Abstract
Objective:
The current recommendation from the UK National Health Service Breast Screening Programme is that digital breast tomosynthesis (DBT) can be used for further assessment of possible screen-detected soft-tissue abnormalities in place of spot compression views and when used should be performed in two projections. The aim of the study was to assess whether two-view DBT is necessary if the abnormality is seen only in one view on initial full-field digital mammography (FFDM).
Methods:
617 cases with possible masses, distortions and asymmetrical densities visualized only in one view on screening FFDM were included. All of these females underwent two-view DBT, clinical examination and ultrasound. The FFDM and DBT findings on each view were compared and correlated with the histological diagnosis.
Results:
586 of 617 cases had normal or benign findings on further assessment, and no additional information was obtained on the other DBT view. There were 31 confirmed cancers. In 26 cases (84%), the cancer was seen on the corresponding DBT view. No cancer was seen on the other DBT view alone. Five cancers (16%) were not seen on either view on DBT owing to technical reasons. No cancers would have been missed if only the corresponding DBT view was performed.
Conclusion:
Two-view DBT may not be necessary when used for further assessment of possible screen-detected soft-tissue abnormalities. Larger studies should be undertaken to investigate this further.
Advances in knowledge:
One-view DBT may be adequate in assessing soft-tissue abnormalities seen only on one FFDM view.
INTRODUCTION
Breast screening is an effective method of early cancer detection with a proven reduction in disease-specific mortality.1 The UK National Health Service Breast Screening Programme (NHSBSP) was started in 1988, and the programme has evolved significantly since its introduction. Technological advances have led to the replacement of film-screen mammography with full-field digital mammography (FFDM) in recent years. Despite the use of modern technology, the overall diagnostic accuracy of FFDM is similar to that of film-screen mammography.2 This is due to the fact that a major limitation of conventional mammography remains, namely the issue of overlapping tissue mimicking or obscuring lesions. This leads both to unnecessary recalls for further assessment with associated psychological stress and to cancers being missed.
Nevertheless, FFDM has been shown to be more accurate in females under the age of 50 years, in females with radiographically dense breasts and in pre-menopausal and perimenopausal females.2 This should prove advantageous in routine screening of females less than 50 years in the NHSBSP and in screening of younger females at increased risk of breast cancer. The advancement in digital technology has also allowed the integration of newer image acquisition techniques such as digital breast tomosynthesis (DBT) into the NHSBSP. DBT uses multiple low-dose X-ray exposures as the X-ray tube moves in an arc. A series of tomographic images are then reconstructed at 1-mm intervals, resulting in a pseudo three-dimensional stack of images which can be scrolled through. This helps to overcome the problem of breast tissue overlap and obscuration of underlying lesions.
There have been a number of large population-based screening studies comparing the use of DBT in addition to FFDM. These have demonstrated increased cancer detection rates of 9.5–40%.3–5 These studies have also demonstrated the added advantage of a significant reduction in the number of false positives, with recall rates reduced by 15–30%.3–5 Moreover, one of the studies5 has shown that the reduction in recall rates occurs across all breast densities and patient ages. This is of particular benefit to the younger females and females with dense breasts. Results from the largest US-based study by Friedewald et al6 have shown similar figures. They showed an increased invasive cancer detection of 41% but no change in the detection rate of ductal carcinoma in situ. There was a relative decrease in recall rate of 15%.
Michell et al7 have studied the use of DBT in the screening assessment clinic and have shown that the addition of DBT increases the accuracy of film-screen mammography and FFDM in the interpretation of soft-tissue abnormalities in females recalled following routine screening. Until recently, the workup of soft-tissue abnormalities on FFDM in screening patients was spot compression views in addition to ultrasound and clinical examination.8 The focal compression is intended to spread out overlying glandular tissue and improve the conspicuity of any underlying lesion. Other studies9,10 have confirmed that DBT is at least as accurate as spot compression view in the assessment of soft-tissue abnormalities. A study by Zuley et al11 has shown that DBT significantly improved diagnostic accuracy and allows better lesion characterization for non-calcified lesions in comparison with supplemental mammographic views.
As a result, DBT has been approved for use in the NHSBSP for the assessment of soft-tissue abnormalities. It has been recommended that DBT is used in conjunction with ultrasound and that two-view DBT should be performed.12 At the time of publication, the expert group that reviewed the evidence for the use of DBT in assessment concluded that DBT has no role to play in the assessment of microcalcification.12 The aim of the present study was to evaluate whether two-view DBT is necessary if an abnormality is seen only in one view on the initial FFDM in screening females who are recalled for further assessment.
