Abstract
Adjuvant systemic therapy along with screening have been key to the observed improvements in disease-free and overall survival (DFS/OS) in breast cancer. Improvements in overall survival already take into account therapy related toxicities that can result in death. However, this measure alone does not adequately capture the impact on health-related quality of life. Therefore, it is important to examine the prevalence, frequency and short/long-term impact of therapy-related toxicities, identify patients who might be at greatest risk. Ultimately decisions regarding expected therapy benefits (relative and absolute percentage improvements in DFS/OS) must be made against a background of known potential harms. For many patients with early breast cancer (EBC), their risk of recurrence is not zero but is small. At the same time, for many therapies for early stage breast cancer, the risk of serious side effects is small but is not zero. As we better understand the long-term side effects of adjuvant chemotherapy and targeted therapy, it becomes critical to integrate our growing understanding of breast cancer biology with standard high-quality histopathologic measures to better identify the patients most likely to benefit from the various options for combined multimodality therapy. Hence, we must strive against the notion of recommending adjuvant systemic chemotherapy “just in case.” This article focuses on the long-term side effects of adjuvant chemotherapy in patients with EBC.
Introduction
Breast cancer outcomes continue to improve, in great part due to the broader use of screening for earlier detection and of more proficient multimodality care that ultimately result in improved local control and in lower risk of systemic recurrence. Chemotherapy remains the primary systemic adjuvant modality for most women with HER2-positive (combined with trastuzumab) and with triple-negative disease, while endocrine therapy is the core of adjuvant therapy for the two-thirds of patients with early breast cancer (EBC) who are diagnosed with hormone receptor-positive (ER-positive) disease. However, starting in the mid-1980s, evidence from large randomized trials and from large systematic reviews/meta-analyses showed a significant relative improvement in the average risk of recurrence offered by chemotherapy, regardless of stage and ER status.1 Policy statements also heavily influenced the adoption of systemic chemotherapy for most patients with EBC. Patients in turn expressed their willingness to consider adjuvant chemotherapy even if the expected absolute survival benefit did not exceed a few percentage points.
In all meta-analyses involving taxane or anthracycline regimens, proportional reductions in early recurrence, any recurrence, and breast cancer mortality were largely independent of age, nodal status, size, differentiation, or ER status (ER-poor or ER-positive). However, a point often missed is that most patients in those exercises had high or intermediate grade tumors, and few patients had low grade tumors. Therefore, even in strongly ER-positive cases, chemotherapy did at least somewhat affect outcome, though not necessarily to exactly the same extent as in less strongly ER-positive disease.1 These nuances were missed by many. As a result, adjuvant chemotherapy became widely adopted for patients with ER-positive breast cancer, including those with small, node-negative tumors.2
However, most patients with EBC, especially those diagnosed with small, stage 1 disease, are expected to survive their cancer diagnosis. Most will live long enough that other competing causes of death such as cardiovascular disease (CVD) eventually become more important than their prior breast cancer diagnosis. Even among patients with ER-negative disease, long-term follow-up data from the observation arms of adjuvant chemotherapy trials conducted 25 years ago show that most are expected to remain disease-free long-term.3
The most commonly used chemotherapy regimens are associated with small, but not insignificant, short- and long-term risk of complications. This is especially important as breast cancer is a disease of older women, many who have other comorbid conditions. Older women are also at greater risk for loss of function complications associated with the use of adjuvant chemotherapy. However, as a group, they are less likely to be offered adjuvant chemotherapy, even though age alone is a poor predictor of complications, and the observed reduction in breast cancer recurrence offered by adjuvant chemotherapy is equally observed across all age groups.4
Large datasets and long term follow-up of adjuvant trials have allowed investigators to ask clinical questions involving both common and rare outcomes that are of direct interest to patients, practicing oncologists, cancer researchers, and policy makers, although few studies included patient-reported outcome measures.5 Younger and otherwise healthier patients are more likely to be offered adjuvant chemotherapy, but many will remain at risk for late complications. Greater awareness of short- and long-term complications from established adjuvant chemotherapy regimens will allow patients and their health care providers to have more careful discussions on the merits of proposed therapies. High quality tools (including standard pathology measures 6–8) and new molecular measures will continue to aid treatment decisions that must also account for individual risk of recurrence, and weigh in the expected absolute improvements in outcome and absolute risks of toxicities.
