Abstract
Background:
Hyperprolactinemia may reflect neuroendocrine stress reaction against acute coronary syndromes.
Aim:
The aim of the present study was evaluation of the serum prolactin level in the acute myocardial infarction (MI) regarding the current pharmacotherapy in management of MI.
Setting and Design:
Cross-sectional clinical based study.
Subjects and Methods:
This cross-sectional clinical study involved all patients with acute MI in a coronary care unit, a total number of 44 patients (45% males and 55% females) with age ranged from 40 to 75 years. A full history for modifiable risk factors and current therapy with aspirin, clopidogrel and or metformin, all patients are nonsmokers. The anthropometric measurements; for estimations of body mass index (kg/m2), electrocardiography was obtained. Fasting blood samples were taken in the morning from all patients and the sera used for estimations of routine investigation and determination of ischemic cardiac biomarkers like cardiac troponin I (cTnI) and serum prolactin level.
Results:
This study shows a significant increase in the serum prolactin in acute MI as compared with the control. In acute MI serum cTnI elevation was correlated with serum prolactin increments. In metformin-treated group, there was a lowest prolactin serum level.
Conclusions:
Serum prolactin level increased in acute MI, and positively correlated with cardiac troponin level and reflects underlying cardiovascular complications.
Keywords: Acute myocardial infarction, metformin, prolactin
INTRODUCTION
Prolactin is a hormone that synthesized and secreted from the anterior pituitary lactotrophic cells,[1] which their numbers do not alter with age, but lactotrophic cells hyperplasia does happen during gestation and lactation, which resolves within months after delivery.[2] Prolactin secretion is stimulated by thyrotropin releasing a hormone (TRH) and inhibited by dopamine.[3] Moreover, extra-pituitary prolactin found in the cerebral cortex, amygdala, myometrium, lacrimal glands, thymus, immune cell, skin fibroblast, sweat gland, and spinal cord this extra-pituitary prolactin is not regulated by dopamine but through independent factors.[4]
Hyperprolactinemia may reflect neuroendocrine stress reaction to acute coronary syndromes, which induces acute endothelial dysfunction, insulin resistance, and induction of vascular immune reactions thus; prolonged hyperprolactinemia lead to arteriosclerosis, augmentation of arterial stiffness, and hypertension.[5] Ex vivo studies shown that prolactin augments adhesion of the immune cells into endothelium through integrin-mediated effects that cause proliferation of vascular smooth muscle cells which may contribute into atherosclerotic expansion and elevation of cardiac risk profile.[6] Many studies link primary hypothyroidism with ischemic heart disease due to an elevation of TRH level which regarded as potent stimulants of prolactin secretion. Hence, peripartum cardiomyopathy was linked to the high serum prolactin level,[7] therefore, physiological prolactin levels stimulate JAK-STAT pathway which stimulated cardiomyocyte hypertrophy, angiogenesis, expression of prolactin receptors, and cardiac protection via upregulation of superoxide dismutase that inhibit free radical formations, but excessive prolactin above physiological level lead to severe inhibition of cardiac metabolism and damaging of cardiac microvasculature's which may prevent via dopamine agonist that inhibit prolactin secretions.[8]
All these observations showed that a high prolactin level plays a potential role in the development of ischemic cardiac disease, also; hyperprolactinemia leads to dyslipidemia, augmentation of platelets aggregation and amplification of vascular thrombosis that leading to the increasing in the risk score of acute coronary syndrome.[9] In addition, high prolactin serum level cause a significant vasoconstriction and induction of oxidative stress in the coronary vessels since; prolactin receptors are overexpressed in atherosclerotic plaque macrophage which indicate the association between prolactin and induction of inflammatory markers that may explain the connection between serum prolactin and cardiovascular mortality.[10]
The pleiotropic effects of prolactin on the inflammatory mediators revealed through T-cell activation, interferon production, and regulation of macrophage function that induce a low-grade vascular inflammation and induction of coronary atherothrombotic complications also, serum prolactin is positively correlated with blood pressure and the risk score of cardiovascular complications.[11] Immunohistochemical studies, implicate prolactin in the pathogenesis of acute coronary syndromes but, serum prolactin levels are not predictive and index factor for development of acute coronary failure, this may explain local paracrine effects of prolactin on vascular smooth muscle hyperplasia in the development of coronary atherosclerosis.[12,13]
Therefore, the aim of the present study was evaluation of the serum prolactin level in the acute myocardial infarction (MI) regarding the current pharmacotherapy in management of MI.
