Abstract
Objective:
The objective of the present study was to identify acute skin toxicity risk factors linked to the anthropometric characteristics of patients with breast cancer treated with radiation therapy.
Methods:
Consecutive patients with breast cancer were enrolled after breast-conserving surgery and before radiotherapy course. Acute skin toxicity was assessed weekly during the 7 weeks of radiotherapy with the International Classification from National Cancer Institute. Grade 2 defined acute skin toxicity. Patient characteristics and anthropometric measurements were collected.
Results:
54 patients were enrolled in 2013. Eight patients (14.8%) had grade ≥2 toxicity. The average weight and chest size were 65.5 kg and 93.6 cm, respectively. Bra cup size is significantly associated with a risk of grade 2 dermatitis [odds ratio (OR) 3.46, 95% confidence interval (CI) (1.29–11.92), p = 0.02]. Anthropometric breast fat mass measurements, such as thickness of left [OR 2.72, 95% CI (1.08–8.26), p = 0.04] and right [OR 2.45, 95% CI (0.99–7.27), p = 0.05] axillary fat, are correlated with an increased risk. Distance between the pectoral muscle and nipple is a reproducible measurement of breast size and is associated with acute skin toxicity with significant tendency (OR = 2.21, 95% CI (0.97–5.98), p = 0.07).
Conclusion:
Breast size and its different anthropometric measurements (thickness of left and right axillary fat, nipple-to-pectoral muscle distance) are correlated with the risk of skin toxicity.
Advances in knowledge:
The present article analyses several characteristics and anthropomorphic measurements of breast in order to assess breast size. A standardized and reproducible protocol to measure breast volume is described.
INTRODUCTION
Breast cancer is the most frequent female cancer in France with 48,763 new cases in 2012.1 It is now considered as a favourable prognosis cancer, since therapeutic progress has increased survival time to at least 5 years for 83% of patients.2 It is estimated that 72.8% of the patients who develop, nowadays, a breast cancer in France will be cured.3 Clearly, one of the next challenges is how to reduce side effects of treatments. Radiotherapy plays a leading role in breast conservation strategy in adjuvant setting, with a significant reduction of local recurrence risk.4,5 Acute radiotherapy skin toxicity, or radiodermatitis, is defined by the occurrence of a dermal lesion in the days or weeks following the beginning of radiation therapy, which will heal in the following months.6,7 Acute radiodermatitis is ranked from one (erythema) to four (necrosis) in terms of severity following the International Classification from the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE).8 Acute skin toxicity is defined by a grade ≥2 toxicity. Acute skin toxicity is a frequent radiotherapy complication: grade 1 radiodermatitis occurs in 50–58% of cases, grade 2 in 32% of cases and grade 3 in 4% of cases.9,10 It can lead to a pause in the treatment, with significant loss of therapeutic benefit.11–13 Radiation parameters, such as total dose, dose per fraction, time between fractions, total treatment time and total treated volume, have an influence on acute skin toxicity.14 Nevertheless, patients receiving similar radiation treatments can develop toxicities of various intensities.15,16 Therefore, factors other than radiation technique can influence the variability of dermal side effects.17 These factors are probably environmental and associated with individual radiosensitivity. Individual radiosensitivity is a complex polygenic phenomenon, resulting from the interaction of numerous genes implicated in numerous intracellular signalling pathways.18 However, it has been shown that patient characteristics, such as smoking, malnutrition or obesity, also has an influence on skin toxicity linked to radiotherapy.19 We know that a high body mass index (BMI) is a predictive indicator of acute skin toxicity [hazard ratio (HR) = 1.09 for 1 kg m−2 with 95% confidence interval (CI) (1.05–1.13)].12 Besides obesity, breast size is significantly correlated with the risk of radiodermatitis.17 This leads us to the necessity of measuring precisely large breasts, in order to identify patients with high-risk toxicity.10,11,20,21 Bra cup sizes vary depending on the models of bras, and patients are generally unfamiliar with this. So cup sizes cannot be a scientific reproducible and reliable breast measure tool. However, no study has yet developed a standardized protocol to measure the breast fat mass. The primary objective of the present observational prospective study was to analyse acute skin toxicity risk factors linked to anthropometric and clinical characteristics of patients with breast cancer treated with radiation therapy. The secondary objective was to develop a reliable and reproducible measure of breast size and breast fat mass, which can help to identify high-risk groups of acute skin toxicity.