Greater Use of Adjuvant Chemotherapy Increases and Improvements in Outcomes
Along with screening, adjuvant systemic therapy has been key to the observed improvements in disease-free and overall survival (DFS/OS) in breast cancer 9 Improvements in survival already take into account death associated with therapy related toxicities. However, complications from systemic therapies like adjuvant chemotherapy may still negatively affect health-related quality of life (HR-QOL). Therefore, it is important to examine the frequency, prevalence, and short/long-term impact on therapy-related toxicities, identify patients who might be at greatest risk, and ultimately individualize decisions regarding expected therapy benefits (relative and absolute percentage improvements in DFS/OS) against known and often fixed rates of potential harms.
Since 1975, there have been marked improvements in breast cancer survival in the US (see Table 1).10 In 2014, there were over 3 million female cancer survivors in the US alone and 41% had a breast cancer diagnosis. Among them, 9% were younger than age 50 while 70% were older than age 60. Most importantly, their overall average 5-year survival was 90%.11 Since the mid-1970s, we have also seen a significant increase in the use of adjuvant chemotherapy, and in 2011 approximately 37% of all patients diagnosed with stage 1–2 breast cancer received it. Not all benefit from it (large “number needed to treat”), and most are expected to survive just with locoregional therapy. Therefore, the decision to offer adjuvant chemotherapy must integrate knowledge about biologic characteristics of an individual tumor, absolute and relative reductions in the hazards of recurrence from potential therapies, known short- and long-term toxicities, and individual comorbidities.
Table 1.
Relative 5-year overall survival rates in the United States 10
| Period | Overall 5-year Relative Survival Rate |
|---|---|
| 1975 – 1977 | 75.8% |
| 2003 – 2009 | 90.3% |
| Stage of Disease | 5-year Relative Survival Rate |
| Local | 98.6% |
| Regional | 84.4% |
| Distant | 24.3% |
An issue with global importance
Cancer’s reach extends beyond developed countries.12 Global surveillance data on cancer survival show a comparable improvement in survival across the world.13 Breast cancer is now the most prevalent adult cancer, even in low- and middle income countries.14 Worldwide, 5.2 million people live with breast cancer, with 40% of them still alive after 4–5 years, compared to ~ 3.2 million survivors each for the 2nd (colorectal) and 3rd (prostate) most prevalent cancers. Data from GLOBOCAN 2012 report an even higher prevalence of 6.23 million cancer survivors, with 3.03 million of them coming from developing nations.15 Therefore, understanding and treating the sequelae of breast cancer therapy becomes even more important in both sheer number and scope.
Chronic/Late Effects from Adjuvant Chemotherapy
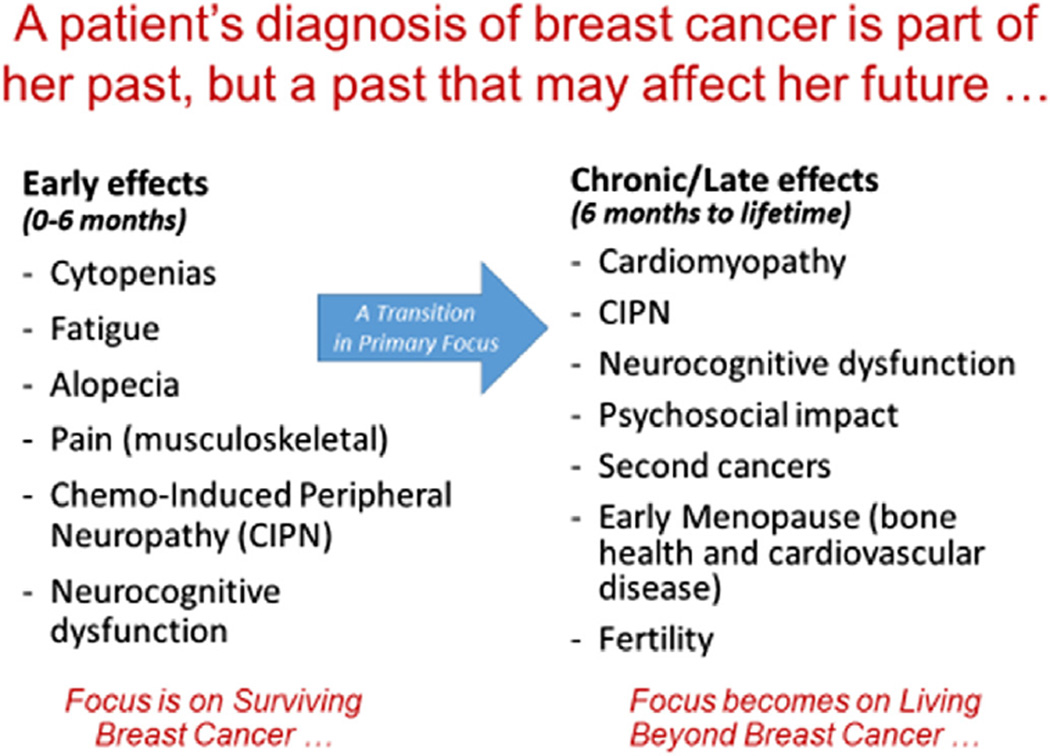
At the time of diagnosis of EBC, patients are understandably concerned about their likelihood of surviving their disease. Decision analysis studies showed early on that patients would be willing to accept a recommendation for adjuvant chemotherapy for a very small absolute percent survival gain.16 At the time of diagnosis, discussions often center on near term toxicities from chemotherapy, like alopecia, nausea and vomiting, fatigue, and myelosupression. But as more patients survived breast cancer, we began to better understand potential long-term and late effects from chemotherapy, including some that could become chronic and quite debilitating (see Figure 1). As such, decisions made about a patient’s treatment not only become part of her past but also heavily influence her future health and HR-QOL. In this article, we review several chronic and late effects from adjuvant chemotherapy.
Figure 1.
Early and Late Toxicities Associated with Adjuvant Chemotherapy
Cardiovascular toxicity