SUBJECTS AND METHODS
This cross-sectional study was done in Department of Clinical Pharmacology, College of Medicine, Al-Mustansiriia University in collaboration with Department of Internal Medicine, Al-Yarmouk Teaching Hospital in Baghdad, Iraq from March to May 2015. This clinical study was mannered according to the guideline on the Declaration of Helsinki and NewYork Heart Association,[14] with specific confined approval from the Ethics Board Review Committee. All patients presented a write knowledgeble consent to this clinical study.
This study involved patients with acute MI in a coronary care unit (CUU), a total number of 44 patients (45% males and 55% females) with age ranged from 40 to 75 years, this compared with 22 normal healthy controlled volunteers.
A full history for modifiable risk factors and current therapy with aspirin, clopidogrel and or metformin, all patients were nonsmokers. The anthropometric measurements; for estimation of body mass index (BMI = kg/m2), electrocardiography was obtained.
Fasting blood samples were taken in the morning from all patients and the sera were used for estimations of routine investigation and determination of ischemic cardiac biomarkers like cardiac troponine I (cTnI) (ELISA kit method Catalog No.: E-EL-R1253 pg/ml), then 100 μl of sera was used for determining serum prolactin level (PRL ELISA kit method INF 5303001GB) in ng/ml with an analytical sensitivity of approximately 0.8 ng/ml.
Depend on specific ELISA kit instruction, serum level of prolactin in ng/ml calculated according to the standard curve [Figure 1].
Figure 1.
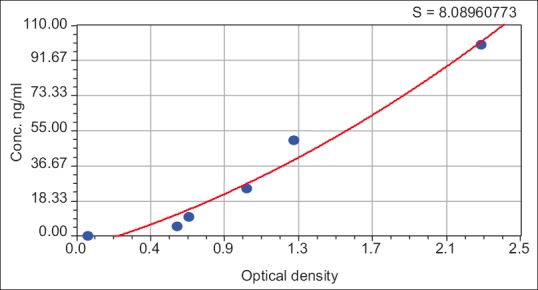
Prolactin hormone standard curve
Determination of lipid profile
Total serum cholesterol, total serum triglyceride (TG) and high-density lipoprotein (HDL) done via a specific kit ELISA method, and then from these parameters we can calculate the followings:[15]
Very low-density lipoprotein (VLDL) = TG/5
Low-density lipoprotein (LDL) = TG−(HDL+VLDL)
Atherogenic index = (TC − HDL)/HDL
Atherogenic ratio = HDL/LDL, LDL/HDL, TC/HDL.
Statistical analysis
All calculations were done via using SPSS software (version 19.0. 2010, IBM Corp., Armonk, NY, USA). All data are expressed as mean ± standard deviation (SD). The unpaired t-test is used to estimate the significant of differences between patients and normal control. The correlation was done for analysis of variables and the differences between groups estimated by ANOVA for correlation scrutiny, two-tailed P < 0.05 was regarded as statistically significant.
RESULTS
Age and gender distribution in this study were selected to exclude gender and physiological changes in the increments of serum prolactin levels [Figure 2].
Figure 2.