METHODS AND MATERIALS
The TOMMY trial (a comparison of TOMosynthesis with digital MammographY in the UK Breast Screening Programme) is a multicentre study involving six different NHSBSP centres in the UK.13 The purpose of the trial was to compare the diagnostic accuracy of DBT in conjunction with two-dimensional (2D) mammography against standard 2D mammography alone and to determine if DBT improves the accuracy of detection of different types of lesions. Females aged 47–73 years who were recalled for further assessment following routine breast screening and females aged 40–49 years with a family history of breast cancer attending annual mammographic screening were invited to enter the trial. The trial was carried out following ethical approval, and written consent was obtained from the females.
As part of the TOMMY trial protocol, all participants underwent two-view 2D FFDM and two-view DBT of both breasts at assessment. The images were taken using the Hologic® Selenia® Dimensions® system (Hologic®, Bedford, MA), which acquires 15 evenly spaced images over an angular range of 15°. One reader reviewed the FFDM and DBT images in the screening assessment clinic, and the females were assessed according to the NHSBSP guidelines, including clinical examination and ultrasound. The data were collected prospectively, and the location, size and type of any perceived abnormality were recorded. Lesion conspicuity and degree of suspicion were also recorded. The features were classified as asymmetry, architectural distortion, mass and microcalcification. An independent, blinded, retrospective reading study was subsequently performed as part of the TOMMY trial but not included in this study. The data were collected and entered into a Microsoft® Excel® (Microsoft, Redmond, WA) spreadsheet. The present study analysed the data collected as part of the TOMMY trial, and no new data were collected.
The Nightingale Centre (University Hospital South Manchester NHS Trust, Manchester, UK) recruited 2079 females to the TOMMY trial from December 2011 to February 2013. Females with an abnormality visible on only one initial screening mammographic view were identified from the TOMMY database and those with microcalcification as the main imaging feature were excluded from the study. 617 cases that had an abnormality only in the mediolateral oblique (MLO) or the craniocaudal (CC) view on FFDM were identified and included in our study for analysis. The type of abnormality detected was categorized as circumscribed mass, spiculated mass, architectural distortion and asymmetrical density. The degree of suspicion on each view was also scored on a 5-point scoring system according to the Royal College of Radiologists Breast group scoring system.14 The imaging findings were correlated with the histology results in females who had undergone biopsy.
RESULTS
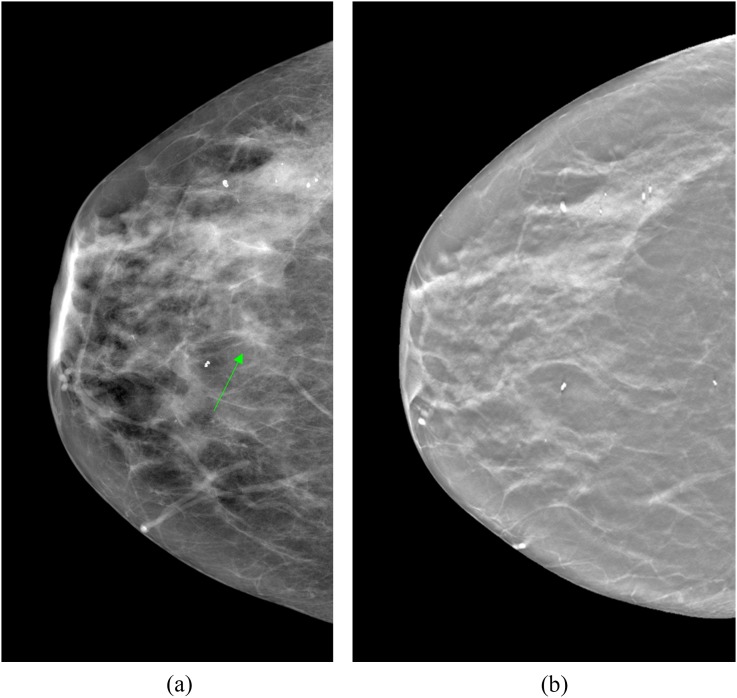
Of the 617 cases with an abnormality on only one view on FFDM, this was seen on the MLO view in 202 cases and on the CC view in 415 cases. Following triple assessment according to the NHSBSP recommendations with DBT, clinical examination and ultrasound, 586 out of the 617 cases had normal or benign findings and were returned to routine screening. The benign findings were confirmed by histological diagnosis. All cases were reviewed by a second consultant radiologist at the time of assessment as required by local policy. Figure 1 is an example of a normal case; a focal asymmetry was detected on a right FFDM CC view (Figure 1a). Subsequent DBT CC imaging was normal (Figure 1b). The DBT MLO imaging and ultrasound were also normal.
