Abstract
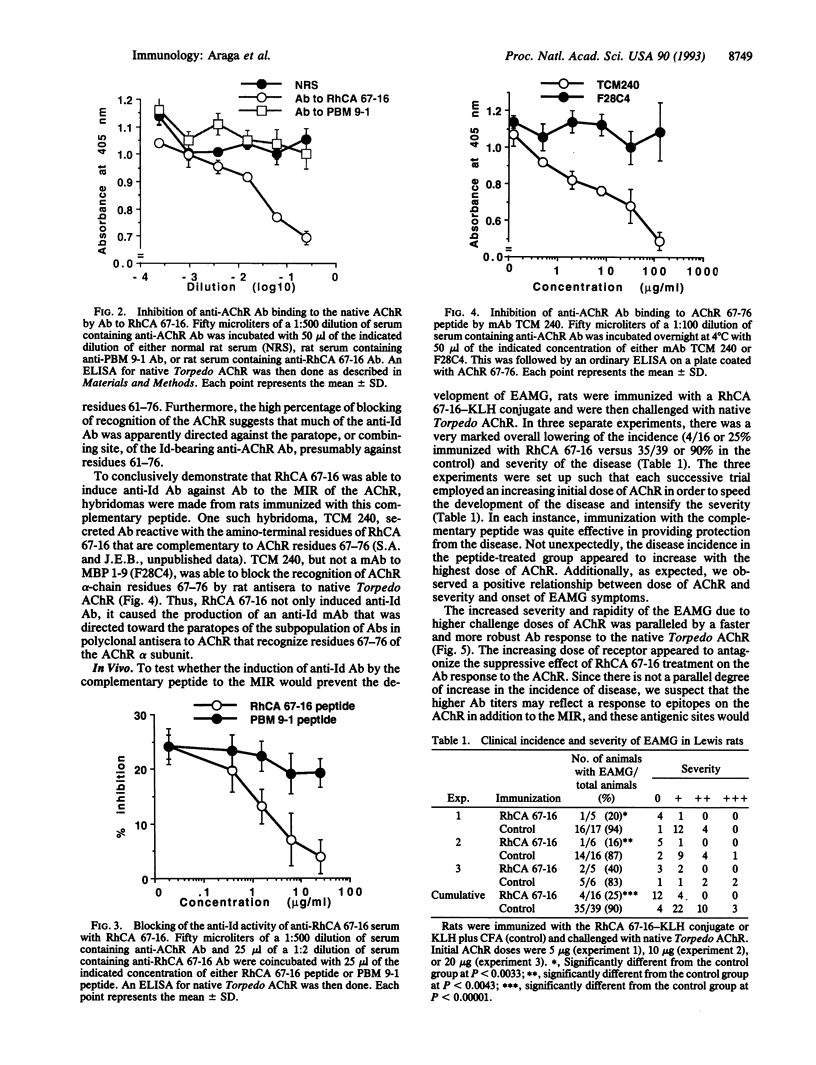
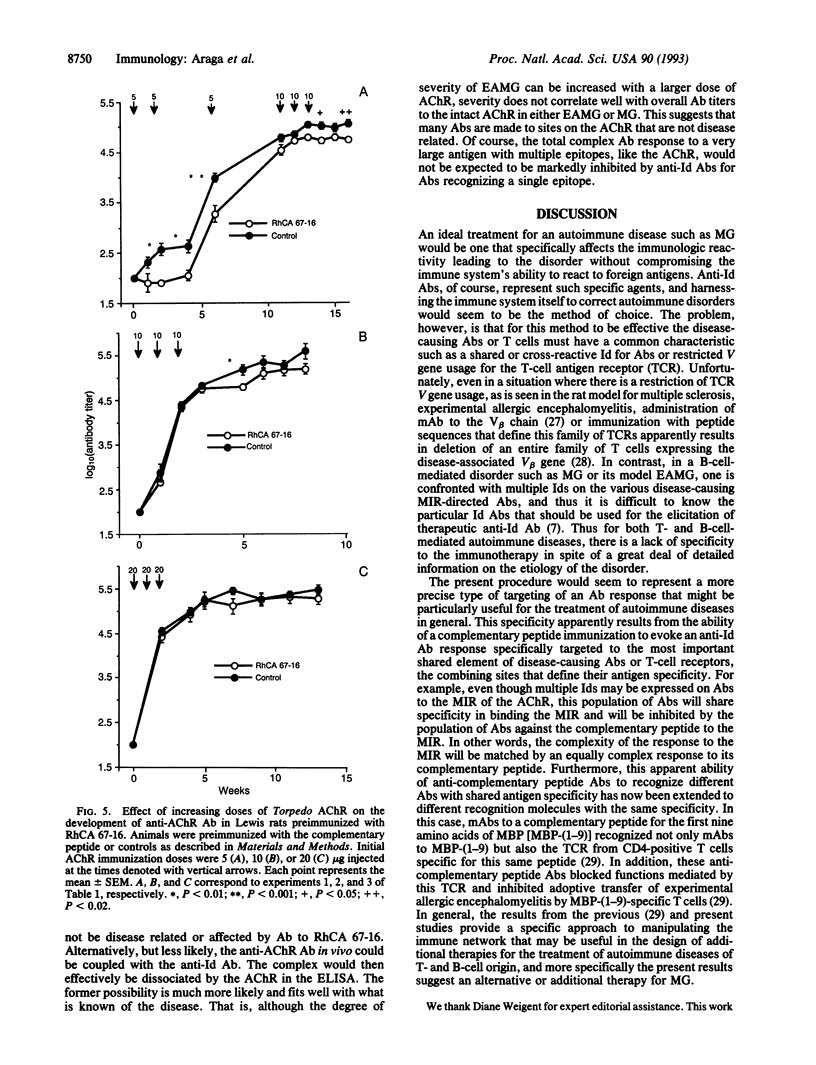
Myasthenia gravis (MG) and experimental autoimmune myasthenia gravis (EAMG) are caused, in part, by the production of autoantibodies against the main immunogenic region, amino acids 61-76, of the alpha chain of the acetylcholine receptor (AChR). Theoretically, induction of anti-idiotypic (Id) antibodies (Abs) should be a highly specific treatment for the disease by virtue of their potential ability to neutralize Abs to the AChR. We have tested this idea by attempting to evoke such anti-Id Abs by immunization with a peptide (termed RhCA 67-16) encoded by RNA complementary to the Torpedo AChR main immunogenic region and determining whether such treatment will prevent the development of EAMG. Immunization with RhCA 67-16, but not a control peptide termed PBM 9-1, was found to elicit the production of anti-Id Abs that blocked recognition of native Torpedo AChR by its Ab. This anti-Id Ab activity was ablated by incubation of the anti-RhCA 67-16 serum with RhCA 67-16, but PBM 9-1, prior to the assay for Ab binding to AChR. The anti-Id Ab-inducing activity of RhCA 67-16 was confirmed by the ability to produce a rat monoclonal Ab to RhCA 67-16 that showed anti-Id activity for polyclonal rat Ab reactive with AChR residues 67-76. Most importantly, RhCA 67-16 immunization also prevented the development of EAMG in Lewis rats challenged with Torpedo AChR (25% incidence versus 90% in the controls) and diminished the AChR Ab levels in animals injected with low doses of AChR. Our results suggest a therapy for MG and perhaps other autoimmune diseases through the induction of anti-Id Abs by peptide immunogens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Barkas T., Gabriel J. M., Mauron A., Hughes G. J., Roth B., Alliod C., Tzartos S. J., Ballivet M. Monoclonal antibodies to the main immunogenic region of the nicotinic acetylcholine receptor bind to residues 61-76 of the alpha subunit. J Biol Chem. 1988 Apr 25;263(12):5916–5920. [PubMed] [Google Scholar]
- Blalock J. E. Complementarity of peptides specified by 'sense' and 'antisense' strands of DNA. Trends Biotechnol. 1990 Jun;8(6):140–144. doi: 10.1016/0167-7799(90)90159-u. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Whitaker J. N., Benveniste E. N., Bost K. L. Use of peptides encoded by complementary RNA for generating anti-idiotypic antibodies of predefined specificity. Methods Enzymol. 1989;178:63–74. doi: 10.1016/0076-6879(89)78006-x. [DOI] [PubMed] [Google Scholar]