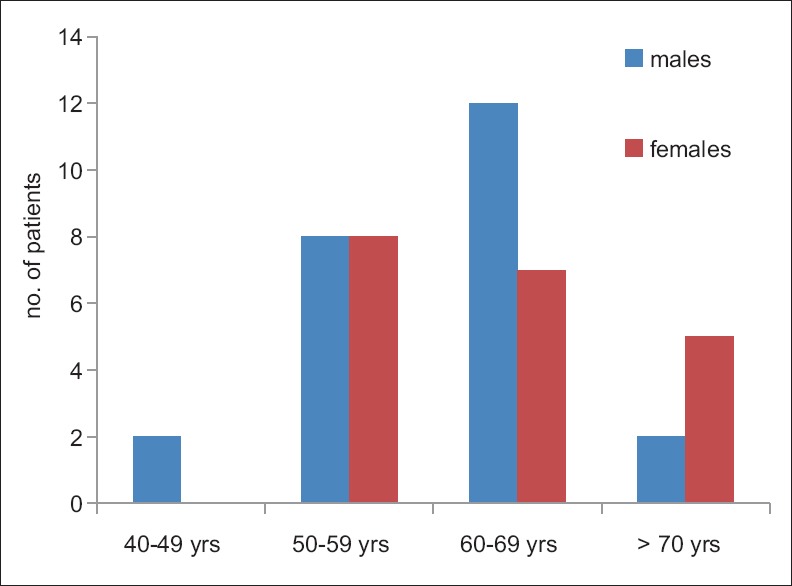
Age and gender distribution of current study
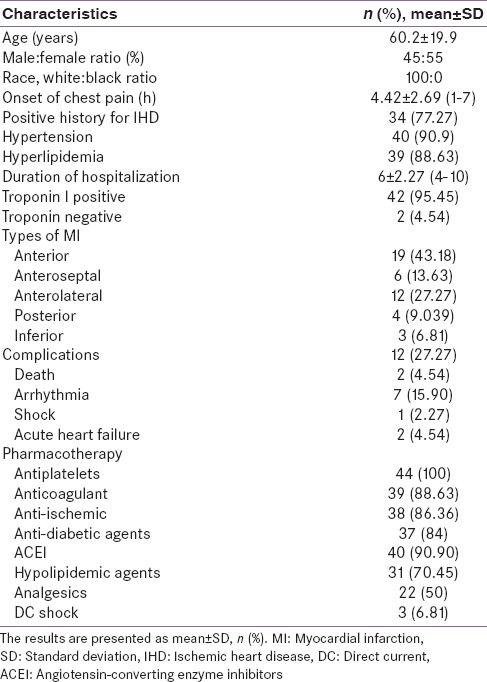
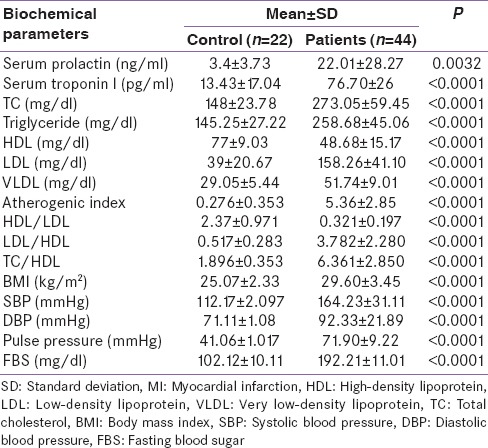
Patients with acute MI presented with diverse clinical and biochemical variables with full previous history [Table 1].
Table 1.
Clinical characteristic of patients presented with acute MI
Anthropometric measurements in patients with acute MI shows overweighted patients (BMI = 29.60 ± 3.45) with other changes [Table 2].
Table 2.
Anthropometric characteristics

A total of 66 subjects (22 control and 44 patients) analyses by Fisher's test for serum prolactin shows 23 subjects above the normal limit (1 control and 22 patients) and 43 subjects (21 control and 22 patients) within normal limit that gave a high significant difference between and within measured groups P = 0.0002 [Table 3].
Table 3.
Comparison between study patient and control groups concerning high prolactin level (ng/ml)

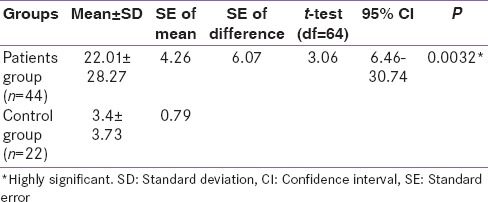
The present study also shows a significant increase in the serum prolactin in acute MI as compared with the control; it increased from 3.4 ± 3.73 ng/ml in controlled groups to 22.01 ± 28.27 ng/ml in patients with acute MI P = 0.0032 [Table 4].
Table 4.
Comparison between patients and control groups concerning the mean prolactin levels (ng/ml)
In the patients presented with acute MI at CCU, there were significant differences in the most biochemical parameters as compared with control.
Serum prolactin level was 22.01 ± 28.27 ng/ml in patients with acute MI as compared with control serum prolactin 3.4 ± 3.73 ng/ml P = 0.0032, this elevation was corresponding with the increments in cTnI from 13.43 ± 17.04 to 76.70 ± 26 pg/ml P < 0.0001. Moreover, blood pressure changes and lipid profile significantly differ from control measures [Table 5].
Table 5.
Biochemical difference between patients with high serum prolactin and control in acute MI
In acute MI, serum cTnI elevation was correlated with serum prolactin increments, this correlation is highly positive R = 1.06 [Figure 3].