Figure 1.
Focal asymmetry on the craniocaudal (CC) view full-field digital mammogram of the right breast at the nipple level (arrow), which is normal on digital breast tomosynthesis (DBT). (a) Right CC view full-field digital mammogram (b) a slice from the corresponding CC DBT imaging.
31 abnormalities were found to be malignant, as confirmed on histology. Of these, 11 were seen only on the MLO view and 20 were seen only on the CC view of the screening FFDM (Tables 1 and 2). In 26 cancers (84%), the abnormality was seen on the corresponding DBT view. 13 cancers (42%) were seen on both DBT MLO and CC views. No cancer was seen on the other DBT view alone. Five cancers (16%) were visible on the CC view on FFDM but were not detected on either view on DBT. These cancers (one architectural distortion and four asymmetric densities) were located posteriorly and not included on either DBT view or the FFDM obtained at the screening assessment clinic. These cases had ultrasound confirming a suspicious lesion and subsequent image-guided biopsy.
Table 1.
Radiological features of the benign and confirmed cancers seen on digital breast tomosynthesis (DBT) mediolateral oblique (MLO) view only when performed in the same projection as the abnormal full-field digital mammography (FFDM) view
| Type of abnormality on FFDM MLO alone | Number of cancers | Number of benign cases | Benign cases—underwent FNA or core biopsy |
||
|---|---|---|---|---|---|
| Total | Seen on both DBT views | Not seen on either DBT view | |||
| Circumscribed mass | 1 | 32 | 12 | 2 | 2 |
| Spiculated mass | 4 | 1 | 1 | 0 | 0 |
| Distortion | 0 | 24 | 0 | – | – |
| Asymmetrical density | 6 | 134 | 5 | 0 | 2 |
| Total | 11 | 191 | 18 | 2 | 4 |
FNA, fine needle aspiration.
Table 2.
Radiological features of the benign and confirmed cancers seen on digital breast tomosynthesis (DBT) craniocaudal (CC) view only when performed in the same projection as the abnormal full-field digital mammography (FFDM) view
| Type of abnormality on FFDM CC alone | Number of cancers | Number of benign cases | Benign cases—underwent FNA or core biopsy |
||
|---|---|---|---|---|---|
| Total | Seen on both DBT views | Not seen on either DBT view | |||
| Circumscribed mass | 2 | 23 | 3 | 1 | 0 |
| Spiculated mass | 2 | 1 | 0 | – | – |
| Distortion | 9 | 73 | 4 | 2 | 2 |
| Asymmetrical density | 7 | 298 | 18 | 4 | 5 |
| Total | 20 | 395 | 25 | 7 | 7 |
FNA, fine needle aspiration.
As expected, six out of the eight (75%) cases classified as spiculated masses were malignant. The other features have lower rates of malignancy: 3/58 (5.2%) circumscribed masses, 9/106 (8.5%) architectural distortions and 13/445 (2.9%) asymmetrical densities were malignant. In some cases, the abnormality descriptor was changed following DBT (presumably because of improved margin assessment); however, the degree of suspicion was the same or increased on DBT. Tables 3 and 4 summarize the degree of suspicion with changes in the type of abnormality if appropriate. The histology and grade are also included. There was 1 case of tubular cancer, 3 cases of lobular cancer and 27 cases of ductal cancer, all being invasive.
Table 3.
