Abstract
Diabetes insipidus (DI) is a hereditary or acquired condition which disrupts normal life of persons with the condition; disruption is due to increased thirst and passing of large volumes of urine, even at night. A systematic search of literature for DI was carried out using the PubMed database for the purpose of this review. Central DI due to impaired secretion of arginine vasopressin (AVP) could result from traumatic brain injury, surgery, or tumors whereas nephrogenic DI due to failure of the kidney to respond to AVP is usually inherited. The earliest treatment was posterior pituitary extracts containing vasopressin and oxytocin. The synthetic analog of vasopressin, desmopressin has several benefits over vasopressin. Desmopressin was initially available as intranasal preparation, but now the oral tablet and melt formulations have gained significance, with benefits such as ease of administration and stability at room temperature. Other molecules used for treatment include chlorpropamide, carbamazepine, thiazide diuretics, indapamide, clofibrate, indomethacin, and amiloride. However, desmopressin remains the most widely used drug for the treatment of DI. This review covers the physiology of water balance, causes of DI and various treatment modalities available, with a special focus on desmopressin.
Keywords: Antidiuretic hormone, desmopressin, polydipsia, polyuria, vasopressin
INTRODUCTION
Diabetes insipidus (DI) is part of a group of hereditary or acquired polyuria and polydipsia diseases. It is associated with inadequate arginine vasopressin (AVP) or antidiuretic hormone (ADH) secretion or renal response to AVP, resulting in hypotonic polyuria and a compensatory/underlying polydipsia.[1] Polyuria (>50mL/kg), dilute urine (osmolality <300 mOsm/L), and increased thirst (water intake of up to 20 L/day) are characteristic of DI.[2] The kidneys pass large amounts of water irrespective of the body's hydration state.[3] Untreated DI can cause hypovolemia, dehydration, and electrolyte imbalances.[4] This article reviews the physiology, causes, and treatment of DI.
REVIEW METHODS
A search of literature on the causes, physiology, and treatment options for DI was carried out using the PubMed database. Only English language articles were included in this review.
PATHOPHYSIOLOGY OF WATER BALANCE
DI can be caused by two fundamentally different defects: Inadequate/impaired secretion of AVP from the posterior pituitary gland and impaired/insufficient renal response to ADH.[3] AVP, a neurohypophyseal nonapeptide, regulates body water and osmotic homeostasis.[5,6] The AVP-neurophysin II gene (AVP-NPII) encodes AVP, which is synthesized in the supraoptic and paraventricular nuclei of the hypothalamus as a precursor complex of AVP, NPII, and glycopeptides copeptin and is released as AVP from neurons in the posterior pituitary. AVP and its protein carrier, NPII, are released by calcium-dependent exocytosis.[6] AVP release may occur due to nonosmotic stimuli also including orthostatic hypotension or emetic reflexes (manifested in nausea and vomiting).[5]
The AVP system [Figure 1] maintains water balance based on serum osmolality and arterial blood volume via the vasopressin-2-receptor (V2R).[5,6,7] AVP activates V2R at the basolateral membrane of the principal cells in the distal convoluted tubule and collecting duct of the kidney. This activates protein kinase A which in turn phosphorylates aquaporin 2 (AQP2) water channels in the intracellular vesicles. Exocytic insertion of AQP2 vesicles into the cell membrane follows, and the collecting duct becomes water permeable, thus concentrating the urine.[6,7]
Figure 1.
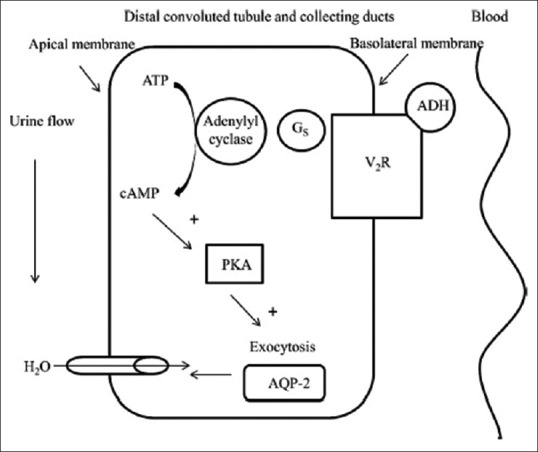
The arginine vasopressin system. cAMP: Cyclic adenosine monophosphate, V2R: Vasopressin-2-receptor, ADH: Antidiuretic hormone, AVP: Arginine vasopressin, Gs: G proteins, PKA: Protein kinase A
EPIDEMIOLOGY OF DIABETES INSIPIDUS
DI is a rare disease, with a prevalence of 1:25,000.[6] DI can present at any age, and the prevalence is equal among males and females. The age of presentation depends on the etiology.[8] Less than 10% of DI is hereditary. X-linked nephrogenic DI (NDI) accounts for 90% of cases of congenital NDI and occurs with a frequency of 4–8/1 million male live births. Autosomal NDI accounts for approximately 10% of the remaining cases.[6]
TYPES OF DIABETES INSIPIDUS
Central diabetes insipidus
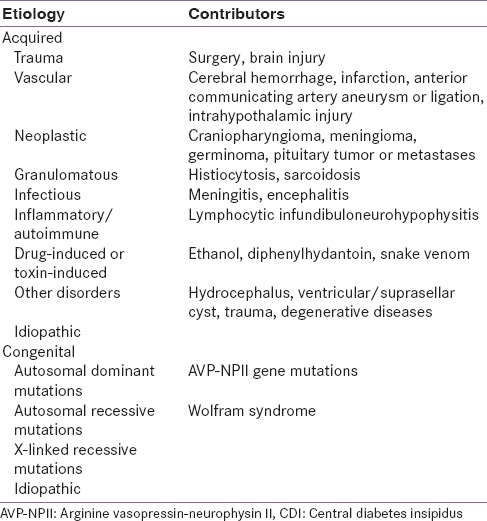
Central DI (CDI) or neurogenic DI is the most common form of DI, occurring in both the sexes equally and at any age. It is caused by inadequate synthesis/release of AVP, often secondary to surgery or head injury which causes traumatic injury to the hypothalamus or posterior pituitary gland[3,4] and destruction/degeneration of neurons originating in the supraoptic and paraventricular nuclei of the hypothalamus.[6] Symptoms of DI manifest after 80–90% of the magnocellular neurons in the hypothalamus are damaged.[1] Damage to the proximal part of the hypothalamo-neurohypophyseal region kills more neurons than do injuries to the distal region. However, proximal injuries account for 30–40% of posttraumatic and postoperative CDI, while distal injuries account for 50–60% of cases.[3] The etiology of CDI is summarized in Table 1. Acquired CDI is more common than congenital CDI, and about 25% of adult CDI cases are idiopathic.[1,6]
Table 1.