METHODS AND MATERIALS
Population
The present study has been performed at the Radiotherapy Department of Lucien Neuwirth Comprehensive Cancer Care Center, Saint Priest on Jarez France. The inclusion criteria were patients with breast cancer treated with conservative surgery and adjuvant radiotherapy. The protocol respected the ethic guidelines enounced in the Helsinki Declaration.
Procedure
Age, medical history, medical treatments (especially hormonal therapy), co-morbidities (especially diabetes, blood pressure, actual or past smoking), breast cancer characteristics (TNM stage, histological type, Scarff–Bloom–Richardson grade, hormonal receptors, Her2 status) and treatment characteristics (type of energy, total dose, bolus dose, dose per fraction, time between fractions, total treatment time) were collected. Clinical morphological characteristics (bra cup, arm size and BMI), Type I skin on Fitzpatrick scale (pale white skin, blue/hazel eyes, blond/red hair) and the last haemoglobin level were also noted. The radiotherapist (SMN) weekly evaluated acute skin toxicity during the 7 weeks of the treatment and at 3 months after the beginning of radiation therapy.
Tools
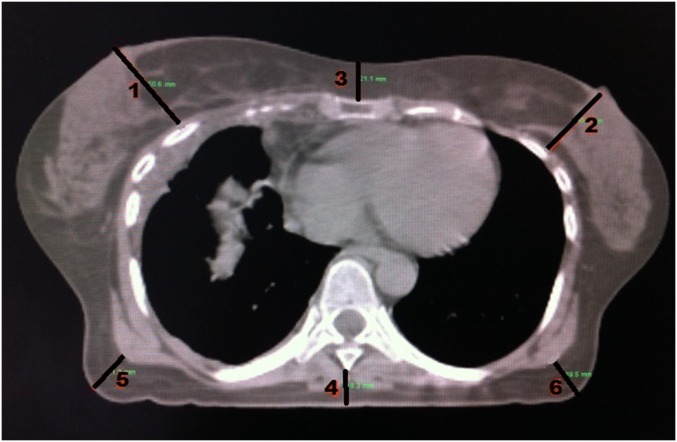
Acute skin toxicity was evaluated with the NCI-CTCAE.8 Acute skin toxicity was defined by a grade ≥2 toxicity.12,20,21 Breast size was systematically evaluated before the beginning of the radiotherapy. Bra cup sizes were collected. Breast anthropometric parameters were measured on simulation CT. Breast size was defined by the distance between the pectoral muscle and the nipple, namely the tangent to the pectoral muscle passing through the nipple. Nipple-to-pectoral muscle distance was measured on the mammography image of the healthy breast nipple, since surgery probably alters breast fat mass. Furthermore, subcutaneous fat thickness was assessed with the distance between skin and spinous process, distance between skin and sternum and the bilateral axillary fat thickness. Axillary fat was assessed as this volume may increase in overweighed females and could cause dose inhomogeneity as it is located at the entrance of radiation therapy external field. Figure 1 is a detailed illustration of these measures. Dosimetric parameters [planning target volume (PTV), clinical target volume, minimum dose (Dmin), maximum dose (Dmax), mean dose (Dmean), skin hot spots, homogeneity index] were reported. Dose calculation was performed systematically on Varian Eclipse™ treatment planning system (Varian® Medical Systems, Palo Alto, CA) and Dosigray® treatment planning system (Dosisoft, Cachan, France). Homogeneity index was based on the Radiation Therapy Oncology Group definition.22 Skin hot spot was defined by a dose >107% of the prescribed dose located in the first centimetre below the skin surface.