Radiological features, degree of suspicion and histological diagnosis of the cancers detected on full-field digital mammography (FFDM) mediolateral oblique (MLO) alone (y = yes, n = no)
| Type of abnormality on FFDM MLO alone | Case No. | Number of lesions | Histology/grade | Degree of suspicion on FFDM | Degree of suspicion on DBT | Seen on DBT MLO | Seen on DBT CC |
|---|---|---|---|---|---|---|---|
| Circumscribed mass | 1 | 2 (seen on FFDM) | Invasive lobular/2 | 5 | 5 | y | n |
| Spiculated mass | 1 | 1 | Invasive ductal/1 | 3 | 5 | y | n |
| 2 | 1 | Invasive ductal/3 | 5 | 5 | y | n | |
| 3 | 2 | Invasive ductal/2 | 5 | 5 | y | n | |
| 4 | 2 (same patient as case 3) | Invasive ductal/2 | 5 | 5 | y | n | |
| Asymmetrical density | 1 | 1 | Invasive lobular/2 | 3 | 5 | y | n |
| 2 | 1 | Invasive ductal/1 | 2 | 4 (switched to distortion on DBT) | y | n | |
| 3 | 1 | Invasive ductal/1 | 3 | 4 (switched to spic mass on DBT) | y | n | |
| 4 | 2 (seen on FFDM) | Invasive ductal/3 | 3 | 5 (switched to spic mass on DBT) | y | n | |
| 5 | 1 | Invasive ductal/2 | 5 | 5 (switched to spic mass on DBT) | y | y | |
| 6 | 1 | Invasive ductal/3 | 3 | 3 | y | n |
CC, craniocaudal; DBT, digital breast tomosynthesis.
Degree of suspicion scores: 1 = normal, 2 = benign, 3 = probably benign, 4 = suspicious, 5 = malignant.
Table 4.
Radiological features, degree of suspicion and histological diagnosis of the cancers detected on full-field digital mammography (FFDM) craniocaudal (CC) alone (y = yes, n = no)
| Type of abnormality on FFDM CC alone | Case No. | Number of lesions | Histology/grade | Degree of suspicion on FFDM | Degree of suspicion on DBT | Seen on DBT CC | Seen on DBT MLO |
|---|---|---|---|---|---|---|---|
| Circumscribed mass | 1 | 1 | Invasive ductal/2 | 5 | 5 (switched to spic mass on DBT) | y | y |
| 2 | 1 | Invasive ductal/1 | 3 | 3 | y | y | |
| Spiculated mass | 1 | 2 (seen on FFDM) | Invasive ductal/1 | 4 | 5 | y | y |
| 2 | 1 | Invasive ductal/2 | 5 | 5 | y | y | |
| Distortion | 1 | 2 (other lesion is ductal in situ in different location) | Invasive ductal/2 | 5 | 5 | y | y |
| 2 | 1 | Invasive ductal/1 | 4 | 4 | y | n | |
| 3 | 1 | Invasive ductal/1 | 3 | 5 (switched to spic mass on DBT) | y | y | |
| 4 | 2 (other lesion seen only on both DBT views) | Invasive lobular/2 | 3 | Not seen | n | n | |
| 5 | 1 | Invasive ductal/2 | 3 | 4 | y | y | |
| 6 | 1 | Invasive ductal/2 | 3 | 5 | y | y | |
| 7 | 1 | Tubular/1 | 4 | 5 | y | y | |
| 8 | 1 | Invasive ductal/1 | 4 | 5 (switched to spic mass on DBT) | y | y | |
| 9 | 1 | Invasive ductal/2 | 4 | 5 (switched to spic mass on DBT) | y | y | |
| Asymmetrical density | 1 | 2 (seen on FFDM) | Invasive ductal/2 | 4 | 3 | y | n |
| 2 | 1 | Invasive ductal/2 | 3 | 5 (switched to spic mass on DBT) | y | y | |
| 3 | 2 (other lesion seen only on both DBT views) | Invasive ductal/2 | 2 | Not seen | n | n | |
| 4 | 2 (seen on FFDM) | Invasive ductal/1 | 2 | Not seen | n | n | |
| 5 | 2 (other lesion seen only on both DBT views) | Invasive ductal/2 | 3 | Not seen | n | n | |
| 6 | 2 (seen on FFDM) | Invasive ductal/2 | 3 | Not seen | n | n | |
| 7 | 3 (seen on FFDM) | Invasive ductal/3 | 3 | 3 | y | n |
DBT, digital breast tomosynthesis; MLO, mediolateral oblique.
Degree of suspicion scores: 1 = normal, 2 = benign, 3 = probably benign, 4 = suspicious and 5 = malignant.