Anthracyclines are established agents of cardiotoxicity, particularly at higher cumulative doses. Epirubicin and doxorubicin may lead to permanent loss of cardiac function, often subclinical, though current adjuvant regimens employ cumulative doses well below the thresholds first established in the late 1970s. 17 The development of adjuvant trastuzumab for HER2-positive breast cancer was initially hampered both by the identification of the HER2 pathway as a critical element in cardiac homeostasis and by excessive cardiotoxicity when co-administered with anthracyclines. Sequential and non-anthracycline trastuzumab-based chemotherapy regimens reduced subsequent risk, though up to 5% of patients initially treated with doxorubicin were then unable to begin trastuzumab therapy due to loss of left ventricular function (LVF), often asymptomatic.18 Reassuringly, long term data from these trials suggest that the cumulative cardiac event rate after 7 years of follow-up is small at ~1.7%.19 Also, while about 8% of adjuvant patients had to interrupt trastuzumab due to cardiac imaging or clinical events, half of them resumed and completed therapy. Older age, low baseline LVF, and history of hypertension are now recognized risk factors associated with an increased risk of cardiotoxicity from trastuzumab following an anthracycline, and many clinicians prefer to avoid anthracycline regimens altogether.20
Still, there is limited long-term knowledge on the late effects from anthracyclines in otherwise asymptomatic patients. An Italian study that randomized 1,000 patients treated with CMF (cyclophosphamide, methotrexate, and 5-fluorouracil) chemotherapy with or without standard doses of doxorubicin located 462 patients who were disease-free after 10 years, and 355 of them underwent echocardiographic evaluation. A higher frequency of asymptomatic systolic dysfunction (8% vs 2%) was detected in those treated with doxorubicin, along with a 1.5% cumulative risk of congestive heart failure (CHF) compared to 0% observed in those treated with just CMF.21 Unfortunately, longer term data on these asymptomatic patients are not available. However, evidence from cardiology studies in non-cancer patients suggests that the presence of asymptomatic LV dysfunction alone increases the risk of death and symptomatic CHF.22 Despite limited data from clinical trials, population-based data using SEER-Medicare showed that older patients have a higher risk of cardiac events when treated with anthracycline-based adjuvant chemotherapy.23, 24 Perhaps more importantly, many patients diagnosed with EBC are at risk for future CVD events, especially those with a high BMI, greater than their risk of cancer recurrence.25
Neurotoxicity and cognitive function
Neurotoxicity is commonly observed with adjuvant taxane regimens, with a frequency of grade 2–4 events that ranges from 13% to 22% in sequential anthracycline-taxane regimens.26 Available evidence indicates no association between toxicity and likelihood of clinical benefit, which then allows clinicians to reduce doses without fear of jeopardizing drug effectiveness.27 Unfortunately, limited options are available to prevent or treat taxane-induced painful neuropathy.28
Cognitive changes have long been observed following adjuvant chemotherapy for breast cancer,29 but studies in this area have been hampered by methodological limitations. In 2011, an international group of investigators issued recommendations on a core set of neuropsychological tests and common criterion for defining cognitive impairment/changes to improve the homogeneity of study methods and study design to allow comparisons across trials including meta-analyses. 30 These barriers were exemplified in a recent systematic review of pharmacologic and non-pharmacologic interventions to manage cognitive alterations, where factors like patient heterogeneity and use of non-standardized neuropsychological outcome measures allowed the authors to only suggest cognitive training and physical activity as promising current interventions.31 A conceptual framework based on models of studying ageing has also been proposed to guide future studies.32