Figure 1.
Anthropological assessment on dosimetric CT scan: 1 and 2: nipple-to-pectoral muscle distance on healthy/treated side; 3: skin-to-xiphoid distance; 4: skin-to-spinous process distance; 5: right axillary fat thickness; 6: left axillary fat thickness.
Statistical analyses
Statistical analyses was performed with R software (The R Foundation for Statistical Computing Vienna, Austria).23 We analysed the correlation between skin toxicity emergence and several risk factors with a univariate logistic regression. The Wald test was also used. The significant value was set to p < 0.05.
RESULTS
Population characteristics
We enrolled 54 patients, with an average age of 62 years. Mean weight was 65.5 kg (47–95 kg), average BMI was 24.9 kg m−2 (19–37.6 kg m−2) and mean chest size was 93.6 cm (80–115 cm). Medical and physical patient characteristics are summarized in Table 1 and Table 2, respectively. Anthropometric parameters of the whole set of patients are described below: skin-to-xiphoid mean distance was 16 mm (5–33 mm), nipple-to-pectoral muscle mean distance on ipsilateral side was 41 mm (14–69 mm), nipple-to-pectoral muscle mean distance on contralateral side was 42 mm (19–77 mm), left axillary fat mean thickness was 20 mm (5–57 mm), right axillary fat mean thickness was 17 mm (6–63 mm) and skin-to-spinous process mean distance was 15 mm (4–38 mm). 32 patients had a Type I skin, 9 patients were active smokers and 11 were former smokers. 9 patients were prescribed hormonotherapy simultaneously with radiation therapy, and 36 patients were prescribed chemotherapy.
Table 1.
Medical patient characteristics
| Medical characteristics | Population (n = 54) |
|---|---|
| Treated breast | |
| Left | 20 |
| Right | 34 |
| Smoker status | |
| Active | 9 |
| Former | 11 |
| No | 34 |
| Fitzpatrick phototyping scale | |
| Pale | 32 |
| Easily tanned skin | 21 |
| Unknown | 1 |
| Familial history of cancer | |
| Yes | 24 |
| No | 29 |
| Unknown | 1 |
| Personal history of cancer | |
| Yes | 14 |
| No | 40 |
| High blood pressure | |
| Yes | 19 |
| No | 34 |
| Unknown | 1 |
| Hormone therapy | |
| Concomitant | 9 |
| After radiation therapy | 30 |
| No | 15 |
| Location | |
| Upper outer quadrant | 25 |
| Upper inner quadrant | 7 |
| Lower outer quadrant | 2 |
| Lower inner quadrant | 3 |
| Other | 17 |
| Axillary lymph node dissection | |
| Yes | 22 |
| No | 24 |
| Unknown | 8 |
| Sentinel lymph node dissection | |
| Yes | 47 |
| No | 4 |
| Unknown | 3 |
| Tumour size | |
| 1 | 43 |
| 2 | 11 |
| Lymph nodes | |
| 0 | 31 |
| 1 | 21 |
| Unknown | 2 |
| Histological type | |
| Ductal invasive cancer | 40 |
| Lobular invasive cancer | 7 |
| Other | 7 |
| Scarff–Bloom–Richardson | |
| 1 | 7 |
| 2 | 23 |
| 3 | 24 |
| Hormone receptor status | |
| Positive | 39 |
| Negative | 11 |
| Unknown | 4 |
| Her2 status | |
| Positive | 11 |
| Negative | 37 |
| Unknown | 6 |
| Adjuvant chemotherapy | |
| Yes | 36 |
| No | 18 |
| Node radiotherapy | |
| Yes | 20 |
| No | 34 |
Table 2.
Physical patient characteristics
| Physical characteristics | Average | Median | Minimum | Maximum |
|---|---|---|---|---|
| Weight (kg) | 65.5 | 62.0 | 47.0 | 95.0 |
| Height (m) | 1.61 | 1.62 | 1.42 | 1.75 |
| BMI (kg m−2) | 24.9 | 23.2 | 18.8 | 37.6 |
| Chest measurement (cm) | 93.6 | 93.0 | 80.0 | 115.0 |
| Brachial perimeter (cm) | 29.4 | 29.0 | 22.0 | 46.0 |
BMI, body mass index.
