[12] However, there is no Indian data available for efficacy and safety of choline fenofibrate in patients of mixed dyslipidemia. Hence, this study was planned to evaluate the efficacy and safety of choline fenofibrate in comparison to micronized fenofibrate among Indian patients of mixed dyslipidemia stabilized on statin therapy.
METHODS
Patients and study sites
Patients with mixed dyslipidemia of either gender, aged 18–70 years having serum TG levels between 150 and 500 mg/dl who were prescribed statin in stable dose for the preceding 8 weeks were enrolled in the study. The primary exclusion criteria included patients with serum LDL-C >160 mg/dl; type 1 diabetes mellitus, thyroid dysfunction, active liver disease, patients with renal dysfunction (serum creatinine more than 1.5 times upper normal limit).
The study was carried out at around 10 centers spread all across the country. The study was conducted according to the protocol and in accordance with the ethical guidelines by Indian Council of Medical Research. The study was conducted at each trial site after receiving approvals from respective Institutional/Independent Ethics Committee and Drug Controller General of India. The study was registered in the clinical trial registry - India (CTRI/2010/091/006086).
Treatment groups and study design
The study design was multicenter, open-label, randomized, active controlled, comparative, and parallel group. Patients meeting eligibility criteria were enrolled in the study and randomized in 1:1 ratio to receive either choline fenofibrate delayed release 135 mg or micronized fenofibrate 160 mg as per computer generated randomization sheet.
There were total six study visits: Screening visit (day − 7–0), enrollment visit (day 1), follow-up visit 1 (day 15), follow-up visit 2 (day 29), follow-up visit 3 (day 57), and end of study visit (day 85). Enrolled patients were provided allocated study medications for 3 months, and they were evaluated during the study period at the end of 2 weeks, 4 weeks, 8 weeks, and 12 weeks for safety and efficacy evaluation. Lipid profile and creatinine phosphokinase (CPK) were repeated at the end of 4 weeks, 8 weeks, and 12 weeks. Laboratory investigations, performed at the time of screening were repeated at the end of the study.
Primary and secondary end points
The primary endpoint was percentage change in serum TG levels at the end of treatment (12 weeks) from baseline between two arms. The secondary efficacy endpoint was percentage change in serum HDL-C levels at the end of treatment from baseline. Change in other lipid parameters was also assessed. The following safety parameters were also evaluated: Any adverse events that were reported voluntarily observed and enquired during the study; any clinically significant change in the value of laboratory results, vital signs, and physical examination during the study compared to baseline.
Sample size determination and statistical methods
Considering the standard deviation of 78 with a power of 80%, approximately 100 patients per group were required to detect a difference of around 31. Considering 12 weeks duration of study and dropout rate, it was planned to enroll 240 patients at the beginning of the study to have data of 200 evaluable patients. The level of significance was 5% for the study.
Evaluation of efficacy and safety was done on the intention to treat population, which consisted of all randomized patients who completed at least one follow-up visit as per protocol. Mean percent change in TG and HDL-C levels at the end of treatment from baseline was statistically evaluated within the group and between the groups by Student's t-test. Within group and between-group comparisons for laboratory results were analyzed using χ2 test or Fisher's exact test. The level of significance was 5%.
RESULTS
A total of 420 patients were screened across all the sites. Of these 226 patients were enrolled in the study - 116 patients were administered choline fenofibrate 135 mg, and 110 were administered micronized fenofibrate 160 mg. Of these 226 enrolled patients, 201 patients who completed at least 1 month follow-up were included for intention to treat principle analysis (105 patients in choline fenofibrate group and 96 patients in micronized fenofibrate group). Number of patients lost to follow-up immediately after randomization and not completed even the second follow-up visit on day 28 was 25. One patient did not take the medication regularly as per protocol, and hence data were not used for analysis.
Baseline characteristics
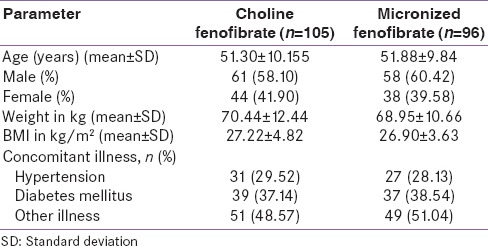
Patients from both groups had matching baseline characteristics in terms of age, gender, weight, and body mass index [Table 1].
Table 1.