Etiology of CDI
Children with acute injury to the nervous system and CDI have a high mortality.[9] Traumatic brain injury (TBI) is associated with high mortality and acute and chronic morbidity. TBI is usually due to road traffic accidents and falls. The direct mechanical impact or the acceleration-deceleration effect of car crashes lead to dysfunction of the hypothalamic-pituitary axis. Trauma may lead to ischemia, hypoxia, alterations of cerebral vascularization/metabolism, and increased intracranial pressure, thus causing pituitary damage.[10] Among patients admitted to the surgical intensive care unit or level 1 trauma center for severe head injuries, CDI occurred in 12.5% of patients with blunt head injuries and 40.9% of patients with penetrating head injuries. Glasgow Coma Scale ≤8, cerebral edema, and an abbreviated injury score >3 were independently associated with development of CDI. However, CDI itself independently led to an almost 4-fold increase in the risk of death.[11]
Among Taiwanese children aged 3 months to 18 years, central nervous system (CNS) infection was a common cause of CDI than blunt head injury (35.2% vs. 18.5%), but the development of CDI within the first 2 days of acute CNS injury resulted in a 5-fold higher risk of mortality.[9] In a larger study, the incidence of CDI after TBI was 18%, and 71.4% of 818 pediatric patients were declared brain dead after CDI diagnosis. A higher mortality was associated with CDI which developed during the first 2 days after head injury.[12]
Pituitary surgery also results in CDI, with a wide range of incidence (1–67%).[13] Minimally invasive surgery has a low incidence of postoperative CDI (transient CDI 13.6% and permanent CDI 2.7%).[14] Abnormalities in the secretion of AVP usually begin during the intraoperative period.[6] The incidence of immediate postoperative DI was higher among patients undergoing surgery by a traditional method than by the endoscopic transsphenoidal method (36% vs. 15%). The incidence of long-term DI did not vary significantly between the groups.[15] CDI that developed postoperatively causes 22% of cases in Indian children, and these cases were diagnosed at a later age (6.9 ± 4.7 years) compared to patients with histiocytosis and CNS malformations (3.7 ± 1.5 years and 0.4 ± 0.1 years, respectively). Compared to those with histiocytosis as a cause, more patients with postoperative CDI had a short stature (92.3% vs. 9.1%).[16]
Tumors have been reported as a cause of CDI, with intracranial tumors accounting for 23% of CDI cases among children and adults in one study.[17] Tumor-associated CDI was not diagnosed in children aged <5 years.[18] Germ cell tumors and Langerhans cell histiocytosis (LCH) accounted for 60% of CDI cases in Taiwanese children, and histiocytosis accounted for 18.6% of cases in Indian children.[16,19] LCH as a cause of CDI has been associated with better survival.[17]
Vascular damage to the CNS has been linked to CDI though the pathophysiology is not completely understood. Magnetic resonance imaging (MRI) studies indicated that abnormal blood supply to the posterior pituitary is associated with what is often termed as “idiopathic” CDI.[1,20] Infections such as cryptococcal meningitis, encephalitis, tuberculosis, tuberculous meningitis, and neurosarcoidosis also contribute to acquired CDI cases.[21,22,23,24,25]
Congenital CDI is a small contributor in the etiology of CDI. Nonetheless, more than 60 gene mutations in the AVP-NPII gene have been identified, most of which are in the NPII gene.[26] In a study of familial CDI, mutations in exon 2 of AVP were detected in two Turkish families, but the age of onset differed (<1 year in one family [G45C mutation] and at 4–7 years in another family [C98X mutation]).[27]
Wolfram or DIDMOAD syndrome (autosomal recessive neurodegenerative disorder resulting in CDI, deafness, optic atrophy, and diabetes mellitus [DM]) is considered a differential diagnosis of CDI that developed in the neonatal period.[28] Mutations have been detected at nucleotide 997 in exon 8 (A997G) and an insertion in exon 8 of wolframin (WFS1) gene, which results in early-onset CDI in children.[29] Wolfram syndrome often results in DI and type 1 DM in children. However, an interesting first case of coexistence of CDI and type 2 DM has been reported in a 56-year-old man though no common cause could be determined.[30]
Nephrogenic diabetes insipidus
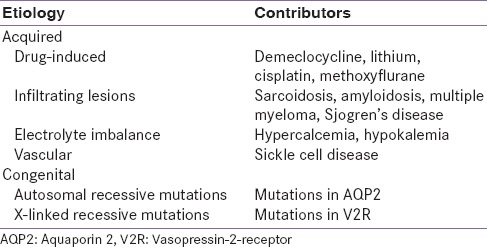
NDI results from the failure of the kidney to respond to AVP. Urine production in patients with NDI is typically 12 L/day. Children usually present with the inherited form whereas adults present with the acquired form of NDI [Table 2].[31] In most cases (90%), inherited NDI is an X-linked condition caused by a loss-of-function mutation in the V2R gene. It is rarely due to mutations in the AQP2 gene (10%).[7] V2R is predominantly expressed in the distal convoluted tubule and renal collecting tubes, and it responds to AVP thus concentrating the urine.[7]
Table 2.
Etiology of nephrogenic diabetes insipidus
In a nationwide survey of NDI patients in Japan, 143/173 patients (82.7%) had congenital NDI whereas the remaining (17.3%) had acquired NDI. Mutations in V2R were more common (74.7%) than mutations in AQP2 (9.2%).[32] In a study of two unrelated Thai children with NDI, the R181C mutation in V2R caused complete NDI whereas the M311V mutation caused mild NDI (V2R retained partial activity).[33]
Drug-induced NDI is most commonly due to lithium, followed by foscarnet and clozapine.[34] Lithium is used to treat mood disorders and is associated with renal injury including impaired urine-concentrating ability. Lithium enters the collecting duct principal cells predominantly via epithelial sodium channels (ENaC). Excess lithium reduces sodium reabsorption. Lithium inhibits AVP-stimulated translocation of cytoplasmic AQP2 to the apical membrane, thus delivering hypo-osmotic fluid. Long-term exposure to lithium may down-regulate AQP2 gene expression.[35,36] Lithium-induced NDI has been reported in adults and geriatric patients.[37,38,39]
Primary polydipsia
Primary polydipsia (PP) results from excessive fluid intake practiced over an extended period. Persons with PP have a defective thirst mechanism or increased the thirst sensation (dipsogenic DI). Patients with an unknown motive for polydipsia are frequently associated with psychiatric disorders (psychogenic polydipsia),[1] caused by schizophrenia, compulsive behavior, or stress reduction. PP also occurs in psychiatric patients to reduce the effect of anticholinergic drugs.[40] Phenothiazine administration causes the sensation of a dry mouth and may result in PP.[4] Excessive fluid consumption resulted in decreased serum osmolality and suppressed release of AVP. Patients exhibit characteristics of DI though pituitary and renal functions are not compromised.[1] Dipsogenic DI can be caused by chronic meningitis, granulomatous diseases, multiple sclerosis, or other diffuse pathology of the brain.[41] Cranial MRI is essential to distinguish PP from CDI.[1]
Gestational diabetes insipidus
Gestational DI occurs in about 1 in 30,000 pregnancies[42] as a result of degradation of AVP by the enzyme, cysteine aminopeptidase.[1] Vasopressinase levels are typically higher in pregnant women (up to 300 times higher), more so in twin pregnancies.[43] However, hormone levels are often normal, suggesting that pregnancy unmasks a subtle underlying deficiency of AVP.[1] Gestational DI typically presents in the third trimester[43] and spontaneously resolves about 2–3 weeks postpartum, but diagnosis to reveal any underlying pathology is necessary.[1] It is usually underdiagnosed since polyuria during pregnancy is considered normal and does not cause complications.[44]
Diagnosis of diabetes insipidus
Onset of CDI can be abrupt (due to insult to the body) or gradual (due to tumor or idiopathic causes). The age of onset and the severity of the disease can differ among patients who have congenital disease.[4,6]
The primary symptoms of DI include persistent polyuria (producing 8–16 L of dilute urine a day) and polydipsia (intake of up to 20 L fluid per day). Other symptoms are dizziness, weakness, nocturia, fatigue, and signs of dehydration (fever, dry skin and mucus membranes, weight loss, poor skin turgor). Hypotension and tachycardia with decreased right atrial and pulmonary artery occlusion pressures and an altered level of consciousness may occur. Young children may present with severe dehydration, vomiting, constipation, fever, irritability, sleep disturbances, retardation of growth, and failure to thrive. Mental retardation can be caused by repeated and unrecognized dehydration.[4,6]
The diagnostic algorithm of CDI and NDI is described in Figure 2. The ability of the CNS to produce AVP and of the kidney to respond to it is measured by the water deprivation test (Hare-Hickey test) and desmopressin challenge test. A 24-h urine volume is used to confirm polyuria. During water deprivation, hourly measurements of body weight and urine osmolality are made, until 2–3 samples vary by <30 mOsm/kg (or <10%), or until the patient loses 5% of his/her body weight. Serum ADH is then measured and ADH/desmopressin (5 units) is injected. Urine osmolality is measured 30–60 min after this.[3,6,41] The test is discontinued if the patient loses >5% of his/her body weight and/or plasma Na+ exceeds 143 mEq/L and/or urine osmolality increases to normal. Response to the administration of desmopressin distinguishes between CDI and NDI. The interpretation of the water deprivation test is described in Table 3.[6]
Figure 2.
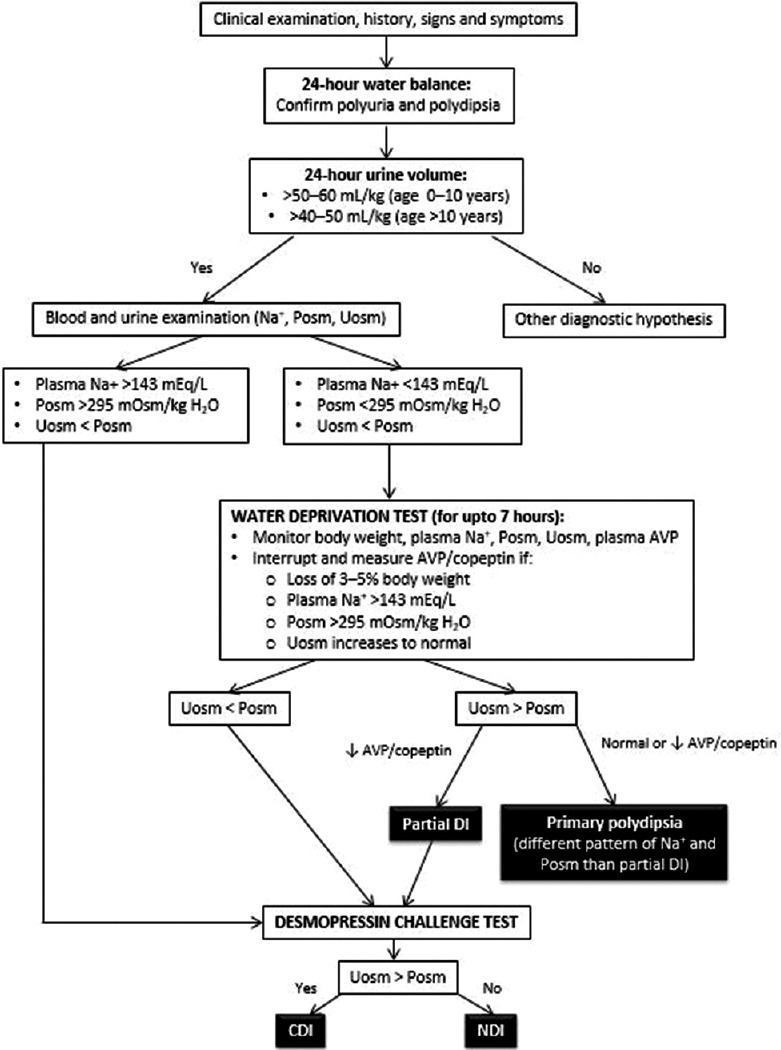
Diagnostic flowchart for central and nephrogenic diabetes insipidus. AVP: Arginine vasopressin, Posm: Plasma osmolality, Uosm: Urine osmolality
Table 3.