Skin toxicity and anthropometric risk factors
Eight patients (14.81%) were diagnosed with a grade ≥2 toxicity (i.e. at least severe erythema). Axillary fat thickness is significantly correlated with acute skin toxicity, whenever ipsilateral or contralateral sides are assessed [left side: odds ratio (OR) 2.72, 95% CI (1.08–8.26), p = 0.04; right side: OR 2.45, 95% CI (0.99–7.27), p = 0.05]. Bra cup size is significantly associated with a risk of radiodermatitis [OR 3.46, 95% CI (1.29–11.92), p = 0.02]. We also found the following trends: radiodermatitis occurrence may be correlated with nipple-to-pectoral muscle distance [OR 2.21, 95% CI (0.97–5.98), p = 0.07], and actual or past smoking may have fostered the appearance of skin toxicity [OR 4.09, 95% CI (0.86–22.89), p = 0.08]. Skin toxicity and correlations data are given in Table 3.
Table 3.
Correlation between patient parameters and acute skin toxicity
| Correlation to grade ≥2 toxicity | Odds ratio | p | 95% confidence interval |
|---|---|---|---|
| Former or active smoker | 4.09 | 0.08 | 0.86–22.89 |
| High blood pressure | 0.62 | 0.58 | 0.08–3.13 |
| Phototype | 0.55 | 0.49 | 0.07–2.77 |
| Chemotherapy | 1.56 | 0.61 | 0.31–11.68 |
| Body mass index | 1.91 | 0.10 | 0.89–4.53 |
| Breast size | 1.10 | 0.10 | 0.98–1.25 |
| Bra cup size | 3.46 | 0.02 | 1.29–11.92 |
| Lymph node treatment | 1.69 | 0.50 | 0.35–8.31 |
| Skin-to-xiphoid distance | 1.02 | 0.95 | 0.45–2.18 |
| Nipple-to-pectoral muscle distance on the healthy side (mm) | 2.21 | 0.07 | 0.97–5.98 |
| Nipple-to-pectoral muscle distance on the treated side | 2.07 | 0.10 | 0.89–5.38 |
| Skin-to-spinous process distance | 1.37 | 0.42 | 0.62–8.26 |
| Left axillary fat thickness | 2.72 | 0.04 | 1.08–8.26 |
| Right axillary fat thickness | 2.45 | 0.05 | 0.99–7.27 |
Data on dosimetry
No hot spot was located within the skin surface. No minor/major deviation was reported based on Radiation Therapy Oncology Group homogeneity index. Mean PTV was 1026.7 cm3 (range: 333–2178 cm3), mean PTV among patients suffering from acute skin toxicity was 1264.9 cm3 (range: 540.5–2106 cm3). Data on dosimetry are reported in Table 4.
Table 4.
Data on dosimetry
| Dosimetric characteristics | Mean (range) |
|---|---|
| PTV | |
| In overall population (cm3) | 1026.7 (333.0–2178.0) |
| In patients with skin toxicity (cm3) | 1264.9 (540.5–2106.0) |
| Dmin (Gy)a | 1.9 (0.0–14.5) |
| Dmax (Gy)a | 68.8 (66.8–70.6) |
| Dmean (Gy)a | 49.5 (36.7–56.6) |
| Homogeneity indexa,b | 1.04 (1.01–1.07) |
| Hot spot within the skina | 0 (0–0) |
Dmin, minimum dose; Dmax, maximum dose; Dmean, mean dose; PTV, planning target volume.
Among overall population.