Baseline characteristics
Primary endpoint
There was a significant reduction in serum TG levels at the end of 12 weeks study period in both groups with respect to baseline value. Serum TG level was reduced by approximately 34.24% in choline fenofibrate group as compared to 38.13% reduction in micronized fenofibrate group at the end of 12 weeks. However, the difference between the group was not statistically significant (P = 0.471). With both investigational products, a large percentage of reduction was evident at 4 weeks after initiation of therapy. This reduction was sustained or increased at the end of 12 weeks in both treatment groups [Figure 1].
Figure 1.
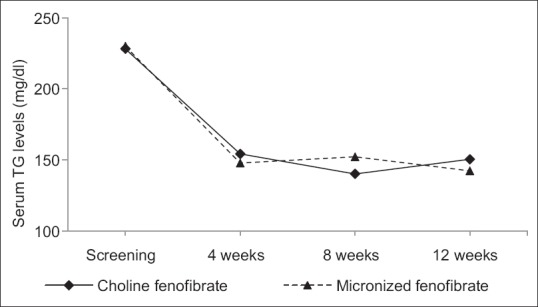
Change in serum triglyceride level in both study groups
Secondary endpoints
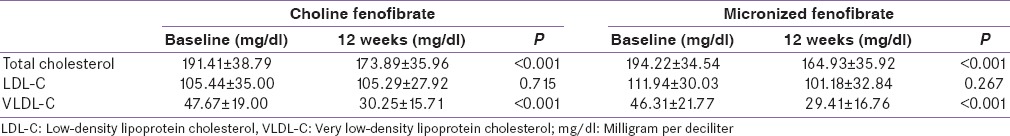
There was a significant increase in serum HDL-C levels at the end of 4 weeks in both the groups as compared to their baseline values. Serum HDL-C level was increased by approximately 10% in choline fenofibrate group as compared to 9% increase in micronized fenofibrate group at the end of 4 weeks. However, the difference between the group was not statistically significant (P = 0.598) [Figure 2]. Increase in serum HDL-C level was faster in choline fenofibrate group as compared to slower increase in micronized fenofibrate group. The increase in serum HDL-C level was maintained until the end of the study period [Figure 2]. Significant reduction in total cholesterol and VLDL-C from baseline was also observed in both the groups [Table 2].
Figure 2.
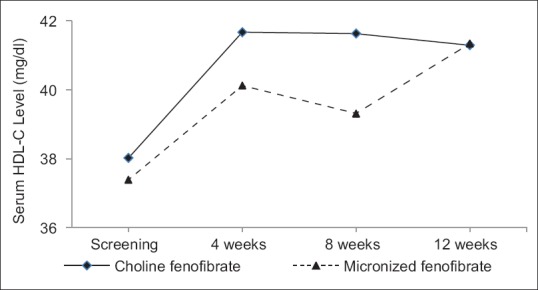
Change in serum high-density lipoprotein cholesterol levels in both study groups
Table 2.
Change in other lipid parameters from baseline
Safety
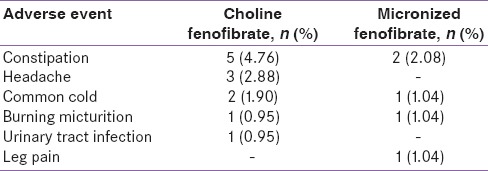
Choline fenofibrate, as well as micronized fenofibrate, were well tolerated. Most commonly reported adverse events during the conduct of study were constipation and headache [Table 3]. One patient in micronized fenofibrate group developed leg pain and slight elevation of CPK levels. Considering the safety of the subject, further treatment was discontinued, and the adverse event was resolved. All adverse events were mild in nature and resolved with the continuation of treatment. No serious or life-threatening adverse event reported during the conduct of the study. No clinical significant abnormality was reported in any of the laboratory investigations done. None of the patients had liver enzymes elevation ≥3 times of upper limit of normal (ULN). Elevation of CPK (≥10 times ULN) was not reported in any of the patients.
Table 3.