Interpretation of the water deprivation test and the desmopressin challenge test in the diagnosis of diabetes insipidus
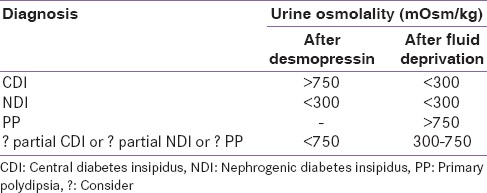
Findings suggestive of PP include the history of psychiatric disease, fluctuating symptoms and gradual onset of polydipsia, posterior pituitary bright spot and normal thickness of the pituitary stalk, and hyponatremia after a therapeutic trial with desmopressin.[1]
New diagnostic markers
Endogenous vasopressin measurement is an important test to differentiate between CDI and NDI. Copeptin (the C-terminal glycoprotein of the AVP prohormone) can be considered a stable surrogate for endogenous plasma AVP. AVP is unstable, largely attached to platelets and is rapidly cleared. The small size of AVP makes estimation difficult.[1,45] In one study, copeptin levels were found to be <2.5 pmol/L in all patients with CDI, suggesting that it could be used to distinguish CDI from PP and NDI.[46]
TREATMENT OF DIABETES INSIPIDUS
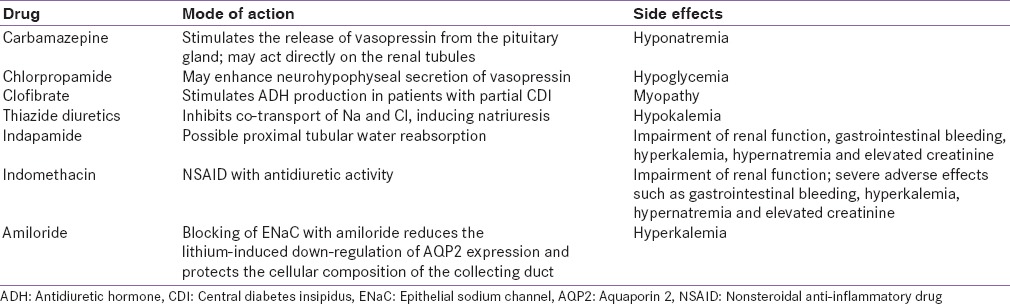
Treatment of DI varies for central or nephrogenic forms. The available medications are described in Table 4.
Table 4.
Summary of drugs used for the treatment of diabetes insipidus
Carbamazepine
Carbamazepine is an anticonvulsant and psychotropic drug used to treat epilepsy or intellectual disabilities. It may stimulate the release of vasopressin from the pituitary gland and act directly on renal tubules. Carbamazepine increased water absorption in the absence of AVP in vitro. The effect was cyclic adenosine monophosphate (cAMP)-dependent. In rats, carbamazepine increased water permeability and absorption in the inner medullary collecting duct, acted directly on the V2R-protein G complex and increased AQP2 expression.[47] Meinders et al., studied six CDI patients treated with 200–800 mg carbamazepine daily. All patients had a reduction in urinary water output (from 8–16 L/day to 1.9–6 L/day) and an increase in urine osmolality (from 60–120 mOsm/kg to 150–532 mOsm/kg). AVP levels were undetectable in DI patients and healthy subjects, suggesting that carbamazepine did not stimulate the release of, or inhibit the breakdown of AVP.[48]
Hyponatremia has been detected in 4.8–40% of patients receiving carbamazepine for neurological disorders[47,49,50] and among patients receiving it for back pain.[51] Prolonged carbamazepine treatment has been linked to reduction in antidiuretic activity possibly due to auto-induction of carbamazepine.[52]
Chlorpropamide
The antidiuretic drug, chlorpropamide, decreases the clearance of solute-free water if the neurohypophysis has the residual secretory capacity and normalizes plasma osmolality.[53] Chlorpropamide administered to children and adults (125–1000 mg daily) reduced urine output from 5.4–10.7 L/day to <2 L/day. Maximal diuresis was achieved in 3–4 days, and the effect was dose-dependent.[54]
Chlorpropamide may enhance the neurohypophyseal secretion of vasopressin, have a vasopressin-like effect or potentiate the effect of minute amounts of vasopressin which persist in DI.[54,55] Chlorpropamide may potentiate vasopressin-induced water transport through activation of renal tubular adenylyl cyclase which generates cAMP rather than affecting water movement.[55]
Clofibrate
Clofibrate is a hypolipidemic agent which stimulates ADH production in patients with partial CDI.[3,56] Clofibrate treatment (500 mg every 6 h) significantly reduced the mean urine clearance from 280 mL/h to 141 mL/h, and the mean free water clearance from 158 mL/h to 10 mL/h in 6 cases of DI. Urine osmolality increased from 152 mOsm/kg to 317 mOsm/kg, with a concomitant decrease in urinary ADH excretion in 4/6 patients. There was a significant antidiuretic activity even in patients who were water-loaded.[56]
Chlorpropamide treatment causes a greater reduction in mean urine volume (59% vs. 47%), and higher urine osmolality compared to clofibrate treatment. However, clofibrate significantly reduces free water clearance. Furthermore, chlorpropamide can cause serious hypoglycemia, unlike clofibrate.[57] Clofibrate is generally safe, but there have been reports of clofibrate-induced myopathy.[58] It is likely that hypothyroidism coexisting in patients with secondary DI may precipitate the development of clofibrate-induced myopathy.[58]
Thiazide diuretics
Thiazide diuretics can be used to treat both NDI and CDI.[3,59] They act at the distal convoluted tubule and inhibit cotransport of sodium and chloride. Prolonged administration reduces extracellular fluid volume, allowing water and sodium reabsorption at the proximal tubules. Ultimately, there is reduction of urine output.[59] An animal model of lithium-induced NDI demonstrated that chronic hydrochlorothiazide treatment up-regulates NCC and ENa C, which enhances sodium reabsorption along the distal segments of the nephron.[60]
Treatment with chlorothiazide (5–10 mg/kg/day) or hydrochlorothiazide (1–2 mg/kg/day) was effective and safe, with hospitalization for hypernatremia required in only 1/13 patients.[61] Treatment with chlorothiazide or hydrochlorothiazide reduced water intake by 23–40% in children with NDI.[62] The combination of amiloride and hydrochlorothiazide was superior to hydrochlorothiazide alone and prevented urinary potassium loss, hypokalemia, and alkalosis.[63] Though hydrochlorothiazide decreases urine volume by up to 50% in pregnant, the risks of hypokalemia and hypovolemia cause it to be used sparingly in gestational DI.[43]
Indapamide
Indapamide is an antihypertensive, diuretic drug which could be considered as an alternative drug for mild CDI.[8] The molecular structure is similar to hydrochlorothiazide and chlorpropamide. As with thiazides, the activity of indapamide may be due to proximal tubular water reabsorption.[64] The first report on the use of indapamide (at 2.5 mg/day) for DI demonstrated a reduction in the 24-h urinary volume (from 5–16 L to 2.3–9.2 L) in 3 patients with CDI.[65]
Among patients with treatment-naïve CDI, there was a smaller reduction in mean urine output volume (48.49% ± 10.69% vs. 40.56% ± 9.7%) in patients receiving indapamide compared to those receiving chlorpropamide. Urine osmolality increased in all nine patients receiving indapamide and in 7/11 patients receiving chlorpropamide, while serum osmolality decreased in 4/9 and 7/11 patients, respectively. Chlorpropamide was discontinued due to hypoglycemia in 2 patients while there were no adverse effects among patients receiving indapamide.[64]
Indomethacin
Indomethacin is a nonsteroidal anti-inflammatory drug which has antidiuretic action.[4] Weinstock and Moses reported that indomethacin effectively treated NDI that persisted after lithium therapy was discontinued. When used along with desmopressin, there was a marked decrease in polyuria.[66] A patient who did not respond to desmopressin, thiazides, and amiloride for the treatment of lithium-induced NDI, responded immediately to indomethacin treatment with a reduction in urine volume from 24 L/day to 12 L/day, and ultimately to 2 L/day.[67] Indomethacin successfully treated streptozocin-induced NDI, with a rapid correction in polyuria that was independent of the glomerular filtration rate.[68] Indomethacin may impair renal function[66] and have severe adverse effects such as gastrointestinal bleeding, hyperkalemia, hypernatremia, and elevated creatinine.[4]