Calculated with Radiation Therapy Oncology Group homogeneity index.22
DISCUSSION
Three new predictive anthropometric characteristics seem to be linked to acute skin toxicity risk: left axillary fat thickness, right axillary fat thickness and nipple-to-pectoral muscle distance. This last measure could be considered as a new, reliable and reproducible assessment of breast size. However, owing to the limited number of patients and of patients with grade ≥2 toxicities, comparison between the groups is difficult and results are to be taken cautiously. Big bra cup size is a well-known predictive factor of acute skin toxicity, yet. When we can foretell the acute skin toxicity risk with those objective anthropometric parameters, we will be able to prescribe radiotherapy schedules precisely adapted to the risk group. A high-risk morphotype group can be defined, with particular characteristics such as big-breasted females, of Type 1 skin on Fitzpatrick scale, former or active smokers. For this group, a specific radiotherapy protocol should be proposed. According to Moody et al,24 the high skin toxicity risk of big-breasted patients is linked to localized hot spots and dose heterogeneity. This might be avoided with a “field-in-field” technique or with intensity-modulated radiation therapy.17,25,26 However, in the present study, homogeneity indexes were excellent and could not be correlated with acute skin toxicity. No hot spots within below the skin surface were reported either. Such results must be taken very cautiously: although dose calculations were performed with two different treatment planning systems, algorithms do not give reliable results within the skin. The hypothesis of dose heterogeneity within the skin cannot be excluded, as intensity-modulated radiation therapy showed interesting clinical results.26 Selecting patients according to the objective and reproducible risk factors is a sine qua non condition to individualize treatments in order to minimize acute skin toxicity of radiation. However, risk factors other than anthropometric risk factors have been shown to play a leading role in the variability of skin toxicities. Phototype is obviously a major predictive factor of skin radiosensitivity.27 Other factors have been described, such as diabetes, immunodepression, malnutrition, smoking or obesity.19 If we find tobacco as a predictive factor of skin toxicity, on the contrary, it is not the case of BMI, probably because of our reduced patient sample. Indeed, it was shown that co-morbidities such as obesity or tobacco act as co-factors which modify radiotoxicity.28 According to the authors,28 this could explain 30% of individual variability to radiations. Moreover, Chang-Claude et al29 showed that the polymorphism of DNA reparation genes (XRCC1 and APEI) seems to be a protective factor against acute radiotherapy skin toxicity, but only among normal-weight females. The genotype's protective effect of XRCC1 and XPD is not found among obese or overweight patients. It seems otherwise that diabetes or autoimmune diseases have an influence on radiosensitivity.30
CONCLUSION
The present study shows the close links between various anthropomorphic parameters and increased risk of skin toxicity. Breast volume is not exactly evaluated using bra cup size, whereas it is a major predictive factor of skin toxicity and represents a particular medical interest. The retrospective nature of the study and the small number of patients are limitations, but the present pilot study provides preliminary results and tendencies in order to design a largest prospective study in a near future. The present study describes a standardized and reproducible protocol to measure breast volume, with breast fat mass and nipple-to-pectoral muscle distance.
Contributor Information
Benoîte Méry, Email: benoite.mery@icloire.fr.
Alexis Vallard, Email: vallardalex@hotmail.com.
Jane-Chloé Trone, Email: jane-chloe.trone@icloire.fr.
Cécile Pacaut, Email: cecile.vassal@icloire.fr.
Jean-Baptiste Guy, Email: jean-baptiste.guy@icloire.fr.
Sophie Espenel, Email: sophie.espenel@icloire.fr.
Julien Langrand-Escure, Email: julien.langrand-escure@icloire.fr.
Edouard Ollier, Email: ed.ollier@gmail.com.
Guoping Wang, Email: guoping.wang@icloire.fr.
Peng Diao, Email: peng.diao@icloire.fr.
Lise Bigot, Email: lise.bigot@icloire.fr.
Sylvie Mengue Ndong, Email: sylvie.menguendong@icloire.fr.
Claire Bosacki, Email: claire.bosacki@icloire.fr.
Majed Ben Mrad, Email: majed.ben-mrad@icloire.fr.
Nicolas Magné, Email: nicolas.magne@icloire.fr.