There were 12 patients with multifocal disease. In seven cases, the second lesions were detected on both FFDM views and DBT views. All of these patients had the same histological cancer type and grade. One patient with distortion confirmed to be cancer had microcalcification which was ductal carcinoma in situ on histological diagnosis. One patient had two lesions, both seen on the same single-view FFDM and corresponding DBT view. In three cases, the second lesion was only detected on DBT and as a result had ultrasound and image-guided biopsy confirmation.
The number of benign cases that had a biopsy and the findings on DBT views are summarized in Tables 1 and 2. As of June 2015, no interval cancer has been reported among the benign cases. No patient required diagnostic surgery to confirm benignity. In the 191 benign cases seen on FFDM MLO, 8 cases were scored as suspicious or malignant. Of these, seven persisted as suspicious/malignant on DBT MLO but were all normal on DBT CC. The other case was normal on both the DBT views.
In the 395 benign cases on FFDM CC, 7 cases were scored as suspicious or malignant. Only one case persisted as high suspicion and was seen on both DBT MLO and CC but proved benign. The other six cases were normal on both the DBT views.
DISCUSSION
In the UK, females who are recalled for further assessment for possible soft-tissue abnormalities on screening mammograms traditionally undergo further mammographic views in the form of repeat full-field views or spot compression views together with clinical examination and ultrasound.8 At present, DBT in two views is advised when used as an assessment tool in place of spot compression views.12 Our study has demonstrated that the second view on DBT did not add any additional information in our group of females, and in particular, no cancers were detected on the second view alone. The use of only one DBT view (in the same projection as the original abnormality was seen) would lead to a decrease in the radiation dose to the females and reductions in the image acquisition and interpretation times.
The addition of two-view DBT to FFDM in large population-based screening studies has produced excellent results with increased cancer detection rates and reduction in recall rates.3–5 DBT has also been found to be superior to FFDM in measuring tumour size15,16 and is at least comparable with supplementary views in the assessment of non-calcified lesions.9–11 The tumour outline can be seen in more cases on DBT as compared with FFDM, which makes local staging of tumours significantly more accurate with DBT.15 The combination of DBT and FFDM has also been found to increase cancer detection and reduce recall rates in females with dense breasts.17 In our study, we illustrated the increased sensitivity of DBT, as there were three cases in which the second lesions were detected only with DBT, on both CC and MLO views. As the second lesions were seen on both views, no cancer would be missed if a single-view DBT was performed.
However, despite the obvious advantages of DBT, there are downsides that have to be taken into consideration. One of the concerns about DBT is the increased radiation dose to the breasts in comparison with FFDM. The dose of a single-view DBT is 2.19 mGy for 50- to 60-mm thick breasts, which is slightly higher than the corresponding one-view FFDM of 1.88 mGy.18 Therefore, the combination of DBT and FFDM will at least double the radiation dose when used as a primary screening tool as compared with FFDM alone. Although the DBT dose is higher, it is still below the current national diagnostic reference level of 3.5 mGy for one-view mammography.19 The higher dose could be justified given the superior performance of DBT over FFDM. Nevertheless, the dose should ideally be kept lower if at all possible.20
A number of large screening trials with DBT evaluated the addition of DBT to FFDM in comparison with FFDM alone.3–5 Although these studies have highlighted the merits of DBT, the females are exposed to a significantly higher radiation dose. Some studies have been undertaken to explore the possibility of replacing the FFDM images with a synthetically reconstructed 2D view from the DBT data set and thereby obviating the need for the extra exposure. A study by Gur et al21 to evaluate the use of synthetically reconstructed 2D has shown promising results with the combination of DBT and synthetic 2D demonstrating comparable specificity but slightly lower sensitivity in comparison with DBT plus FFDM. A more recent publication by Skaane et al22 in a large screening population has demonstrated that the combination of reconstructed 2D images combined with DBT performed comparably to FFDM plus DBT.
One of the possible ways of reducing the radiation dose is by reducing the number of views taken with DBT. Research has been undertaken comparing the use of two-view FFDM to one-view DBT and the opposite-view FFDM. An early study23 has shown significantly better diagnostic accuracy with combined one-view DBT and opposite-view FFDM compared with FFDM alone. However, there was no significant difference between one-view DBT and FFDM alone. In another study24, the combination of one-view DBT and opposite-view FFDM showed better diagnostic performance than two-view FFDM in dense breasts with a small increase in average glandular dose. One-view DBT and opposite-view FFDM have also shown better lesion detection and characterization than two-view FFDM and are non-inferior to two-view FFDM in cancer detection and malignant lesion characterization.25 The radiation dose from one-view DBT and opposite-view FFDM is similar to that of two-view FFDM alone.