Figure 3.
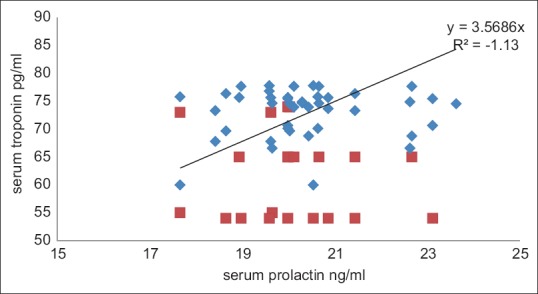
Correlation between serum prolactin and serum troponin in acute myocardial infarction
Moreover, serum prolactin in this study is correlated with serum cholesterol and TG positively R = 0.173 and R = 0.2, respectively, thus, serum prolactin is more correlated with serum cholesterol than with TG.
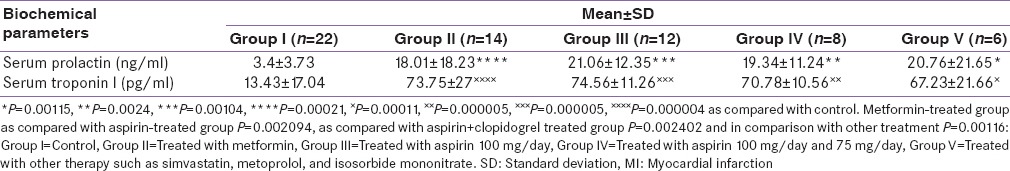
The differential effects of current therapy for acute MI on serum levels of both prolactin and cTnI. In previously treated groups of metformin and currently with soluble insulin at the time of diagnosis, serum prolactin level was 18.01 ± 18 ng/ml, while serum troponin was 73.75 ± 27 pg/ml in comparison with control P = 0.00021 for prolactin and P = 0.000004 for cTnI. While, patients treated with aspirin 100 mg/day, serum prolactin and cardiac troponin levels were 21.06 ± 12.35 ng/ml and 74.56 ± 11.26 pg/ml with P = 0.00104 and P = 0.000005, respectively in comparison with control. Furthermore, combined aspirin and clopidogrel treatment show significant increments in both prolactin and cTnI P = 0.0024 and P = 0.000005, respectively. Regarding other types of therapy currently and previously used in patients with acute MI such as simvastatin, isosorbide mononitrate, and metoprolol serum levels of both prolactin and cTnI were 20.76 ± 21.65 ng/ml P = 0.00115 and 67.23 ± 21.66 pg/ml P = 0.000005, respectively as compared with control.
Because, in the metformin-treated group, there was a lowest prolactin serum level 18.01 ± 18.23 ng/ml the differences with other treated group was significant P = 0.002094 in comparison with the aspirin-treated group, in comparison with aspirin + clopidogrel-treated group P = 0.002402 in comparison with other treatment group P = 0.00116 [Table 6].
Table 6.
Differential effects of previous treatments on serum prolactin and cardiac troponin in acute MI
Regarding gender differences in serum prolactin, higher serum prolactin level showed in females in comparison with male P = 0.0063, while, in cTnI serum level, there is insignificant gender variation P = 0.526 [Table 7].
Table 7.
Gender differences in serum prolactin level in acute MI

DISCUSSION
The present study shows elevation in the serum prolactin hormone levels in patients presented with acute MI, significantly as compared with healthy control, this increment in serum prolactin is positively correlated with cTnI, serum cholesterol, and TG levels. This clinical study also demonstrated that current and previous pharmacotherapy for managements of acute MI may affect serum prolactin levels, metformin mainly decreased prolactin levels in acute MI.
The present study revealed that higher levels of serum prolactin encountered in patients with acute MI pretreated with aspirin. This may be attributed to the influence of aspirin therapy on prostaglandin generation in the hypothalamic gland. It's well known that hypothalamic prostaglandin participate in regulation of dopamine release. So inhibiting of hypothalamic prostaglandin synthesis by aspirin might interfere with normal release of dopamine (prolactin inhibitor factor) and this in turn leads to increase in serum prolactin levels.[16]
Unlike aspirin, clopidogrel did not affect serum prolactin.[17] Moreover, countless clinical studies showed dose-dependent effects of metformin in the reduction of serum prolactin significantly in polycystic ovary syndromes this suggesting that a vague signal pathway to prolactin secretion could be under the influence of insulin,[18] this corresponded with our findings that show a low serum prolactin in the metformin-treated group.