Amiloride
Amiloride is a diuretic that is useful in the treatment of lithium-induced NDI. It reduces transcellular lithium transport, intracellular lithium concentration and lithium-induced inactivation of GSK-3-xβ. Blocking of ENaC with amiloride reduces the lithium-induced down-regulation of AQP2 expression and protects the cellular composition of the collecting duct.[69] In a study of the effect of amiloride versus placebo on lithium-induced NDI, amiloride administered for 6 weeks increased maximal urine osmolality and AQP2 excretion.[35]
Desmopressin
Vasopressin was first used to treat CDI in 1913, using posterior pituitary extracts containing oxytocin and vasopressin. Pitressin (vasopressin tannate in oil) became available for clinical use in the 1930s and was the principal drug for the treatment of DI almost until the 1970s when desmopressin was introduced. Substitution of the 8th residue of vasopressin with the D-isomer of arginine diminished the pressor effect of vasopressin.[70,71] This synthetic analog of vasopressin, 1-deamino-8-D-AVP(DDAVP), or desmopressin, has increased and prolonged antidiuretic effect, decreased vasoconstrictive and oxytocic effects, reduced side effects (hyponatremia and convulsions), and enhanced resistance to vasopressinase. It is convenient to administer, being available as an intranasal spray, oral tablet, and orally disntegrating tablet (ODT).[70,71,72]
Pharmacokinetics
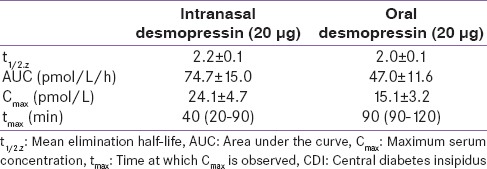
Desmopressin formulations include intranasal spray, oral tablet, and orally disintegrating tablet (ODT)/oral lyophilisate/melt. Due to safety issues, desmopressin intranasal spray is no longer approved by the United States Food and Drug Administration. This led to withdrawal of the spray and switch to the tablet or melt formulation in a majority of countries; however, the spray continues to be available in India.[73] The pharmacokinetic properties or oral DDAVP among adult patients with CDI uncontrolled on intranasal DDAVP are described in Table 5.[74]
Table 5.
Pharmacokinetic properties of the oral and intranasal forms of desmopressin in adults with CDI
The antidiuretic activity of the various formulations is described in Figure 3. The mean urine osmolality at 4 h was only marginally higher for the intranasal compared to the oral formulation. There was no significant difference in the mean urine flow rates for up to 8 h. Increasing the dose of desmopressin from 200 to 400 mg did not significantly increase the maximal antidiuretic activity.[74] Among children with CDI, free water clearance decreased rapidly with both intranasal (10–20 μg) and oral (200–400 μg) formulations. Doses of the oral formulation were approximately 20 times greater than those for the nasal formulation.[75]
Figure 3.
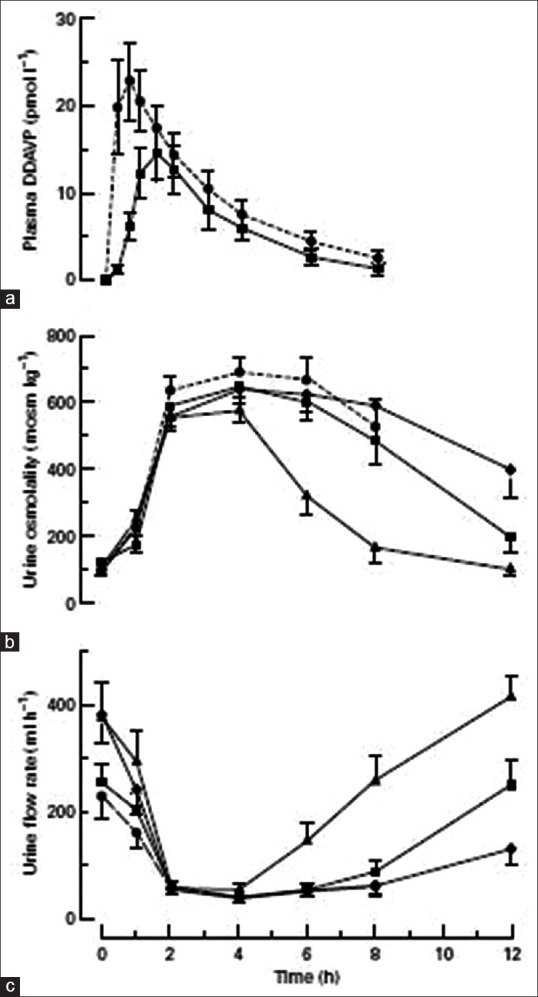
(a) Plasma 1-deamino-8-D-arginine-vasopressin concentrations (n = 9), (b) urine osmolality, and (c) urine flow rate at varying intervals after the administration of intranasal or oral desmopressin in 10 adult patients with central diabetes insipidus
Large individual variations in the absorption of orally administered desmopressin have been reported in studies with adults[74] and children.[75] In healthy, overhydrated adults who were administered intravenous desmopressin (30–500 ng), the increase in plasma desmopressin and urine osmolality was proportional to the dose. Cmax occurred at or within 15 min of the end of a 2-h infusion though the individual Cmax varied over a 2-fold or 3-fold range. Urine osmolality increases were 350 mOsm/kg in the 60 ng group and 700 mOsm/kg in the 500 ng group. The individual variation in time required for a given level of plasma desmopressin to exert its maximum antidiuretic effect could be due to individual differences in the kinetics of one or more intracellular mechanisms that promote the reabsorption of solute-free water by principal cells in renal collecting tubules.[76]
The gender difference in the activity of desmopressin has been reported in a study of ODT desmopressin for nocturia and among healthy volunteers. At lower doses (25 μg), the decrease in nocturia was larger for women than men. Among patients aged >50 years, women had 2-fold greater decreases in serum sodium levels than men, at doses of 25–50 μg, but a 5-fold greater risk of hyponatremia at 50 μg. Overall, sensitivity to the antidiuretic effect of desmopressin with females was higher than males.[77]
Oral tablet formulation of desmopressin
Oral tablets have advantages over the intranasal formulation as they can be used easily in infants, elderly patients and patients with visual disturbances and chronic rhinitis. Desmopressin is absorbed unaltered from the gastrointestinal tract after oral administration.[78] Fukuda et al., studied the use of oral desmopressin (100 μg) in patients with CDI (age 17–36 years) switching from the intranasal formulation. Plasma concentrations of desmopressin peaked (14.7 ± 5.4 pg/mL) at 90 min, and then decreased gradually in the subsequent 3 h. Urine volumes decreased 1 h after administration, with diuretic activity remaining low from 3 h to 6 h after administration. Urine osmolality increased progressively from 1 h after administration, reaching a maximum at 4 h (732 ± 21 mOsm/kg).[78] Promising results for the oral formulation emerged from a similar study involving a switch from intranasal to oral formulation. Both formulations controlled urine volume (1958 ± 758 vs. 1720 ± 378 mL, respectively) and corrected urine specific gravity to normal (1101 ± 6 vs. 1101 ± 4, respectively). While the spray had a more rapid onset of action and a longer duration of action, the tablet was more available and much more easily consumed according to patients (P = 0.00067). The dose equivalence ratio for intranasal to 100 μg oral desmopressin was 1:18.[79]
The long-term use of oral desmopressin has been evaluated. The mean ratio of oral to intranasal doses required to maintain antidiuretic activity was 19 ± 2 (range 15–30). Daily urine volumes were well-maintained (mean volume 1739 ± 68 mL/day, range 1500–1930 mL/day) and urine osmolality was 275–899 mOsm/kg. Importantly, there was no adverse reaction or loss of desmopressin activity over the study period. The oral formulation was as effective as the intranasal formulation in controlling CDI over a 5-year period and was a safe treatment modality.[78]
A study on oral desmopressin in children with CDI determined that doses of 100–400 μg achieved a mean antidiuretic activity of 8 h. The antidiuretic activity was dose-dependent. The effect on urine osmolality is described in Figure 4. Most patients were discharged from hospital with an initial dose of 100–400 μg thrice daily. The activity of desmopressin correlated with body weight (r = 0.61, P < 0.05). There were no adverse reactions to any dosage of desmopressin and no significant changes in body weight, blood pressure, or heart rate. During a follow-up period of 6 months, the mean daily oral dose was 550 μg while the mean intranasal dose was 25 μg. Higher initial intranasal doses corresponded to higher oral dose requirements (r = 0.79, P < 0.5). Oral desmopressin was considered safe and efficacious in the management of CDI in children in the long-term.[80]
Figure 4.