REFERENCES
- 1.Institut Nationnal du Caner. Epidémiologie du cancer du sein en France métropolitaine—Incidence et mortalité. [Updated 16 March 2015.] Available at: http://lesdonnees.e-cancer.fr/les-fiches-de-synthese/1-types-cancer/9-cancer-sein/1-epidemiologie-du-cancer-du-sein-en-france-metropolitaine-incidence-et-mortalite.html.
- 2.Belot A, Grosclaude P, Bossard N, Jougla E, Benhamou E, Delafosse P, et al. Cancer incidence and mortality in France over the period 1980-2005. Rev Epidemiol Sante Publique 2008; 56: 159–75. doi: 10.1016/j.respe.2008.03.117 [DOI] [PubMed] [Google Scholar]
- 3.Francisci S, Capocaccia R, Grande E, Santaquilani M, Simonetti A, Allemani C, et al. ; EUROCARE Working Group. The cure of cancer: a European perspective. Eur J Cancer 2009; 45: 1067–79. doi: 10.1016/j.ejca.2008.11.034 [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–41. doi: 10.1056/NEJMoa022152 [DOI] [PubMed] [Google Scholar]
- 5.Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost. EORTC 22881-10882 trial. J Clin Oncol 2007; 25: 3259–65. doi: 10.1200/JCO.2007.11.4991 [DOI] [PubMed] [Google Scholar]
- 6.Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014; 14: 53. doi: 10.1186/1471-2407-14-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modesto A, Faivre JC, Granel-Brocard F, Tao YG, Pointreau Y. Evaluation and management of acute radiation dermatitis. [In French.] Cancer Radiother 2012; 16: 456–61. doi: 10.1016/j.canrad.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 8.Common Terminology Criteria for Adverse Events v3.0 (CTCAE). [Updated 16 March 2015; cited 9 August 2006.] Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 9.Maroun P, Jacob J, Roberti E, Bourgier C, Heymann S. Gated radiotherapy in breast cancer. [In French.] Cancer Radiother 2012; 16: 697–701. doi: 10.1016/j.canrad.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 10.Bentzen SM, Overgaard J. Patient-to-patient variability in the expression of radiation-induced normal tissue injury. Semin Radiat Oncol 1994; 4: 68–80. doi: 10.1053/SRAO00400068 [DOI] [PubMed] [Google Scholar]
- 11.Fisher J, Scott C, Stevens R, Marconi B, Champion L, Freedman GM, et al. Randomized phase III study comparing Best Supportive Care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys 2000; 48: 1307–10. doi: 10.1016/S0360-3016(00)00782-3 [DOI] [PubMed] [Google Scholar]
- 12.Twardella D, Popanda O, Helmbold I, Ebbeler R, Benner A, von Fournier D, et al. Personal characteristics, therapy modalities and individual DNA repair capacity as predictive factors of acute skin toxicity in an unselected cohort of breast cancer patients receiving radiotherapy. Radiother Oncol 2003; 69: 145–53. doi: 10.1016/S0167-8140(03)00166-X [DOI] [PubMed] [Google Scholar]
- 13.Huang EY, Chen HC, Wang CJ, Sun LM, Hsu HC. Predictive factors for skin telangiectasia following post-mastectomy electron beam irradiation. Br J Radiol 2002; 75: 444–7. doi: 10.1259/bjr.75.893.750444 [DOI] [PubMed] [Google Scholar]
- 14.Mettler F. Medical effects of ionizing radiation. 2nd edn. Philadelphia, PA. WB Saunders Co.; 1995. [Google Scholar]
- 15.Fernando IN, Ford HT, Powles TJ, Ashley S, Glees JP, Torr M, et al. Factors affecting acute skin toxicity in patients having breast irradiation after conservative surgery: a prospective study of treatment practice at the Royal Marsden Hospital. Clin Oncol (R Coll Radiol) 1996; 8: 226–33. doi: 10.1016/S0936-6555(05)80657-0 [DOI] [PubMed] [Google Scholar]