Animal models study showed that α-2 antagonist yohimbine lead significant increase in the serum prolactin,[19] while; nonselective β-antagonist like propranolol inhibits basal prolactin secretion thus; β-blocker produced insignificant effects at physiological prolactin level but it reduced prolactin significantly at pathological and stress induced hyperprolactinemia,[20] which may explained the effects of antihypertensive agent that commonly used in the cardiovascular complications on serum prolactin measurement in acute coronary syndrome but, there is a little association between high prolactin level and antihypertensive agents and most of the antihypertensive agent in this study were uncontrolled and unremarkable.
Therefore, most of the currents pharmacotherapy decreases serum prolactin levels in acute MI; this may be due to amelioration of cardiac function and oxidative stress since; during acute MI serum prolactin levels increased as a stress response, this cardiac necrosis leads to depletion of antioxidant STAT3 level which led to the provoking of cardiomyocyte oxidative response that in consequence activate cathepsin D enzyme which plays an essential role in conversion of 23 kDa prolactin into anti-angiogenic and pro-inflammatory 16 kDa subfraction which leads to chemokine and cytokine overexpressions with NFκB activation all these changes leading to coronary vasoconstriction, apoptosis, and inflammatory cardiomyocyte injury,[21] unfortunately, 16kDa subfraction was not measured separately in this study.
Moreover, dopamine agonist agents like bromocriptine reduce infarction size and improves cardiac function after ischemic reperfusion injury in animal model study, which implicate the prolactin in progressions of acute MI.[22]
Therefore, reductions of oxidative stress by current pharmacotherapy prevent activation of harmful 16 kDa prolactin subfraction which per se reduces the body stress response and then reduction of serum prolactin levels during the recovery period from MI.[23]
In MI-induced hyperprolactinemia, there is increasing in the level of C-reactive protein (CRP) which indicate for inflammation and vascular injury, so, CRP regarded as independent risk factor for cardiovascular morbidity,[24] also, bromocriptine decreases CRP level after 8 weeks of therapy via inhibition of prolactin secretion thus, during amenorrhea and menopause the level of CRP elevated which indicating increasing in the level of serum prolactin.[25] Therefore, prolactin was implicated in the inflammatory changes that occur to acute attack of MI, and the correlation between high prolactin and CRP level is well documented.[26]
In addition, serum prolactin was linked with hypertension, arterial stiffens, peripheral vascular resistance, activations of vascular sympathetic tone, vascular smooth muscle cell proliferations, and low-grade inflammatory process,[27] all these effects leading to the alteration in endothelial integrity which reflected the relationship between prolactin and vascular damage, but prolactin was mainly correlated with arteriosclerosis than with atherosclerosis.[28] These, findings correlated with this study result which shows a link between hypertension and serum prolactin in acute MI, since in most hypertensive patients circadian prolactin secretion is blunted and become higher at morning[29] this may give important explanations of high prolactin levels in acute MI because of most of the samples were collected in the morning of this study.
High serum prolactin level showed a positive correlation with dyslipidemia, a study on females with prolactinoma revealed a decrease in the HDL, but other lipid profile parameters was similar to the control group thus; exogenous estrogen or bromocriptine improves lipid profile during the menopause this per se explained the role of prolactin in the induction of dyslipidemia.[30] In our study, a high prolactin serum level is associated with hyperlipidemia as compared with control and there is a significant correlation between serum prolactin serum level and serum TG and cholesterol.
As well, prolactin induces expression of P-selectin in the platelet and augment of adenosine diphosphate-induced platelet aggregation leading to thrombosis in acute coronary syndromes mainly in acute MI, also there was an association between high prolactin level and stroke,[26] which corresponded with our findings that show a potential link between prolactin serum level and incidence of acute MI.