Marrow neoplasm after adjuvant chemotherapy
Leukemia after breast cancer were first reported in 1980s. In the early 2000s, the National Surgical Adjuvant Breast and Bowel Project (NSABP) reported a 0.27% 8-year cumulative incidence of myelodysplastic syndrome (MDS) and/or acute myelogenous leukemia (AML) among patients treated with standard doses of adjuvant doxorubicin and cyclophosphamide (AC).33 Topoisomerase II–targeting and DNA alkylating drugs are known carcinogens with a median latency of 1–3 and 4–6 years, respectively, with the later often preceded by a MDS prodrome. Recently, our group reported that the risk of marrow neoplasm (MN) after standard AC chemotherapy is about twice that previously reported, and with a cumulative incidence that doubled between years 5 and 10 (0.24% to 0.48%) and no apparent plateau.5 Consequently, we concluded that while the MN risk after adjuvant chemotherapy was low, it was higher than previously described. Also, this risk continued to increase beyond 5 years. In the meantime, little information is available regarding the risk of marrow neoplasm with non-anthracycline regimens like docetaxel/cyclophosphamide.
Cessation of menses, menopause, and fertility
Type of adjuvant chemotherapy and age are factors associated with risk of cessation of menses in premenopausal women. On average, approximately 35% of women treated with AC will report amenorrhea 12 months after completion of chemotherapy, a figure that increases to 45% among those also treated with a taxane and to 60% for those treated with CMF.34 Most women younger than age 35 are likely to fully recover menses, while most above age 40 are not. Cessation of menses is associated with a negative impact on HR-QOL in terms of hot flashes, GU symptoms, and sexual dysfunction. Recent data suggest a potential role for LH-RH agonists administered during chemotherapy for women with ER-negative disease in modestly increasing their chances of continuing to menstruate.35, 36 However, chemotherapy-induced amenorrhea (CIA) does not equate menopause, and many patients with CIA remain fertile and resume menses when exposed to an aromatase inhibitor.37
Evidence suggest that women who reported an episode of menstrual bleeding during the 2nd year following start of adjuvant chemotherapy will on average become menopausal three years earlier than planned.38 While limited evidence exist among breast cancer survivors, data from prospective cohorts like the Nurses’ Health Study show a significant increase in the hazard for all-cause mortality and CVD events among women without breast cancer who underwent bilateral oophorectomy before age 50.39 Our analyses of the National Health and Nutrition Examination Survey (NHANES III) also showed a higher age-standardized mortality rate (all-cause and CVD) in women without breast cancer who had bilateral oophorectomy, a risk that was higher in those with a body mass index (BMI) above 30 kg/m2 and especially if they had an oophorectomy before age 40.40 We also observed a higher rate of low bone mineral density in women who had oophorectomies done before age 45 and of arthritis following oophorectomies at any age.41
Ovarian Suppression as an Alternative to Chemotherapy
Almost 120 years ago, Sir George Beatson first suggested that oophorectomy might be used to treat breast cancer.42 Unfortunately, historical accidents favored the use of chemotherapy in the premenopausal setting, and it was not until the late 1980s that the ovarian suppressive effects of chemotherapy in young women were fully recognized. Investigators then postulated that the adjuvant benefits of chemotherapy could in part be due to indirect effects on the ovary leading to estrogen deprivation. This then supported a generation of randomized trials that assessed the efficacy of ovarian suppression compared with chemotherapy or added to chemotherapy.43 Results from the Suppression of Ovarian Function Trial (SOFT) 44 and the Triptorelin with Exemestane or Tamoxifen (TEXT) trial 45 were recently reported.