Other researchers have studied the addition of a single-view DBT to two-view FFDM in the screening population following the excellent results from screening with two-view DBT and FFDM. The combination of one-view DBT and FFDM has shown to improve diagnostic accuracy and decrease recall rate when compared with FFDM alone. However, the addition of two-view tomosynthesis to FFDM provided twice the performance gain in diagnostic accuracy and further reduced the recall rate. The diagnostic sensitivity for invasive cancers increased by 12% with one-view tomosynthesis and by 21.7% for two-view tomosynthesis.26 A larger study from the Malmö breast tomosynthesis screening trial has shown more promising results. Results from the analysis of 7500 females have shown 43% increase in cancer detection rate with one-view DBT (MLO) alone when compared with two-view FFDM.27
In our study, of the cancers detected on FFDM MLO view (Table 3), the abnormality is seen in both views on DBT in only 1 case (1/11 i.e. not seen on DBT CC view in 10/11 cases), whereas the cancers detected on the FFDM CC view (Table 4) are seen on both views in 12 cases (12/20 i.e. not seen on DBT MLO view in 8/20 cases). This may be due to greater coverage with MLO projections. These findings complement the findings of the Malmö trial in which DBT MLO alone was used.
A study by Waldherr et al28 assessed the diagnostic value of one-view DBT vs two-view FFDM, and vs a combination of both modalities in the diagnostic workup of females with abnormal mammograms. The authors found that one-view DBT had better sensitivity and negative-predictive value than FFDM alone in fatty and dense breasts.
The recommendation from the NHSBSP about the use of DBT in screening assessment females is based largely on an earlier study from Michell et al.7 More recent work from the same group found that two-view mammography with one-view DBT showed significantly improved accuracy compared with two-view mammography and cone compression magnification mammography (CCMM or spot views) and concluded that DBT can be used effectively in the further evaluation of mammographic abnormalities found at screening and in symptomatic diagnostic practice.29 Although the authors of that study have altered their imaging protocol to include two-view DBT in place of CCMM for all soft-tissue lesions requiring further mammographic assessment, their findings offer further evidence that one-view DBT may be sufficient.
There are some limitations to our study. This is a retrospective review of the TOMMY trial data and the imaging was not reviewed again for this study. DBT was not assessed as a stand-alone modality but in combination with repeat FFDM, clinical examination and ultrasound. Additional diagnostic confidence may therefore have been obtained from the repeat FFDM views in addition to the DBT. The total number of cancers included in this study is small. A larger study with more cancers should be carried out to investigate this further. There were five cancers that were not detected on tomosynthesis. The cause for this is poor positioning as the cancers were located close to the chest wall. This study does not include females with microcalcification as the abnormal finding on FFDM, even though some of these had an associated soft-tissue abnormality. Lastly, there is no formal follow-up of the normal or benign cases; however, no interval cancer has been reported in these cases at the time of data analysis (15 months after the last TOMMY participant underwent assessment). The small possibility, however, that there were some females with cancers that were not diagnosed at assessment does not affect the conclusion of the study.
In conclusion, our study has shown that one-view DBT (in the same projection as the original mammographic abnormality) may be adequate for the assessment of possible screen-detected soft-tissue abnormalities seen only on one FFDM view. The alternate DBT view did not show any cancers that are not otherwise visible, and the current recommendation from the NHSBSP for two-view DBT may not be necessary. As the number of females included in this study is limited, a larger study should be carried out to substantiate these findings.
Acknowledgments
ACKNOWLEDGMENTS
We would like to thank Professor Stephen Duffy for his help.
Contributor Information
Rabea Haq, Email: rabea.haq1@gmail.com.
Yit Y Lim, Email: yit.lim@uhsm.nhs.uk.
Anthony J Maxwell, Email: anthony.maxwell@uhsm.nhs.uk.
Emma Hurley, Email: emma.hurley@uhsm.nhs.uk.
Ursula Beetles, Email: ursula.beetles@uhsm.nhs.uk.
Sara Bundred, Email: sara.bundred@uhsm.nhs.uk.
Mary Wilson, Email: mary.wilson@uhsm.nhs.uk.
Susan Astley, Email: sue.astley@manchester.ac.uk.
Fiona J Gilbert, Email: fjg28@medschl.cam.ac.uk.