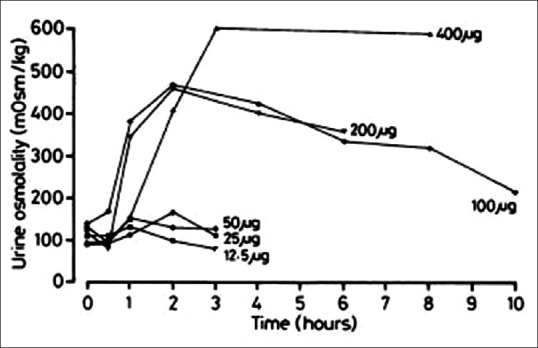
Effect of single rising doses of oral desmopressin on urine osmolality in the seven pediatric patients in the study
A case of gestational DI was successfully treated with desmopressin (vasopressinase resistant) but not AVP (vasopressinase sensitive). Subcutaneous administration resulted in a prompt decrease in oral fluid intake (85 mL/h) and urine output (66 mL/h) and an increase in urine osmolality (656 mOsm/L). Subsequent treatment with intravenous and intranasal forms also controlled the symptoms, and desmopressin was weaned gradually from 6 weeks postpartum while her condition resolved.[81] A large study in Sweden found that the use of desmopressin during pregnancy was not a risk to infants since the duration of pregnancy and infant birth weight were not affected by the treatment.[82]
New formulation of desmopressin: Orally disintegrating tablet
The ODT formulation of desmopressin was launched in 2005 for the treatment of nocturnal enuresis. It is a wafer-like formulation which dissolves instantly in the mouth with no need for water,[70] thus avoiding the ingestion of extra fluids and overcoming difficulties related to swallowing tablets.[83] It is advantageous over the intranasal formulation which requires cold storage for long-term stability. Absorption of intranasal desmopressin may be erratic due to changes in nasal mucosa due to atrophy, scarring, congestion, or discharge.[84]
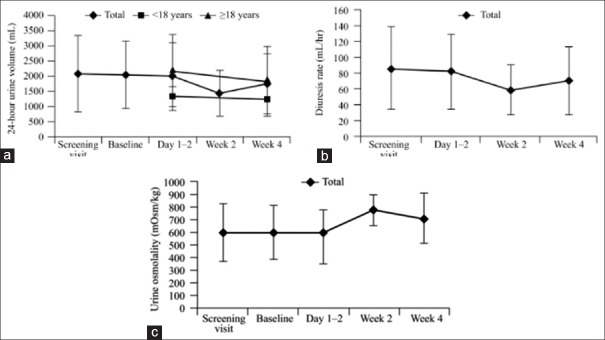
The efficacy of switching to from the intranasal to the ODT formulation was demonstrated in a recent multicenter, open-label, dose-titration study in CDI patients. After 4 weeks of ODT treatment, there was no significant difference between the 24-h urine volume, hourly diuresis rate [Figure 5], urine specific gravity, and urine osmolality compared to the baseline values. More patients had urine osmolality and specific gravity within the normal range [Figure 5c]. Patients received desmopressin ODT 120–360 μg/day in either two or three doses. Treatment-emergent adverse effects were seen in 11 patients, and hyponatremia was the most common adverse effect. The ratio of ODT to intranasal desmopressin dose was 24. Desmopressin ODT achieved sufficient antidiuretic control compared to intranasal therapy and was well-tolerated over long-term treatment. Individual titration of dosing is important as 6 patients in the study required dose modification due to altered urine volume or sodium levels.[83]
Figure 5.
Changes in (a) 24-h urine volume and (b) hourly diuresis rate and (c) urine osmolality over time
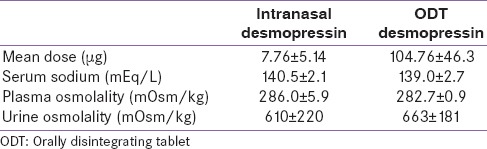
The ODT formulation was safe and effective in adults with CDI switching from intranasal spray/drops to 60 μg ODT desmopressin. The mean daily dosage of the formulations and the laboratory characteristics are described in Table 6. The ratio of nasal to ODT desmopressin dose was 17.0 ± 7.6 in all patients, 11.7 ± 6.5 in the nasal spray group, and 21.0 ± 5.5 in the nasal drop group. The difference between the ratio for the drop and spray groups was statistically significant. Prior dosage of intranasal desmopressin significantly correlated with the required ODT dose. No events of hyponatremia or water toxicity were reported.[84]
Table 6.
Characteristics of the two formulations of desmopressin
The potential of desmopressin ODT in very young infants with CDI was recently described. A preterm male infant was administered 60 μg desmopressin ODT, resulting in an increase in urine osmolality within 3 h. The bioavailability of desmopressin was maintained over time, but several dose adjustments were required due to variability in urine output and body weight. After 125 days, 60 μg desmopressin ODT was administered daily, together with a normal fluid intake, resulting in stable random sodium concentrations. In a full-term female infant, 15 μg of desmopressin ODT increased the urine osmolality to >400 mOsm/kg within 3 h of administration and the effect was maintained for 12 h. By the age of 12 months, DI was managed with 45 μg/day desmopressin ODT.[70]
Quality of life (QOL) has been assessed using the Peds QL and the Adult Hypopituitarism Questionnaire, but neither is specific for CDI. A new tool, the Nagasaki DI Questionnaire, included 10 questions directly related to CDI symptoms and two questions about the consistency of the effect of and the patient's satisfaction with desmopressin has recently been tested. Patients receiving intranasal spray were either switched to desmopressin ODT or continued the nasal spray. Newly diagnosed patients were treated with desmopressin ODT. The frequency of hyponatremia was lower during ODT than intranasal treatment (13.3% vs. 21.1%), but clinical significance was lacking. Changing from intranasal to ODT desmopressin improved the overall QOL, except the frequency of urination in the daytime. Among patients with newly diagnosed CDI, there were improvements in QOL for each question, without statistical difference.[85]
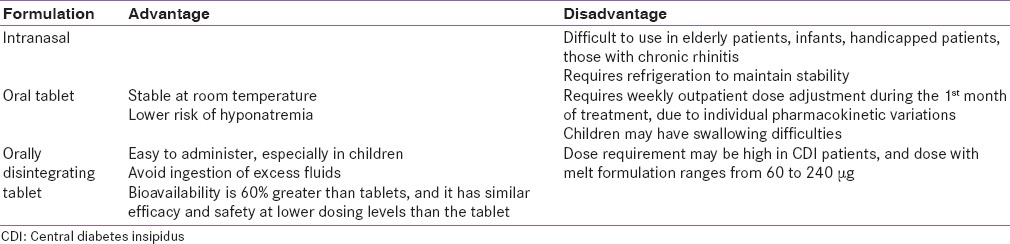
Pharmacovigilance data show that hyponatremia is very rare (<1 in 10,000). The lower risk of hyponatremia for the oral formulations makes them preferable over nasal sprays.[86] The pros and cons of the different formulations of desmopressin are listed in Table 7.
Table 7.
Advantages and disadvantages of various formulations of desmopressin
GUIDELINES FOR THE MANAGEMENT OF DIABETES INSIPIDUS
The book, Medicine Update Volume 23, published by the Association of Physicians of India in 2013, had a theme of “Practical Indian Guidelines and Protocols for all the Common Diseases.” It described an algorithm for diagnosis of polyuria [Figure 6].[41] The Indian Society for Paediatric and Adolescent Endocrinology has enlisted instructions for patients on desmopressin treatment. These include:[87]
Figure 6.