- 16.Gray JR, McCormick B, Cox L, Yahalom J. Primary breast irradiation in large-breasted or heavy women: analysis of cosmetic outcome. Int J Radiat Oncol Biol Phys 1991; 21: 347–54. doi: 10.1016/0360-3016(91)90781-X [DOI] [PubMed] [Google Scholar]
- 17.De Langhe S, Mulliez T, Veldeman L, Remouchamps V, van Greveling A, Gilsoul M, et al. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer 2014; 14: 711. doi: 10.1186/1471-2407-14-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lilla C, Ambrosone CB, Kropp S, Helmbold I, Schmezer P, von Fournier D, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat 2007; 106: 143–50. doi: 10.1007/s10549-006-9480-9 [DOI] [PubMed] [Google Scholar]
- 19.Ginot A, Doyen J, Hannoun-Lévi JM, Courdi A. Normal tissue tolerance to external beam radiation therapy: skin. [In French.] Cancer Radiother 2010; 14: 379–85. doi: 10.1016/j.canrad.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 20.Bentzen SM, Overgaard M. Relationship between early and late normal-tissue injury after postmastectomy radiotherapy. Radiother Oncol 1991; 20: 159–65. doi: 10.1016/0167-8140(91)90092-U [DOI] [PubMed] [Google Scholar]
- 21.Saksornchai K, Rojpornpradit P, Shotelersak K, Lertbutsayanukul C, Chakkabat C, Raiyawa T. Skin toxicity and cosmesis after hypofractionated whole breast irradiation for early breast cancer. J Med Assoc Thai 2012; 95: 229–40. [PubMed] [Google Scholar]
- 22.Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 1993; 27: 1231–9. doi: 10.1016/0360-3016(93)90548-A [DOI] [PubMed] [Google Scholar]
- 23.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. Available from: http://www.R-project.org. [Google Scholar]
- 24.Moody AM, Mayles WP, Bliss JM, A'Hern RP, Owen JR, Regan J, et al. The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiother Oncol 1994; 33: 106–12. doi: 10.1016/0167-8140(94)90063-9 [DOI] [PubMed] [Google Scholar]
- 25.Heymann S, Verstraet R, Pichenot C, Vergne E, Lefkopoulos D, Husson F, et al. Intensity modulation in breast radiotherapy: development of an innovative field-in-field technique at Institut Gustave-Roussy. [In French.] Cancer Radiother 2011; 15: 663–9. doi: 10.1016/j.canrad.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008; 26: 2085–92. doi: 10.1200/JCO.2007.15.2488 [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki H, Yoshida K, Nishimura T, Kobayashi K, Tsubokura T, Kodani N, et al. Association between skin phototype and radiation dermatitis in patients with breast cancer treated with breast-conserving therapy: suntan reaction could be a good predictor for radiation pigmentation. J Radiat Res. 2011; 52: 496–501. doi: 10.1269/jrr.10169 [DOI] [PubMed] [Google Scholar]
- 28.Turesson I, Nyman J, Holmberg E, Odén A. Prognostic factors for acute and late skin reactions in radiotheraphy patients. Int J Radiat Oncol Biol Phys 1996; 36: 1065–75. doi: 10.1016/S0360-3016(96)00426-9 [DOI] [PubMed] [Google Scholar]
- 29.Chang-Claude J, Popanda O, Tan XL, Kropp S, Helmbold I, von Fournier D, et al. Association between polymorphisms in the DNA repair genes, XRCC1, APE1, and XPD and acute side effects of radiotherapy in breast cancer patients. Clin Cancer Res 2005; 11: 4802–9. doi: 10.1158/1078-0432.CCR-04-2657 [DOI] [PubMed] [Google Scholar]
- 30.Twardella D, Chang-Claude J. Studies on radiosensitivity from an epidemiological point of view—overview of methods and results. Radiother Oncol 2002; 62: 249–60. doi: 10.1016/S0167-8140(01)00491-1 [DOI] [PubMed] [Google Scholar]