Number of patients with adverse events
DISCUSSION
High serum TG, low HDL-C levels, and often nonoptimal serum LDL-C levels are the hallmarks of mixed dyslipidemia.[3] The presence of high TG and low HDL-C levels are independently associated with increased coronary heart disease risk.[13,14] When compared with elevated LDL-C alone, the combination of high TG and/or low HDL-C in addition to suboptimal LDL-C poses a significantly higher risk for cardiovascular events.[15]
Fenofibrate is a prodrug and undergoes rapid hydrolysis at the ester bond to form fenofibric acid, which is the active metabolite.[16] Fenofibrate is a neutral, lipophilic compound and is practically insoluble in water; which makes it difficult to consistently attain therapeutic levels. Novel fenofibrate formulations were developed with different approaches to overcome the challenges with solubility.[17] Initially, micronized formulations that increased solubility by reducing particle size, and increasing surface area were introduced.[18] A formulation of the active metabolite (fenofibric acid) with a choline salt form was developed resulting in a hydrophilic compound with the greatest bioavailability of the available formulations.[12]
The results of our study indicated that there was a significant reduction in the serum TG levels observed with 12 weeks treatment of fenofibrate (34.24% in choline fenofibrate group vs. 38.13% in micronized fenofibrate group). There was also a significant increase in the serum HDL-C levels observed with 12 weeks treatment of fenofibrate (8.6% in choline fenofibrate group vs. 10.56% in micronized fenofibrate group). Results of our study were similar to the pooled subgroup analysis of three randomized controlled phase III clinical trials of choline fenofibrate was.[19] In the pooled analysis, the addition of fibrates to moderate dose of statin yielded approximately 43% reduction in serum TG levels and 16% increase in HDL-C levels over a period of 12 weeks.
For patients with type 2 diabetes, statin monotherapy is recommended to achieve their LDL-C goal. However, statin alone often does not normalize other lipid parameters in patients having mixed dyslipidemia. Recent retrospective analyses showed that in majority of patients with mixed dyslipidemia on statin monotherapy have suboptimal serum levels of LDL-C, HDL-C, and TG.[20,21,22] In type 2 diabetes patients with dyslipidemia, a considerable residual risk persists even after statin therapy. In patients not achieving the target goals for lipid parameters, one of the options is to increase statin dose, but increasing statin dose increases the risk of side effects like myopathy. In such group of patients with below normal HDL-C and TG levels, addition of fibrates has been shown to provide additional benefit.[23] Other lipid parameters were also improved with use of both the drugs in the patients. Serum total cholesterol and very LDL-C levels were also reduced with use of both the drugs during the study period. The result of our clinical study was comparable with previously reported results available in the literature.
Adverse effects involving skeletal muscle are associated with use of statins. Concomitant use of statins and fibrates is believed to further increase the incidence of myalgia and muscle damage.[23] However, in our study concomitant use of fibrate with statin was found to be safe, and no increased incidence of muscle damage was reported. A meta-analysis which included six clinical trials and 1628 subjects reported no cases of myopathy or muscle damage but showed a significant increase in elevation of liver enzymes in statin-fibrate combination therapy when compared to statin monotherapy. The study concluded that statin-fibrate combination therapy was well tolerated.[24]
All the reported adverse events were mild in nature. No hepatotoxicity was reported in any of the patients treated. Except in a single case who complained of myalgia in micronized fenofibrate group, all others tolerated it very well. In none of the patients, clinically significant elevation of liver enzymes or CPK was observed.
CONCLUSION
Overall, results of the current study concluded that 135 mg of choline fenofibrate is as safe and effective as 160 mg of micronized fenofibrate in Indian patients with mixed dyslipidemia. Both study drugs, i.e., choline fenofibrate and micronized fenofibrate were well tolerated and resulted in more comprehensive improvement in the abnormal lipid profile.
Financial support and sponsorship
This study was funded by Intas Pharmaceuticals Limited, Ahmedabad.
Conflicts of interest
Dr. Piyush Patel and Dr. Hanmant Barkate are the employees of Intas Pharmaceuticals Limited.
Acknowledgment
We would like to acknowledge following investigators for their continuous support in this research study: Dr. Asha N. Shah, M.D. (Medicine); Dr. Nehal Sadhu, M.D. (Medicine); Dr. Vaishali Deshmukh, D.M. (Endocrinology); Dr. J. L. Shah, M.D. (Medicine); Dr. Rajesh Deshmane, MBBS, FCPS, Diploma in Diabetology; Dr. Manoj Vithalani, M.D. (Medicine); Dr. Dimple Patel, M.D. (Medicine),
Dr. Raxit Brahmabhatt, M.D. (Medicine); Dr. Sanjay Godbole, M.D. (Medicine); Dr. Dhiren Punatar, M.D. (Medicine). We would also like to thank Dr. Dimple Shah and Dr. Falgun Vyas for their contribution in this research study.