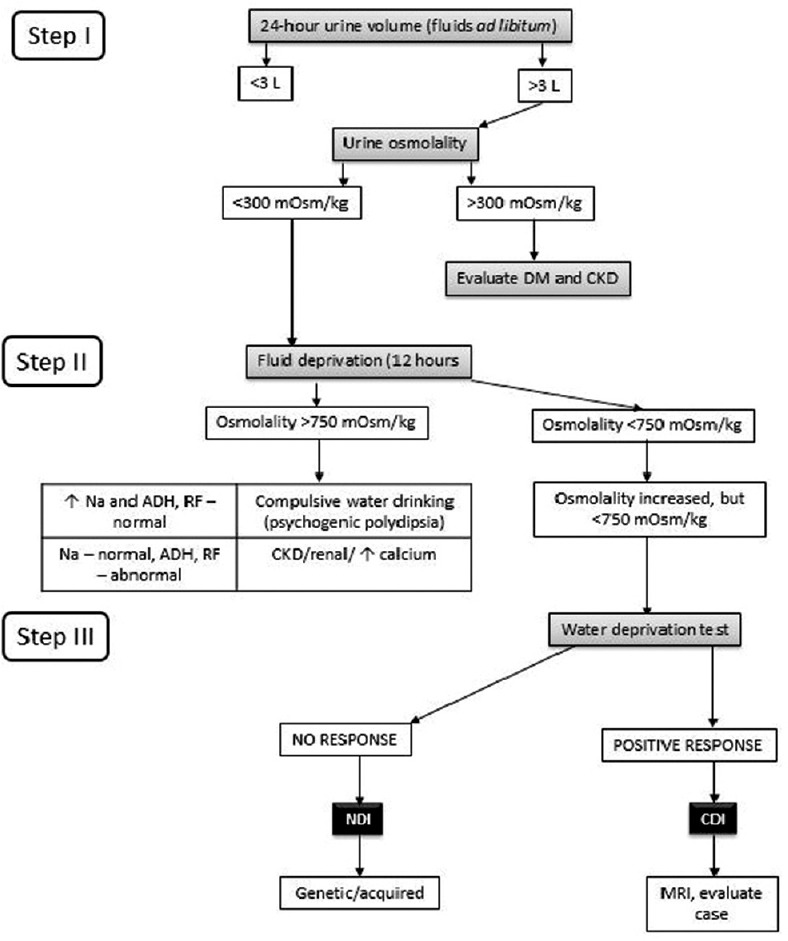
Diagnostic flowchart for polyuria. DM: Diabetes mellitus, CKD: Chronic kidney disease, ADH: Antidiuretic hormone, RF: Renal function, NDI: Nephrogenic diabetes insipidus, CDI: Central diabetes insipidus, MRI: Magnetic resonance imaging
Never change dosage of medicine without the advice of doctor
Give next dose of desmopressin only when the effect of the previous day's dose has finished, and the patient begins passing urine frequently for at least 2 h
On days of cold and stuffy nose, the effectiveness of the desmopressin is low, so the patient will pass larger amount of urine. Give oral fluids according to thirst. Do not try to double the dose of desmopressin
On days of fever, loose motions, and vomiting, desmopressin should be continued. Extraoral fluids should be given according to thirst
If the patient becomes unconscious, consult a local doctor immediately.
SUMMARY
DI is a disorder that can be debilitating. It is important to identify the etiology of DI to be able to treat is appropriately. Since its introduction in 1972, desmopressin has been demonstrated as a safe and effective drug for the management of DI in several short-term and long-term studies. Newer preparations including oral and ODT formulations are advantageous over nasal preparations as they are useful in patients who are elderly, mentally, or physically handicapped or have chronic rhinitis. Desmopressin continues to be a valuable drug after more than 35 years since its introduction and provides an effective therapeutic option for DI.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Harshad Malve is working as a Lead Medical, Asia Pacific region with Ferring Pharmaceuticals Pvt. Ltd.
REFERENCES
- 1.Fenske W, Allolio B. Clinical review: Current state and future perspectives in the diagnosis of diabetes insipidus: A clinical review. J Clin Endocrinol Metab. 2012;97:3426–37. doi: 10.1210/jc.2012-1981. [DOI] [PubMed] [Google Scholar]
- 2.Grace M, Balachandran V, Preethy, Menon S. Idiopathic central diabetes insipidus. Indian J Med Sci. 2011;65:452–5. [PubMed] [Google Scholar]
- 3.Makaryus AN, McFarlane SI. Diabetes insipidus: Diagnosis and treatment of a complex disease. Cleve Clin J Med. 2006;73:65–71. doi: 10.3949/ccjm.73.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Crawford A, Harris H. Water world, part 2: Understanding diabetes insipidus in adults. Nurs Crit Care. 2012;7:12–6. [Google Scholar]
- 5.Juul KV, Bichet DG, Nielsen S, Nørgaard JP. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol. 2014;306:F931–40. doi: 10.1152/ajprenal.00604.2013. [DOI] [PubMed] [Google Scholar]
- 6.Di Iorgi N, Napoli F, Allegri AE, Olivieri I, Bertelli E, Gallizia A, et al. Diabetes insipidus – Diagnosis and management. Horm Res Paediatr. 2012;77:69–84. doi: 10.1159/000336333. [DOI] [PubMed] [Google Scholar]
- 7.García Castaño A, Pérez de Nanclares G, Madariaga L, Aguirre M, Chocron S, Madrid A, et al. Novel mutations associated with nephrogenic diabetes insipidus. A clinical-genetic study. Eur J Pediatr. 2015;174:1373–85. doi: 10.1007/s00431-015-2534-4. [DOI] [PubMed] [Google Scholar]
- 8.Saifan C, Nasr R, Mehta S, Sharma Acharya P, Perrera I, Faddoul G, et al. Diabetes insipidus: A challenging diagnosis with new drug therapies. ISRN Nephrol 2013. 2013:797620. doi: 10.5402/2013/797620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang YH, Lin JJ, Hsia SH, Wu CT, Wang HS, Hung PC, et al. Central diabetes insipidus in children with acute brain insult. Pediatr Neurol. 2011;45:377–80. doi: 10.1016/j.pediatrneurol.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Capatina C, Paluzzi A, Mitchell R, Karavitaki N. Diabetes insipidus after traumatic brain injury. J Clin Med. 2015;4:1448–62. doi: 10.3390/jcm4071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadjizacharia P, Beale EO, Inaba K, Chan LS, Demetriades D. Acute diabetes insipidus in severe head injury: A prospective study. J Am Coll Surg. 2008;207:477–84. doi: 10.1016/j.jamcollsurg.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Alharfi IM, Stewart TC, Foster J, Morrison GC, Fraser DD. Central diabetes insipidus in pediatric severe traumatic brain injury. Pediatr Crit Care Med. 2013;14:203–9. doi: 10.1097/PCC.0b013e31827127b5. [DOI] [PubMed] [Google Scholar]
- 13.Schreckinger M, Szerlip N, Mittal S. Diabetes insipidus following resection of pituitary tumors. Clin Neurol Neurosurg. 2013;115:121–6. doi: 10.1016/j.clineuro.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Sigounas DG, Sharpless JL, Cheng DM, Johnson TG, Senior BA, Ewend MG. Predictors and incidence of central diabetes insipidus after endoscopic pituitary surgery. Neurosurgery. 2008;62:71–8. doi: 10.1227/01.NEU.0000311063.10745.D8. [DOI] [PubMed] [Google Scholar]
- 15.Shah S, Har-El G. Diabetes insipidus after pituitary surgery: Incidence after traditional versus endoscopic transsphenoidal approaches. Am J Rhinol. 2001;15:377–9. [PubMed] [Google Scholar]
- 16.Bajpai A, Kabra M, Menon PS. Central diabetes insipidus: Clinical profile and factors indicating organic etiology in children. Indian Pediatr. 2008;45:463–8. [PubMed] [Google Scholar]
- 17.Varan A, Atas E, Aydin B, Yalçin B, Akyüz C, Kutluk T, et al. Evaluation of patients with intracranial tumors and central diabetes insipidus. Pediatr Hematol Oncol. 2013;30:668–73. doi: 10.3109/08880018.2013.816984. [DOI] [PubMed] [Google Scholar]
- 18.Maghnie M, Cosi G, Genovese E, Manca-Bitti ML, Cohen A, Zecca S, et al. Central diabetes insipidus in children and young adults. N Engl J Med. 2000;343:998–1007. doi: 10.1056/NEJM200010053431403. [DOI] [PubMed] [Google Scholar]
- 19.Liu SY, Tung YC, Lee CT, Liu HM, Peng SF, Wu MZ, et al. Clinical characteristics of central diabetes insipidus in Taiwanese children. J Formos Med Assoc. 2013;112:616–20. doi: 10.1016/j.jfma.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Maghnie M, Altobelli A, Di Iorgi N, Genovese E, Meloni G, Manca-Bitti ML, et al. Idiopathic central diabetes insipidus is associated with abnormal blood supply to the posterior pituitary gland caused by vascular impairment of the inferior hypophyseal artery system. J Clin Endocrinol Metab. 2004;89:1891–6. doi: 10.1210/jc.2003-031608. [DOI] [PubMed] [Google Scholar]
- 21.Woredekal Y. Recurrent central diabetes insipidus secondary to cryptococcal meningitis. J Assoc Acad Minor Phys. 1998;9:22–4. [PubMed] [Google Scholar]
- 22.Kawamoto S, Hatanaka K, Imakita M, Tamaki T. Central diabetes insipidus in an HHV6 encephalitis patient with a posterior pituitary lesion that developed after tandem cord blood transplantation. Intern Med. 2013;52:1107–10. doi: 10.2169/internalmedicine.52.9432. [DOI] [PubMed] [Google Scholar]
- 23.Domiciano DS, de Carvalho JF, Macedo AR, Laurindo IM. Central diabetes insipidus induced by tuberculosis in a rheumatoid arthritis patient. Acta Reumatol Port. 2010;35:232–5. [PubMed] [Google Scholar]
- 24.Mishra OP, Singh SK, Das BK, Agrawal JK. Partial central diabetes insipidus following tuberculous meningitis. J Assoc Physicians India. 1993;41:757. [PubMed] [Google Scholar]