An interesting observation from the initial published results from SOFT is that in the subgroup of patients selected for chemotherapy based on persistent premenopausal status, ovarian suppression improved outcomes when added to tamoxifen compared to tamoxifen alone, especially among younger patients. Unfortunately, the related Premenopausal Endocrine Responsive Chemotherapy (PERCHE) trial that attempted to answer whether premenopausal women would benefit from the addition of chemotherapy to ovarian suppression with tamoxifen or exemestane (NCT00066807) could not be completed due to poor accrual. Still, available evidence from studies like IBCSG Trial VIII indicate that ovarian function fully recovers after temporary suppression with an LH-RH agonist,46 in contrast to the permanent ablation effects often observed with chemotherapy. This suggests that optimal dual endocrine therapy with an LH-RH agonist is a feasible strategy, as it exploits the breast cancer-related benefits from the temporary suppression of ovarian function but with fewer long-term health-related complications associated with earlier onset of menopause.
Options to Avoid or Reduce Exposure to Adjuvant Chemotherapy
Dual endocrine therapy (LH-RH agonist with tamoxifen or with an aromatase inhibitor), while associated with more acute symptoms from estrogen suppression, could be a reasonable alternative to chemotherapy-based regimens for women with ER-positive early stage breast cancer, particularly for those with strong ER expression who are expected to have endocrine responsive disease. Even before prospective-retrospective data on the clinical utility from a gene expression profiling assay became available,47 data from studies like IBCSG Trial IX had already shown that most patients with ER-positive, node-negative disease gained little benefit from the addition of chemotherapy to endocrine therapy.46, 48
Reducing exposure to chemotherapy is also feasible for carefully selected patients with HER2-positive breast cancer. While only limited data exist on the absolute improvement in DFS/OS offered by adjuvant trastuzumab in node-negative disease, retrospective studies suggest that patients with stage 1 disease (especially those with tumors greater than 0.5 cm) are at risk for recurrence.49 However, tumor size still matters as part of our increased focus on biologically-driven, decision-making in the adjuvant therapy. Consequently, recent efforts to eliminate exposure to anthracyclines while still offering the benefits from combining trastuzumab with adjuvant chemotherapy (in this case, paclitaxel 50) were received with widespread interest.
Triple negative breast cancer is clearly not a homogenous entity, but our ability to individually tailor treatment decisions in this setting remains limited. Although existing guidelines recommend that patients with T1bN0 triple-negative cancers be considered for adjuvant chemotherapy, and those with T1cN0 tumors be offered adjuvant chemotherapy,51 long-term data from trials like NSABP B13 showed that about 50% of patients treated with local therapy alone without chemotherapy remained relapse-free and ~70% were alive after 14 years of follow-up.3
Conclusion
Advances in screening and adjuvant therapy have translated into improved outcomes and the ranks of those with a previous diagnosis of breast cancer continues to build. Many women will survive breast cancer, and be at risk for other comorbid conditions such as CVD, some of which may be a direct sequelae. For many patients with early stage breast cancer, their risk of recurrence is not zero but is very small. At the same time, for many therapies for early stage breast cancer, the risk of side effects is very small but is not zero, and we must improve our ability to identify patients at greater risk for toxicities. As we better understand the long-term side effects of adjuvant chemotherapy in patients with early breast cancer, it is critical that we integrate our growing understanding of breast cancer biology along with standard high-quality histopathologic measures to better identify the patients most likely to benefit from the various combined multimodality therapies available. In regards to adjuvant therapy, we must strive against the notion of recommending adjuvant systemic chemotherapy “just in case.”
Acknowledgement
Support
Supported in part by National Cancer Institute grant CA006973 to the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center and Susan G. Komen for the Cure Grant No. SAC110053 to ACW. We thank Drs. Stuart Russell (Johns Hopkins University, Baltimore, MD) and Ann Partridge (Dana Farber Cancer Institute, Boston, MA) for their helpful discussions with the senior author.