REFERENCES
- 1.Best JD, O’Neal DN. Diabetic dyslipidaemia: Current treatment recommendations. Drugs. 2000;59:1101–11. doi: 10.2165/00003495-200059050-00006. [DOI] [PubMed] [Google Scholar]
- 2.Szapary PO, Rader DJ. The triglyceride-high-density lipoprotein axis: An important target of therapy? Am Heart J. 2004;148:211–21. doi: 10.1016/j.ahj.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 4.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 5.Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. PROVE IT-TIMI Investigators. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–30. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care. 2002;25:1198–202. doi: 10.2337/diacare.25.7.1198. [DOI] [PubMed] [Google Scholar]
- 7.Durrington PN, Tuomilehto J, Hamann A, Kallend D, Smith K. Rosuvastatin and fenofibrate alone and in combination in type 2 diabetes patients with combined hyperlipidaemia. Diabetes Res Clin Pract. 2004;64:137–51. doi: 10.1016/j.diabres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol. 2005;45:1649–53. doi: 10.1016/j.jacc.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial) Am J Cardiol. 2005;95:462–8. doi: 10.1016/j.amjcard.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Davidson MH. Statin/fibrate combination in patients with metabolic syndrome or diabetes: Evaluating the risks of pharmacokinetic drug interactions. Expert Opin Drug Saf. 2006;5:145–56. doi: 10.1517/14740338.5.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Jones PH, Davidson MH, Kashyap ML, Kelly MT, Buttler SM, Setze CM, et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: A phase 3 study. Atherosclerosis. 2009;204:208–15. doi: 10.1016/j.atherosclerosis.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 12.North Chicago (IL): Abbott Laboratories; 2008. [Last accessed on 2015 Dec 05]. Trilipix [package insert] Available from: http://www.trilipixpro.com/ [Google Scholar]
- 13.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 14.Assmann G, Schulte H, von Eckardstein A, Huang Y. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 1996;124(Suppl):S11–20. doi: 10.1016/0021-9150(96)05852-2. [DOI] [PubMed] [Google Scholar]
- 15.Stanek EJ, Sarawate C, Willey VJ, Charland SL, Cziraky MJ. Risk of cardiovascular events in patients at optimal values for combined lipid parameters. Curr Med Res Opin. 2007;23:553–63. doi: 10.1185/030079906x167660. [DOI] [PubMed] [Google Scholar]
- 16.Adkins JC, Faulds D. Micronised fenofibrate: A review of its pharmacodynamic properties and clinical efficacy in the management of dyslipidaemia. Drugs. 1997;54:615–33. doi: 10.2165/00003495-199754040-00007. [DOI] [PubMed] [Google Scholar]
- 17.Vogt M, Kunath K, Dressman JB. Dissolution enhancement of fenofibrate by micronization, cogrinding and spray-drying: Comparison with commercial preparations. Eur J Pharm Biopharm. 2008;68:283–8. doi: 10.1016/j.ejpb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Rawat N, Kumar SM, Mahadevan N. Solubility: Particle size reduction is a promising approach to improve the bioavailability of lipophillic drugs. Int J Recent Adv Pharm Res. 2011;1:8–18. [Google Scholar]
- 19.Ballantyne CM, Jones PH, Kelly MT, Setze CM, Lele A, Thakker KM, et al. Long-term efficacy of adding fenofibric acid to moderate-dose statin therapy in patients with persistent elevated triglycerides. Cardiovasc Drugs Ther. 2011;25:59–67. doi: 10.1007/s10557-011-6280-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsheikh-Ali AA, Lin JL, Abourjaily P, Ahearn D, Kuvin JT, Karas RH. Extent to which accepted serum lipid goals are achieved in a contemporary general medical population with coronary heart disease risk equivalents. Am J Cardiol. 2006;98:1231–3. doi: 10.1016/j.amjcard.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 21.Stacy TA, Egger A. Results of retrospective chart review to determine improvement in lipid goal attainment in patients treated by high-volume prescribers of lipid-modifying drugs. J Manag Care Pharm. 2006;12:745–51. doi: 10.18553/jmcp.2006.12.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toth PP, Zarotsky V, Sullivan JM, Laitinen D. Lipid therapy utilization rates in a managed-care mixed dyslipidemia population. J Clin Lipidol. 2008;2:365–74. doi: 10.1016/j.jacl.2008.08.443. [DOI] [PubMed] [Google Scholar]
- 23.Agouridis AP, Rizos CV, Elisaf MS, Filippatos TD. Does combination therapy with statins and fibrates prevent cardiovascular disease in diabetic patients with atherogenic mixed dyslipidemia? Rev Diabet Stud. 2013;10:171–90. doi: 10.1900/RDS.2013.10.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, Meng F, Ma N, Li C, Ding Z, Wang H, et al. Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyperlipidemia. Am J Cardiol. 2012;110:1296–301. doi: 10.1016/j.amjcard.2012.06.050. [DOI] [PubMed] [Google Scholar]