Furthermore, prolactin serum level effects correlated significantly with platelet P-selectin expression in acute MI.[31] Therefore, prolactin regarded as a novel platelet activator via specific isoform of prolactin receptor and there is a possible link between high serum prolactin level and venous thromboembolism,[32] but, unfortunately, the thromboembolic profile was not measured in this study. Therefore, the effects of antiplatelet agents on the prolactin induced platelet aggregations were investigated in vitro, shown that clopidogrel produced significant inhibition whereas; aspirin produced no effects. Consequently serum prolactin level may be regarded as a novel biomarker for prediction of antiplatelet agents selection during acute management of MI at the CUU.[33]
Moreover, dopamine agonist decreases the risk and occurrence of cardiometabolic risk that linked with visceral obesity and metabolic syndrome, this gives an idea about the link between hyperprolactinemia with metabolic syndrome that induced coronary complications and MI.[34] This is corresponding with our findings that showed a link between BMI and serum prolactin, also; long-term effects of high serum prolactin level contribute to the development and progression of angiogenesis, which lead to cardiomyopathy, inhibits angiogenesis through antiangiogenic and profibrinolytic activities.[35]
Regarding, other cardiac biomarkers in acute MI, cTnI released into the bloodstream as binary or tertiary complex earlier than other type of troponin, it gives an idea about myocardial ischemia while; second phase increase in 3 days reflects the infarction size.[36]
About 50% of patients with septic shock may develop acute heart failure lead to a rise in the level of cTnI this indicating that cardiac troponin may be released due to increasing oxygen demand as in anemia, hypoxia, stroke, and pulmonary embolism thus, myocardial necrosis regardless of its cause lead to elevations in serum troponin.[37]
Furthermore, cardiac troponin is an extremely sensitive but; not specific to the myocardial damage it also elevates to noncardiac conditions as in vigorous exercise which leads to stress-induced myocardial systolic dysfunction, but; it's extremely realistic to exclude non-ST elevation MI but; cannot differentiate between thrombotic and nonthrombotic acute coronary syndrome.[38]
Thus, coexistences elevation of both cardiac troponin and serum prolactin in acute MI give an idea about the underlying predisposing factor of occurrences of MI, and the elevated troponin revealed myocardial necrosis due to the coronary vessel complications that appeared in association with dyslipidemia which linked to high prolactin level.[39]
Therefore, a positive correlation between cardiac troponin and serum prolactin in this study elucidate that acute MI occurrence is due to long-term vascular complications.
CONCLUSION
Serum prolactin level increased in acute MI, and positively correlated with cardiac troponin level and reflects underlying cardiovascular complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to express thanks to the Research Committees and Professor Sadiq M. Alhamash, Dean of College of Medicine, Al-Mustansiriya University, Iraq, for his enormous maintaining in the march of scientific advancement and scientific reality in the Faculty of Medicine.
REFERENCES
- 1.Kokot I, Pawlik-Sobecka L, Placzkowska S, Piwowar A. Prolactin as an immunomodulatory factor in psoriatic arthritis. Postepy Hig Med Dosw (Online) 2013;67:1265–72. doi: 10.5604/17322693.1079893. [DOI] [PubMed] [Google Scholar]
- 2.Southgate TD, Windeatt S, Smith-Arica J, Gerdes CA, Perone MJ, Morris I, et al. Transcriptional targeting to anterior pituitary lactotrophic cells using recombinant adenovirus vectors in vitro and in vivo in normal and estrogen/sulpiride-induced hyperplastic anterior pituitaries. Endocrinology. 2000;141:3493–505. doi: 10.1210/endo.141.9.7639. [DOI] [PubMed] [Google Scholar]
- 3.Jin J, Hashizume T. Effects of hypothalamic dopamine on growth hormone-releasing hormone-induced growth hormone secretion and thyrotropin-releasing hormone-induced prolactin secretion in goats. Anim Sci J. 2015;86:634–40. doi: 10.1111/asj.12333. [DOI] [PubMed] [Google Scholar]