- 25.Non L, Brito D, Anastasopoulou C. Neurosarcoidosis-associated central diabetes insipidus masked by adrenal insufficiency. BMJ Case Rep 2015. 2015:pii, Bcr2014206390. doi: 10.1136/bcr-2014-206390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Fost M, van Trotsenburg AS, van Santen HM, Endert E, van den Elzen C, Kamsteeg EJ, et al. Familial neurohypophyseal diabetes insipidus due to a novel mutation in the arginine vasopressin-neurophysin II gene. Eur J Endocrinol. 2011;165:161–5. doi: 10.1530/EJE-11-0048. [DOI] [PubMed] [Google Scholar]
- 27.Turkkahraman D, Saglar E, Karaduman T, Mergen H. AVP-NPII gene mutations and clinical characteristics of the patients with autosomal dominant familial central diabetes insipidus. Pituitary. 2015;18:898–904. doi: 10.1007/s11102-015-0668-z. [DOI] [PubMed] [Google Scholar]
- 28.Ghirardello S, Dusi E, Castiglione B, Fumagalli M, Mosca F. Congenital central diabetes insipidus and optic atrophy in a Wolfram newborn: Is there a role for WFS1 gene in neurodevelopment? Ital J Pediatr. 2014;40:76. doi: 10.1186/s13052-014-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrotta S, Di Iorgi N, Ragione FD, Scianguetta S, Borriello A, Allegri AE, et al. Early-onset central diabetes insipidus is associated with de novo arginine vasopressin-neurophysin II or Wolfram syndrome 1 gene mutations. Eur J Endocrinol. 2015;172:461–72. doi: 10.1530/EJE-14-0942. [DOI] [PubMed] [Google Scholar]
- 30.Shin HJ, Kim JH, Yi JH, Han SW, Kim HJ. Polyuria with the concurrent manifestation of Central Diabetes Insipidus (CDI) and type 2 Diabetes Mellitus (DM) Electrolyte Blood Press. 2012;10:26–30. doi: 10.5049/EBP.2012.10.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bockenhauer D, Bichet DG. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol. 2015;11:576–88. doi: 10.1038/nrneph.2015.89. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto M, Okada S, Kawashima Y, Nishimura R, Miyahara N, Kawaba Y, et al. Clinical overview of nephrogenic diabetes insipidus based on a nationwide survey in Japan. Yonago Acta Med. 2014;57:85–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Sahakitrungruang T, Tee MK, Rattanachartnarong N, Shotelersuk V, Suphapeetiporn K, Miller WL. Functional characterization of vasopressin receptor 2 mutations causing partial and complete congenital nephrogenic diabetes insipidus in Thai families. Horm Res Paediatr. 2010;73:349–54. doi: 10.1159/000308167. [DOI] [PubMed] [Google Scholar]
- 34.Bendz H, Aurell M. Drug-induced diabetes insipidus: Incidence, prevention and management. Drug Saf. 1999;21:449–56. doi: 10.2165/00002018-199921060-00002. [DOI] [PubMed] [Google Scholar]
- 35.Bedford JJ, Weggery S, Ellis G, McDonald FJ, Joyce PR, Leader JP, et al. Lithium-induced nephrogenic diabetes insipidus: Renal effects of amiloride. Clin J Am Soc Nephrol. 2008;3:1324–31. doi: 10.2215/CJN.01640408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behl T, Kotwani A, Kaur I, Goel H. Mechanisms of prolonged lithium therapy-induced nephrogenic diabetes insipidus. Eur J Pharmacol. 2015;755:27–33. doi: 10.1016/j.ejphar.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 37.Imam SK, Hasan A, Shahid SK. Lithium-induced nephrogenic diabetes insipidus. J Pak Med Assoc. 2005;55:125–7. [PubMed] [Google Scholar]
- 38.Eustatia-Rutten CF, Tamsma JT, Meinders AE. Lithium-induced nephrogenic diabetes insipidus. Neth J Med. 2001;58:137–42. doi: 10.1016/s0300-2977(00)00104-2. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay D, Gokulkrishnan L, Mohanaruban K. Lithium-induced nephrogenic diabetes insipidus in older people. Age Ageing. 2001;30:347–50. doi: 10.1093/ageing/30.4.347. [DOI] [PubMed] [Google Scholar]
- 40.Kohli A, Verma S, Jr, Sharma A., Jr Psychogenic polydipsia. Indian J Psychiatry. 2011;53:166–7. doi: 10.4103/0019-5545.82554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarma KV. Algorithmic approach for the diagnosis of polyuria. In: Muruganathan A, editor. Medicine Update. Vol. 23. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2013. pp. 311–3. [Google Scholar]
- 42.Weinberg LE, Dinsmoor MJ, Silver RK. Severe hydramnios and preterm delivery in association with transient maternal diabetes insipidus. Obstet Gynecol. 2010;116(Suppl 2):547–9. doi: 10.1097/AOG.0b013e3181e6c683. [DOI] [PubMed] [Google Scholar]
- 43.Ananthakrishnan S. Diabetes insipidus in pregnancy: Etiology, evaluation, and management. Endocr Pract. 2009;15:377–82. doi: 10.4158/EP09090.RA. [DOI] [PubMed] [Google Scholar]
- 44.Aleksandrov N, Audibert F, Bedard MJ, Mahone M, Goffinet F, Kadoch IJ. Gestational diabetes insipidus: A review of an underdiagnosed condition. J Obstet Gynaecol Can. 2010;32:225–31. doi: 10.1016/s1701-2163(16)34448-6. [DOI] [PubMed] [Google Scholar]
- 45.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–9. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 46.de Fost M, Oussaada SM, Endert E, Linthorst GE, Serlie MJ, Soeters MR, et al. The water deprivation test and a potential role for the arginine vasopressin precursor copeptin to differentiate diabetes insipidus from primary polydipsia. Endocr Connect. 2015;4:86–91. doi: 10.1530/EC-14-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Bragança AC, Moyses ZP, Magaldi AJ. Carbamazepine can induce kidney water absorption by increasing aquaporin 2 expression. Nephrol Dial Transplant. 2010;25:3840–5. doi: 10.1093/ndt/gfq317. [DOI] [PubMed] [Google Scholar]
- 48.Meinders AE, Cejka V, Robertson GL. The antidiuretic action of carbamazepine in man. Clin Sci Mol Med. 1974;47:289–99. doi: 10.1042/cs0470289. [DOI] [PubMed] [Google Scholar]
- 49.Henry DA, Lawson DH, Reavey P, Renfrew S. Hyponatraemia during carbamazepine treatment. Br Med J. 1977;1:83–4. doi: 10.1136/bmj.1.6053.83-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Amelsvoort T, Bakshi R, Devaux CB, Schwabe S. Hyponatremia associated with carbamazepine and oxcarbazepine therapy: A review. Epilepsia. 1994;35:181–8. doi: 10.1111/j.1528-1157.1994.tb02930.x. [DOI] [PubMed] [Google Scholar]
- 51.Kamiyama T, Iseki K, Kawazoe N, Takishita S, Fukiyama K. Carbamazepine-induced hyponatremia in a patient with partial central diabetes insipidus. Nephron. 1993;64:142–5. doi: 10.1159/000187295. [DOI] [PubMed] [Google Scholar]
- 52.Radó JP. Decreased antidiuretic response in diabetes insipidus during prolonged treatment with carbamazepine presumably due to enzym-induction. Endokrinologie. 1976;67:322–30. [PubMed] [Google Scholar]
- 53.Hocken AG, Longson D. Reduction of free water clearance by chlorporpamide. Br Med J. 1968;1:355–6. doi: 10.1136/bmj.1.5588.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cushard WG, Jr, Beauchamp CJ, Martin ND. Oral therapy of diabetes insipidus with chlorpropamide. Calif Med. 1971;115:1–5. [PMC free article] [PubMed] [Google Scholar]
- 55.Wales JK. Mode of action of chlorpropamide in the treatment of diabetes insipidus. Proc R Soc Med. 1971;64:1070–1. doi: 10.1177/003591577106401031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moses AM, Howanitz J, van Gemert M, Miller M. Clofibrate-induced antidiuresis. J Clin Invest. 1973;52:535–42. doi: 10.1172/JCI107213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson P, Jr, Earll JM, Schaaf M. Comparison of clofibrate and chlorpropamide in vasopressin-responsive diabetes insipidus. Metabolism. 1977;26:749–62. doi: 10.1016/0026-0495(77)90062-2. [DOI] [PubMed] [Google Scholar]
- 58.Matsukura S, Matsumoto J, Chihara K, Yoshimoto Y, Fujita T. Clofibrate-induced myopathy in patients with diabetes insipidus. Endocrinol Jpn. 1980;27:401–3. doi: 10.1507/endocrj1954.27.401. [DOI] [PubMed] [Google Scholar]