REFERENCES
- 1.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson S, Wlicox M. The benefits and harms of breast cancer screening: an independent review: a report jointly commissioned by Cancer Research UK and the Department of Health (England) October 2012. Br J Cancer 2013; 108: 2205–40. doi: 10.1038/bjc.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005; 353: 1773–83. doi: 10.1056/NEJMoa052911 [DOI] [PubMed] [Google Scholar]
- 3.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267: 47–56. doi: 10.1148/radiol.12121373 [DOI] [PubMed] [Google Scholar]
- 4.Ciatto S, Houssami N, Bernardi D, Caumo F, Peelegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14: 583–9. doi: 10.1016/S1470-2045(13)70134-7 [DOI] [PubMed] [Google Scholar]
- 5.Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology 2013; 269: 694–700. doi: 10.1148/radiol.13130307 [DOI] [PubMed] [Google Scholar]
- 6.Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311: 2499–507. doi: 10.1001/jama.2014.6095 [DOI] [PubMed] [Google Scholar]
- 7.Michell M, Iqbal A, Wasan RK, Evans DR, Peacock C, Lawinski CP, et al. A comparison of the accuracy of film screen mammography, full-field digital mammography and digital breast tomosynthesis. Clin Radiol 2012; 67: 976–81. doi: 10.1016/j.crad.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 8.Wilson R, Liston J. (eds). Clinical Guidelines for Breast Cancer Screening Assessment. NHSBSP Publication No 49 (3rd edn). 2010; Sheffield, UK: NHS Cancer Screening Programmes. [Google Scholar]
- 9.Tagliafico A, Astengo D, Cavagnetto F, Rosasco R, Rescinito G, Monetti F, et al. One-to-one comparison between digital spot compression view and digital breast tomosynthesis. Eur Radiol 2012; 22: 539–44. doi: 10.1007/s00330-011-2305-1 [DOI] [PubMed] [Google Scholar]
- 10.Brandt KR, Craig DA, Hoskins TL, Henrichsen TL, Bendel EC, Brandt SR, et al. Can digital breast tomosynthesis replace conventional diagnostic mammography views for screening recalls without calcifications? A comparison study in a simulated clinical setting. AJR Am J Roentgenol 2013; 200: 291–8. doi: 10.2214/AJR.12.8881 [DOI] [PubMed] [Google Scholar]
- 11.Zuley ML, Bandos AI, Ganott MA, Sumkin JH, Kelly AE, Catullo VJ, et al. Digital breast tomosynthesis versus supplemental diagnostic mammographic views for evaluation of noncalcified breast lesions. Radiology 2013; 266: 89–95. doi: 10.1148/radiol.12120552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NHS Cancer Screening Programmes. Current position on use of tomosynthesis (DBT) in the NHS Breast Screening Programme. 2013.Available from: http://www.cancerscreening.nhs.uk/breastscreen/digital-mammography.html [Google Scholar]
- 13.Gilbert FJ, Tucker L, Gillan MG, Willsher P, Cooke J, Duncan KA, et al. The TOMMY trial: a comparison of TOMosynthesis with digital MammographY in the UK NHS Breast Screening Programme—a multicentre retrospective reading study comparing the diagnostic performance of digital breast tomosynthesis and digital mammography with digital mammography alone. Health Technol Assess 2015; 19: 1–136. doi: 10.3310/hta19040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell AJ, Ridley NT, Rubin G, Wallis MG, Gilbert FJ, Michell MJ. The Royal College of Radiologists Breast Group imaging classification. Clin Radiol 2009; 64: 624–7. doi: 10.1016/j.crad.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Fornvik D, Zackrisson S, Ljungberg O, Svahn T, Timberg P, Tingberg A, et al. Breast tomosynthesis: accuracy of tumor measurement compared with digital mammography and ultrasonography. Acta Radiol 2010; 51: 240–7. [DOI] [PubMed] [Google Scholar]
- 16.Mun HS, Kim HH, Shin HJ, Cha JH, Ruppel PL, Oh MY, et al. Assessment of extent of breast cancer: comparison between digital breast tomosynthesis and full-filed digital mammography. Clin Radiol 2013; 68: 1254–9. doi: 10.1016/j.crad.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 17.Rafferty E, Niklason L. FFDM vs FFDM with tomosynthesis for women with radiographically dense breasts: An enriched retrospective reader study. In: Radiological Society of North America 2011 Scientific Assembly and Annual Meeting; 6 November–2 December 2011; Chicago, IL: Radiological Society of North America.