- Bost K. L., Smith E. M., Blalock J. E. Similarity between the corticotropin (ACTH) receptor and a peptide encoded by an RNA that is complementary to ACTH mRNA. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1372–1375. doi: 10.1073/pnas.82.5.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. S., Bradley R. J., Urquhart C. K., Kearney J. F. Naturally occurring anti-idiotypic antibodies in myasthenia gravis patients. Nature. 1983 Feb 17;301(5901):611–614. doi: 10.1038/301611a0. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M., Drachman D. B., Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science. 1973 Oct 19;182(4109):293–295. doi: 10.1126/science.182.4109.293. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Rafto S. Comparison of the subunits of Torpedo californica acetylcholine receptor by peptide mapping. Biochemistry. 1979 Jan 23;18(2):301–307. doi: 10.1021/bi00569a011. [DOI] [PubMed] [Google Scholar]
- Genkins G., Kornfeld P., Papatestas A. E., Bender A. N., Matta R. J. Clinical experience in more than 2000 patients with myasthenia gravis. Ann N Y Acad Sci. 1987;505:500–513. doi: 10.1111/j.1749-6632.1987.tb51318.x. [DOI] [PubMed] [Google Scholar]
- Hashim G. A., Vandenbark A. A., Galang A. B., Diamanduros T., Carvalho E., Srinivasan J., Jones R., Vainiene M., Morrison W. J., Offner H. Antibodies specific for VB8 receptor peptide suppress experimental autoimmune encephalomyelitis. J Immunol. 1990 Jun 15;144(12):4621–4627. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Lennon V. A., Lindstrom J. M., Seybold M. E. Experimental autoimmune myasthenia: A model of myasthenia gravis in rats and guinea pigs. J Exp Med. 1975 Jun 1;141(6):1365–1375. doi: 10.1084/jem.141.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Pascual D. W., Bost K. L. Anti-peptide antibodies recognize anti-substance P antibodies in an idiotypic fashion. Pept Res. 1989 May-Jun;2(3):207–212. [PubMed] [Google Scholar]
- Patrick J., Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973 May 25;180(4088):871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Use of glutaraldehyde as a coupling agent for proteins and peptides. Methods Enzymol. 1980;70(A):159–165. doi: 10.1016/s0076-6879(80)70047-2. [DOI] [PubMed] [Google Scholar]