- 59.Abraham MB, Rao S, Price G, Choong CS. Efficacy of hydrochlorothiazide and low renal solute feed in neonatal central diabetes insipidus with transition to oral desmopressin in early infancy. Int J Pediatr Endocrinol 2014. 2014:11. doi: 10.1186/1687-9856-2014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim GH, Lee JW, Oh YK, Chang HR, Joo KW, Na KY, et al. Antidiuretic effect of hydrochlorothiazide in lithium-induced nephrogenic diabetes insipidus is associated with upregulation of aquaporin-2, Na-Cl co-transporter, and epithelial sodium channel. J Am Soc Nephrol. 2004;15:2836–43. doi: 10.1097/01.ASN.0000143476.93376.04. [DOI] [PubMed] [Google Scholar]
- 61.Al Nofal A, Lteif A. Thiazide diuretics in the management of young children with central diabetes insipidus. J Pediatr. 2015;167:658–61. doi: 10.1016/j.jpeds.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 62.O’doherty NJ, Rosser J, Slater RJ. Diabetes insipidus: The influence of chlorothiazide therapy in affected children. Can Med Assoc J. 1962;86:559–67. [PMC free article] [PubMed] [Google Scholar]
- 63.Alon U, Chan JC. Hydrochlorothiazide-amiloride in the treatment of congenital nephrogenic diabetes insipidus. Am J Nephrol. 1985;5:9–13. doi: 10.1159/000166896. [DOI] [PubMed] [Google Scholar]
- 64.Tetiker T, Sert M, Koçak M. Efficacy of indapamide in central diabetes insipidus. Arch Intern Med. 1999;159:2085–7. doi: 10.1001/archinte.159.17.2085. [DOI] [PubMed] [Google Scholar]
- 65.Koçak M, Karademir BM, Tetiker T. Antidiuretic effect of indapamide in central diabetes insipidus. Acta Endocrinol (Copenh) 1990;123:657–60. doi: 10.1530/acta.0.1230657. [DOI] [PubMed] [Google Scholar]
- 66.Weinstock RS, Moses AM. Desmopressin and indomethacin therapy for nephrogenic diabetes insipidus in patients receiving lithium carbonate. South Med J. 1990;83:1475–7. doi: 10.1097/00007611-199012000-00026. [DOI] [PubMed] [Google Scholar]
- 67.Lam SS, Kjellstrand C. Emergency treatment of lithium-induced diabetes insipidus with nonsteroidal anti-inflammatory drugs. Ren Fail. 1997;19:183–8. doi: 10.3109/08860229709026274. [DOI] [PubMed] [Google Scholar]
- 68.Delaney V, de Pertuz Y, Nixon D, Bourke E. Indomethacin in streptozocin-induced nephrogenic diabetes insipidus. Am J Kidney Dis. 1987;9:79–83. doi: 10.1016/s0272-6386(87)80166-x. [DOI] [PubMed] [Google Scholar]
- 69.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, et al. Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int. 2009;76:44–53. doi: 10.1038/ki.2009.91. [DOI] [PubMed] [Google Scholar]
- 70.De Waele K, Cools M, De Guchtenaere A, Van de Walle J, Raes A, Van Aken S, et al. Desmopressin lyophilisate for the treatment of central diabetes insipidus: First experience in very young infants. Int J Endocrinol Metab. 2014;12:e16120. doi: 10.5812/ijem.16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qureshi S, Galiveeti S, Bichet DG, Roth J. Diabetes insipidus: Celebrating a century of vasopressin therapy. Endocrinology. 2014;155:4605–21. doi: 10.1210/en.2014-1385. [DOI] [PubMed] [Google Scholar]
- 72.Kauli R, Laron Z. A vasopressin analogue in treatment of diabetes insipidus. Arch Dis Child. 1974;49:482–5. doi: 10.1136/adc.49.6.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Guchtenaere A, Van Herzeele C, Raes A, Dehoorne J, Hoebeke P, Van Laecke E, et al. Oral lyophylizate formulation of desmopressin: Superior pharmacodynamics compared to tablet due to low food interaction. J Urol. 2011;185:2308–13. doi: 10.1016/j.juro.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 74.Lam KS, Wat MS, Choi KL, Ip TP, Pang RW, Kumana CR. Pharmacokinetics, pharmacodynamics, long-term efficacy and safety of oral 1-deamino-8-D-arginine vasopressin in adult patients with central diabetes insipidus. Br J Clin Pharmacol. 1996;42:379–85. doi: 10.1046/j.1365-2125.1996.39914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fjellestad-Paulsen A, Tubiana-Rufi N, Harris A, Czernichow P. Central diabetes insipidus in children. Antidiuretic effect and pharmacokinetics of intranasal and peroral 1-deamino-8-D-arginine vasopressin. Acta Endocrinol (Copenh) 1987;115:307–12. [PubMed] [Google Scholar]
- 76.Juul KV, Erichsen L, Robertson GL. Temporal delays and individual variation in antidiuretic response to desmopressin. Am J Physiol Renal Physiol. 2013;304:F268–78. doi: 10.1152/ajprenal.00502.2012. [DOI] [PubMed] [Google Scholar]
- 77.Juul KV, Klein BM, Sandström R, Erichsen L, Nørgaard JP. Gender difference in antidiuretic response to desmopressin. Am J Physiol Renal Physiol. 2011;300:F1116–22. doi: 10.1152/ajprenal.00741.2010. [DOI] [PubMed] [Google Scholar]
- 78.Fukuda I, Hizuka N, Takano K. Oral DDAVP is a good alternative therapy for patients with central diabetes insipidus: Experience of five-year treatment. Endocr J. 2003;50:437–43. doi: 10.1507/endocrj.50.437. [DOI] [PubMed] [Google Scholar]
- 79.Larijani B, Tabatabaei O, Soltani A, Taheri E, Pajouhi M, Bastanhagh MH, et al. Comparison of desmopressin (DDAVP) tablet and intranasal spray in the treatment of central diabetes insipidus. RJMS. 2004;11:289–97. [Google Scholar]
- 80.Westgren U, Wittström C, Harris AS. Oral desmopressin in central diabetes insipidus. Arch Dis Child. 1986;61:247–50. doi: 10.1136/adc.61.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallia A, Bizhanova A, Huang W, Goldsmith SL, Gossett DR, Kopp P. Acute diabetes insipidus mediated by vasopressinase after placental abruption. J Clin Endocrinol Metab. 2013;98:881–6. doi: 10.1210/jc.2012-3548. [DOI] [PubMed] [Google Scholar]
- 82.Källén BA, Carlsson SS, Bengtsson BK. Diabetes insipidus and use of desmopressin (Minirin) during pregnancy. Eur J Endocrinol. 1995;132:144–6. doi: 10.1530/eje.0.1320144. [DOI] [PubMed] [Google Scholar]
- 83.Arima H, Oiso Y, Juul KV, Nørgaard JP. Efficacy and safety of desmopressin orally disintegrating tablet in patients with central diabetes insipidus: Results of a multicenter open-label dose-titration study. Endocr J. 2013;60:1085–94. doi: 10.1507/endocrj.ej13-0165. [DOI] [PubMed] [Google Scholar]
- 84.Murakami T, Hatoko T, Nambu T, Matsuda Y, Matsuo K, Yonemitsu S, et al. Desmopressin orally disintegrating tablet in Japanese patients with central diabetes insipidus: A retrospective study of switching from intranasal desmopressin. Endocr J. 2014;61:773–9. doi: 10.1507/endocrj.ej14-0097. [DOI] [PubMed] [Google Scholar]
- 85.Nozaki A, Ando T, Akazawa S, Satoh T, Sagara I, Horie I, et al. Quality of life in the patients with central diabetes insipidus assessed by Nagasaki Diabetes Insipidus Questionnaire. Endocrine. 2015. [Last accessed on 2015 Oct 16]. Epub ahead of print. doi:101007/s12020-015-0637-3. Available from: http://linkspringercom/article/101007%2Fs12020-015-0637-3 . [DOI] [PubMed]
- 86.Vande Walle J, Stockner M, Raes A, Nørgaard JP. Desmopressin 30 years in clinical use: A safety review. Curr Drug Saf. 2007;2:232–8. doi: 10.2174/157488607781668891. [DOI] [PubMed] [Google Scholar]
- 87.Instructions for Patient on Desmopressin Treatment for Diabetes Insipidus. Chennai (TN): He Indian Society for Pediatric and Adolescent Endocrinology. c2015. [Last accessed on 2015 Jul 27; Last cited on 2015 Jul 27]. Available from: http://www.ispae.org.in/html_pages/diabetes_insipidus.php .