Antonio Wolff has received grants/research supports from BMS and Myriad and received honoraria from Mersana.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers
No conflicts of interest to declare.
Authorship
All authors have directly participated in the planning and data collection. The last author wrote the first draft and all authors edited and approved the final version submitted.
Conflict of interest statement
All other authors have no conflict of interest to disclose.
References
- 1.Early Breast Cancer Trialists' Collaborative G. Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Dignam J, Tan-Chiu E, et al. Prognosis and Treatment of Patients With Breast Tumors of One Centimeter or Less and Negative Axillary Lymph Nodes. Journal of the National Cancer Institute. 2001;93:112–120. doi: 10.1093/jnci/93.2.112. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Jeong JH, Dignam J, et al. Findings from recent national surgical adjuvant breast and bowel project adjuvant studies in stage I breast cancer. J Natl Cancer Inst Monogr. 2001:62–66. doi: 10.1093/oxfordjournals.jncimonographs.a003463. [DOI] [PubMed] [Google Scholar]
- 4.Muss HB. Adjuvant Chemotherapy in Older Women With Breast Cancer: Who and What? Journal of Clinical Oncology. 2014;32:1996–2000. doi: 10.1200/JCO.2013.54.8586. [DOI] [PubMed] [Google Scholar]
- 5.Wolff AC, Blackford AL, Visvanathan K, et al. Risk of marrow neoplasms after adjuvant breast cancer therapy: the national comprehensive cancer network experience. J Clin Oncol. 2015;33:340–348. doi: 10.1200/JCO.2013.54.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 8.Wolff AC, Hammond ME, Hicks DG, et al. Reply to E.A. Rakha et al. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.59.7559. [DOI] [PubMed] [Google Scholar]
- 9.Berry DA, Cronin KA, Plevritis SK, et al. Effect of Screening and Adjuvant Therapy on Mortality from Breast Cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 10.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. Apr, http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- 11.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GF, Chu E. Expanding priorities--confronting chronic disease in countries with low income. N Engl J Med. 2007;356:209–211. doi: 10.1056/NEJMp068182. [DOI] [PubMed] [Google Scholar]
- 13.Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2014 doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. [accessed on 14 March 2015];IARC CancerBase No. 11 [Internet] 2013 Available from: http://globocan.iarc.fr,
- 16.Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol. 1998;16:515–521. doi: 10.1200/JCO.1998.16.2.515. [DOI] [PubMed] [Google Scholar]
- 17.Von Hoff DD, Layard MW, Basa P, et al. Risk Factors for Doxorubicin-Induced Congestive Heart Failure. Annals of Internal Medicine. 1979;91:710. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 18.Perez EA, Suman VJ, Davidson NE, et al. Cardiac Safety Analysis of Doxorubicin and Cyclophosphamide Followed by Paclitaxel With or Without Trastuzumab in the North Central Cancer Treatment Group N9831 Adjuvant Breast Cancer Trial. Journal of Clinical Oncology. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romond EH, Jeong J-H, Rastogi P, et al. Seven-Year Follow-Up Assessment of Cardiac Function in NSABP B-31, a Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Paclitaxel (ACP) With ACP Plus Trastuzumab As Adjuvant Therapy for Patients With Node-Positive, Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. Journal of Clinical Oncology. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slamon D, Eiermann W, Robert N, et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. New England Journal of Medicine. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zambetti M, Moliterni A, Materazzo C, et al. Long-Term Cardiac Sequelae in Operable Breast Cancer Patients Given Adjuvant Chemotherapy With or Without Doxorubicin and Breast Irradiation. Journal of Clinical Oncology. 2001;19:37–43. doi: 10.1200/JCO.2001.19.1.37. [DOI] [PubMed] [Google Scholar]