- 4.Bu G, Liang X, Li J, Wang Y. Extra-pituitary prolactin (PRL) and prolactin-like protein (PRL-L) in chickens and Zebrafish. Gen Comp Endocrinol. 2015;220:143–53. doi: 10.1016/j.ygcen.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Medic-Stojanoska M, Icin T, Pletikosic I, Bajkin I, Novakovic-Paro J, Stokic E, et al. Risk factors for accelerated atherosclerosis in young women with hyperprolactinemia. Med Hypotheses. 2015;84:321–6. doi: 10.1016/j.mehy.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 6.McCoy JM, Walkenhorst DE, McCauley KS, Elaasar H, Everett JR, Mix KS. Orphan nuclear receptor NR4A2 induces transcription of the immunomodulatory peptide hormone prolactin. J Inflamm (Lond) 2015;12:13. doi: 10.1186/s12950-015-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch LA. Progress for peripartum cardiomyopathy. Trends Cardiovasc Med. 2015;25:407–8. doi: 10.1016/j.tcm.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–21. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson DC, Doraiswamy PM. Prolactin-related and metabolic adverse effects of atypical antipsychotic agents. J Clin Psychiatry. 2008;69(Suppl 1):32–44. [PubMed] [Google Scholar]
- 10.Reuwer AQ, van Eijk M, Houttuijn-Bloemendaal FM, van der Loos CM, Claessen N, Teeling P, et al. The prolactin receptor is expressed in macrophages within human carotid atherosclerotic plaques: A role for prolactin in atherogenesis? J Endocrinol. 2011;208:107–17. doi: 10.1677/JOE-10-0076. [DOI] [PubMed] [Google Scholar]
- 11.Glintborg D, Altinok M, Mumm H, Buch K, Ravn P, Andersen M. Prolactin is associated with metabolic risk and cortisol in 1007 women with polycystic ovary syndrome. Hum Reprod. 2014;29:1773–9. doi: 10.1093/humrep/deu133. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Wong AO. Signal transduction mechanisms for autocrine/paracrine regulation of somatolactin – A secretion and synthesis in carp pituitary cells by somatolactin-a and-ß. Am J Physiol Endocrinol Metab. 2013;304:E176–86. doi: 10.1152/ajpendo.00455.2012. [DOI] [PubMed] [Google Scholar]
- 13.Jayasena CN, Comninos AN, Clarke H, Donaldson M, Meeran K, Dhillo WS. The effects of long-term growth hormone and insulin-like growth factor-1 exposure on the development of cardiovascular, cerebrovascular and metabolic co-morbidities in treated patients with acromegaly. Clin Endocrinol (Oxf) 2011;75:220–5. doi: 10.1111/j.1365-2265.2011.04019.x. [DOI] [PubMed] [Google Scholar]
- 14.Ganiats TG, Browner DK, Dittrich HC. Comparison of quality of well-being scale and NYHA functional status classification in patients with atrial fibrillation. NewYork Heart Association. Am Heart J. 1998;135(5 Pt 1):819–24. doi: 10.1016/s0002-8703(98)70040-7. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors – Results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55. doi: 10.1016/j.ijcard.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gözü H, Sezgin O, et al. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. Eur J Endocrinol. 2003;149:187–93. doi: 10.1530/eje.0.1490187. [DOI] [PubMed] [Google Scholar]
- 18.Kalra S, Dhamija P, Das AK. Metformin: Midlife maturity, maiden charm. Indian J Endocrinol Metab. 2012;16:1015–8. doi: 10.4103/2230-8210.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber U, Reitinger A, Szusz R, Hellmich C, Steinlechner B, Hager H, et al. Emergency ambulance transport induces stress in patients with acute coronary syndrome. Emerg Med J. 2009;26:524–8. doi: 10.1136/emj.2008.059212. [DOI] [PubMed] [Google Scholar]
- 20.Ugarte-Gil MF, Gamboa-Cárdenas RV, Zevallos F, Medina M, Cucho-Venegas JM, Perich-Campos RA, et al. High prolactin levels are independently associated with damage accrual in systemic lupus erythematosus patients. Lupus. 2014;23:969–74. doi: 10.1177/0961203314531083. [DOI] [PubMed] [Google Scholar]
- 21.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 22.Chopra S, Verghese PP, Jacob JJ. Bromocriptine as a new therapeutic agent for peripartum cardiomyopathy. Indian J Endocrinol Metab. 2012;16(Suppl 1):S60–2. doi: 10.4103/2230-8210.94261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta V, Patil S, Raval D, Gopani P. Pituitary apoplexy presenting as myocardial infarction. Indian J Endocrinol Metab. 2014;18:232–3. doi: 10.4103/2230-8210.129119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cincotta AH, Meier AH, Cincotta M., Jr Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: A new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8:1683–1707. doi: 10.1517/13543784.8.10.1683. [DOI] [PubMed] [Google Scholar]