- Smith L. R., Bost K. L., Blalock J. E. Generation of idiotypic and anti-idiotypic antibodies by immunization with peptides encoded by complementary RNA: a possible molecular basis for the network theory. J Immunol. 1987 Jan 1;138(1):7–9. [PubMed] [Google Scholar]
- Souroujon M. C., Fuchs S. Antiidiotypic antibodies in the regulation of experimental autoimmune myasthenia gravis. Ann N Y Acad Sci. 1987;505:676–682. doi: 10.1111/j.1749-6632.1987.tb51336.x. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Benda P., Meunier J. C., Changeux J. P. Immunological characterisation of the cholinergic receptor protein from Electrophorus electricus. FEBS Lett. 1973 Sep 1;35(1):124–128. doi: 10.1016/0014-5793(73)80592-7. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Kokla A., Walgrave S. L., Conti-Tronconi B. M. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67-76 of the alpha subunit. Proc Natl Acad Sci U S A. 1988 May;85(9):2899–2903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. N., Sparks B. E., Walker D. P., Goodin R., Benveniste E. N. Monoclonal idiotypic and anti-idiotypic antibodies produced by immunization with peptides specified by a region of human myelin basic protein mRNA and its complement. J Neuroimmunol. 1989 Apr;22(2):157–166. doi: 10.1016/0165-5728(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Wofsy L., Burr B. The use of affinity chromatography for the specific purification of antibodies and antigens. J Immunol. 1969 Aug;103(2):380–382. [PubMed] [Google Scholar]
- Zhou S. R., Whitaker J. N. An idiotype shared by monoclonal antibodies to different peptides of human myelin basic protein. J Immunol. 1990 Oct 15;145(8):2554–2560. [PubMed] [Google Scholar]
- Zhou S. R., Whitaker J. N., Dwyer D. The modulatory effect of anti-idiotypic antibody on hybridoma cells secreting antibody to human myelin basic protein peptide 80-89. J Neuroimmunol. 1990 Sep-Oct;29(1-3):113–124. doi: 10.1016/0165-5728(90)90153-e. [DOI] [PubMed] [Google Scholar]
- Zhou S. R., Whitaker J. N. Interstrain cross-reactive idiotypes on monoclonal antibodies to an encephalitogenic myelin basic protein peptide. Clin Immunol Immunopathol. 1992 Apr;63(1):74–83. doi: 10.1016/0090-1229(92)90096-7. [DOI] [PubMed] [Google Scholar]
- Zhou S. R., Whitaker J. N. Specific modulation of T cells and murine experimental allergic encephalomyelitis by monoclonal anti-idiotypic antibodies. J Immunol. 1993 Feb 15;150(4):1629–1642. [PubMed] [Google Scholar]