- 18.Dance DR, Strudley CJ, Young KC, Oduko JM, Whelehan PJ, Mungutroy EHL. Comparison of breast doses for digital tomosynthesis estimated from patient exposures and using pmma breast phantoms. In: Maidment AD, Bakic PR, Gavenonis S, eds. Proceedings of the 11th international conference on breast imaging (IWDM 2012). Berlin, Germany: Springer; 2012. pp. 316–21. [Google Scholar]
- 19.Department of Health (2000). Guidance on the establishment and use of diagnostic reference levels (DRLs) as the term is applied in the Ionising Radiation (Medical Exposure) Regulations 2000. Available from: http://www.dh.gov.uk/en/Publicationsandstatistics/
- 20.The ionising radiations regulations 1999 (IRR'99). Available from: http://www.legislation.gov.uk/uksi/1999/3232/contents/made [PubMed] [Google Scholar]
- 21.Gur D, Zuley ML, Anello MI, Rathfon GY, Chough DM, Ganott MA, et al. Dose reduction in digital breast tomosynthesis (DBT) screening using synthetically reconstructed projection images: an observer performance study. Acad Radiol 2012; 19: 166–71. doi: 10.1016/j.acra.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skaane P, Bandos AI, Eben EB, Jebsen IN, Krager M, Haakenaasen U, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology 2014; 271: 655–63. doi: 10.1148/radiol.13131391 [DOI] [PubMed] [Google Scholar]
- 23.Svahn T, Andersson I, Chakraborty D, Svensson S, Ikeda D, Förnvik D, et al. The diagnostic accuracy of dual-view digital mammography, single-view breast tomosynthesis and a dual-view combination of breast tomosynthesis and digital mammography in a free-response observer performance study. Radiat Prot Dosimetry 2010; 139: 113–17. doi: 10.1093/rpd/ncq044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin SU, Chang JM, Bae MS, Lee SH, Cho N, Seo M, et al. Comparative evaluation of average glandular dose and breast cancer detection between single-view digital breast tomosynthesis (DBT) plus single-view digital mammography (DM) and two-view DM: correlation with breast thickness and density. Eur Radiol 2015; 25: 1–8. doi: 10.1007/s00330-014-3399-z [DOI] [PubMed] [Google Scholar]
- 25.Gennaro G, Hendrick RE, Toledano A, Paquelet JR, Bezzon E, Chersevani R, et al. Combination of one-view digital breast tomosynthesis with one-view digital mammography versus standard two-view digital mammography: per lesion analysis. Eur Radiol 2013; 23: 2087–94. doi: 10.1007/s00330-013-2831-0 [DOI] [PubMed] [Google Scholar]
- 26.Rafferty EA, Park JM, Philpotts LE, Poplack SP, Sumkin JH, Halpern EF, et al. Diagnostic accuracy and recall rates for digital mammography and digital mammography combined with one-view and two-view tomosynthesis: results of an enriched reader study. AJR Am J Roentgenol 2014; 202: 273–81. doi: 10.2214/AJR.13.11240 [DOI] [PubMed] [Google Scholar]
- 27.Lång K, Andersson I, Rosso A, Tinberg A, Timberg P, Zackrisson S. Performance of one-view breast tomosynthesis as a stand-alone breast cancer screening modality: results from the Malmö Breast Tomosynthesis Screening Trial, a population-based study. Eur Radiol 2015; 1–8. doi: 10.1007/s00330-015-3803-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waldherr C, Cerny P, Altermatt HJ, Berclaz G, Ciriolo M, Buser K, et al. Value of one-view breast tomosynthesis versus two-view mammography in diagnostic workup of women with clinical signs and symptoms and in women recalled from screening. AJR Am J Roentgenol 2013; 200: 226–31. doi: 10.2214/AJR.11.8202 [DOI] [PubMed] [Google Scholar]
- 29.Morel JC, Iqbal A, Wasan RK, Peacock C, Evans DR, Rahim R, et al. The accuracy of digital breast tomosynthesis compared with cone compression magnification mammography in the assessment of abnormalities found on mammography. Clin Radiol 2014; 69: 1112–16. doi: 10.1016/j.crad.2014.06.005 [DOI] [PubMed] [Google Scholar]