- 25.Bakowska JC, Morrell JI. The distribution of mRNA for the short form of the prolactin receptor in the forebrain of the female rat. Brain Res Mol Brain Res. 2003;116:50–8. doi: 10.1016/s0169-328x(03)00213-4. [DOI] [PubMed] [Google Scholar]
- 26.Wallaschofski H, Lohmann T, Hild E, Kobsar A, Siegemund A, Spilcke-Liss E, et al. Enhanced platelet activation by prolactin in patients with ischemic stroke. Thromb Haemost. 2006;96:38–44. doi: 10.1160/TH05-09-0634. [DOI] [PubMed] [Google Scholar]
- 27.Stuijver DJ, Debeij J, van Zaane B, Dekkers OM, Smit JW, Büller HR, et al. Levels of prolactin in relation to coagulation factors and risk of venous thrombosis. Results of a large population-based case-control study (MEGA-study) Thromb Haemost. 2012;108:499–507. doi: 10.1160/TH12-04-0225. [DOI] [PubMed] [Google Scholar]
- 28.Wallaschofski H, Eigenthaler M, Kiefer M, Donné M, Hentschel B, Gertz HJ, et al. Hyperprolactinemia in patients on antipsychotic drugs causes ADP-stimulated platelet activation that might explain the increased risk for venous thromboembolism: Pilot study. J Clin Psychopharmacol. 2003;23:479–83. doi: 10.1097/01.jcp.0000088914.24613.51. [DOI] [PubMed] [Google Scholar]
- 29.Haring R, Friedrich N, Völzke H, Vasan RS, Felix SB, Dörr M, et al. Positive association of serum prolactin concentrations with all-ause and cardiovascular mortality. Eur Heart J. 2014;35:1215–21. doi: 10.1093/eurheartj/ehs233. [DOI] [PubMed] [Google Scholar]
- 30.Bihoreau C, Rasolonjanahary R, Gerozissis K, Clauser H, Kordon C. Arachidonate metabolism in the anterior pituitary: Effect of arachidonate inhibitors on basal and stimulated secretion of prolactin, growth hormone and luteinizing hormone. II. Hormone release from dispersed pituitary cells. J Neuroendocrinol. 1990;2:445–52. doi: 10.1111/j.1365-2826.1990.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 31.Velija-Ašimi Z. Evaluation of endocrine changes in women with the polycystic ovary syndrome during metformin treatment. Bosn J Basic Med Sci. 2013;13:180–5. doi: 10.17305/bjbms.2013.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Székács D, Bodnár I, Mravec B, Kvetnansky R, Vizi ES, Nagy GM, et al. The peripheral noradrenergic terminal as possible site of action of salsolinol as prolactoliberin. Neurochem Int. 2007;50:427–34. doi: 10.1016/j.neuint.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Haanwinckel MA, Antunes-Rodrigues J, De Castro e Silva E. Role of central beta-adrenoceptors on stress-induced prolactin release in rats. Horm Metab Res. 1991;23:318–20. doi: 10.1055/s-2007-1003686. [DOI] [PubMed] [Google Scholar]
- 34.Tzeng CR, Chang YC, Chang YC, Wang CW, Chen CH, Hsu MI. Cluster analysis of cardiovascular and metabolic risk factors in women of reproductive age. Fertil Steril. 2014;101:1404–10. doi: 10.1016/j.fertnstert.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Sugi T. Kininogen-dependent antiphosphatidylethanolamine antibodies and autoantibodies to factor XII in patients with recurrent pregnancy losses. J Obstet Gynaecol Res. 2013;39:1223–9. doi: 10.1111/jog.12110. [DOI] [PubMed] [Google Scholar]
- 36.Aydin S, Kuloglu T, Aydin S, Kalayci M, Yilmaz M, Çakmak T, et al. Elevated adropin: A candidate diagnostic marker for myocardial infarction in conjunction with troponin-I. Peptides. 2014;58:91–7. doi: 10.1016/j.peptides.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Markou N, Gregorakos L, Myrianthefs P. Increased blood troponin levels in ICU patients. Curr Opin Crit Care. 2011;17:454–63. doi: 10.1097/MCC.0b013e3283491f0d. [DOI] [PubMed] [Google Scholar]
- 38.Dumaine R, Gibson CM, Murphy SA, Southard M, Ly HQ, McCabe CH, et al. Association of a history of systemic hypertension with mortality, thrombotic, and bleeding complications following non-ST-segment elevation acute coronary syndrome. J Clin Hypertens (Greenwich) 2006;8:315–22. doi: 10.1111/j.1524-6175.2006.05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam S, Pathan F, Ahmed T. Clinical and biochemical characteristics of polycystic ovarian syndrome among women in Bangladesh. Mymensingh Med J. 2015;24:310–8. [PubMed] [Google Scholar]