- 22.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 23.Doyle JJ, Neugut AI, Jacobson JS, et al. Chemotherapy and Cardiotoxicity in Older Breast Cancer Patients: A Population-Based Study. Journal of Clinical Oncology. 2005;23:8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 24.Pinder MC, Duan Z, Goodwin JS, et al. Congestive Heart Failure in Older Women Treated With Adjuvant Anthracycline Chemotherapy for Breast Cancer. Journal of Clinical Oncology. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 25.Bardia A, Arieas ET, Zhang Z, et al. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat. 2012;131:907–914. doi: 10.1007/s10549-011-1843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider BP, Zhao F, Wang M, et al. Neuropathy Is Not Associated With Clinical Outcomes in Patients Receiving Adjuvant Taxane-Containing Therapy for Operable Breast Cancer. Journal of Clinical Oncology. 2012;30:3051–3057. doi: 10.1200/JCO.2011.39.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 29.Brezden CB, Phillips K-A, Abdolell M, et al. Cognitive Function in Breast Cancer Patients Receiving Adjuvant Chemotherapy. Journal of Clinical Oncology. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 30.Wefel JS, Vardy J, Ahles T, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology. 2011;12:703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 31.Chan RJ, McCarthy AL, Devenish J, et al. Systematic review of pharmacologic and non-pharmacologic interventions to manage cognitive alterations after chemotherapy for breast cancer. European journal of cancer. 2015 doi: 10.1016/j.ejca.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Ahles TA, Root JC, Ryan EL. Cancer- and Cancer Treatment–Associated Cognitive Change: An Update on the State of the Science. Journal of Clinical Oncology. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith RE. Risk for the development of treatment-related acute myelocytic leukemia and myelodysplastic syndrome among patients with breast cancer: review of the literature and the National Surgical Adjuvant Breast and Bowel Project experience. Clin Breast Cancer. 2003;4:273–279. doi: 10.3816/cbc.2003.n.032. [DOI] [PubMed] [Google Scholar]
- 34.Petrek JA, Naughton MJ, Case LD, et al. Incidence, Time Course, and Determinants of Menstrual Bleeding After Breast Cancer Treatment: A Prospective Study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 35.Del Mastro L, Boni L, Michelotti A, et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269–276. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 36.Moore HC, Unger JM, Phillips KA, et al. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med. 2015;372:923–932. doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith IE, Dowsett M, Yap Y-S, et al. Adjuvant Aromatase Inhibitors for Early Breast Cancer After Chemotherapy-Induced Amenorrhoea: Caution and Suggested Guidelines. J Clin Oncol. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 38.Partridge A, Gelber S, Gelber RD, et al. Age of menopause among women who remain premenopausal following treatment for early breast cancer: Long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007 doi: 10.1016/j.ejca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Parker WH, Feskanich D, Broder MS, et al. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses' health study. Obstet Gynecol. 2013;121:709–716. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy AM, Menke A, Ouyang P, et al. Bilateral oophorectomy, body mass index, and mortality in U.S. women aged 40 years and older. Cancer Prev Res (Phila) 2012;5:847–854. doi: 10.1158/1940-6207.CAPR-11-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy A, Visvanathan K. Oophorectomy is associated with a higher prevalence of arthritis and lower bone mineral density in women 40 years and older (Abstract P4--11-01) San Antonio Breast Cancer Symposium. 2011 [Google Scholar]
- 42.Beatson G. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:104–107. [PMC free article] [PubMed] [Google Scholar]
- 43.Wolff AC, Davidson NE. Still Waiting After 110 Years: The Optimal Use of Ovarian Ablation As Adjuvant Therapy for Breast Cancer. J Clin Oncol. 2006;24:4949–4951. doi: 10.1200/JCO.2006.07.9160. [DOI] [PubMed] [Google Scholar]
- 44.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.International Breast Cancer Study G. Castiglione-Gertsch M, O'Neill A, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2003;95:1833–1846. doi: 10.1093/jnci/djg119. [DOI] [PubMed] [Google Scholar]
- 47.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 48.Wolff AC, Abeloff MD. Adjuvant chemotherapy for postmenopausal lymph node-negative breast cancer: it ain't necessarily so. J Natl Cancer Inst. 2002;94:1041–1043. doi: 10.1093/jnci/94.14.1041. [DOI] [PubMed] [Google Scholar]
- 49.Curigliano G, Viale G, Bagnardi V, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27:5693–5699. doi: 10.1200/JCO.2009.22.0962. [DOI] [PubMed] [Google Scholar]
- 50.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer. N Engl J Med. 2015;372:134–141. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gradishar WJ, Anderson BO, Blair SL, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw. 2014;12:542–590. doi: 10.6004/jnccn.2014.0058. [DOI] [PubMed] [Google Scholar]