Abstract
Purpose of review
HIV persists in cellular and anatomical reservoirs during antiretroviral therapy (ART). Viral persistence is ensured by a variety of mechanisms including ongoing viral replication and proliferation of latently infected cells. In this review, we summarize recent findings establishing a link between the unresolved levels of inflammation observed in virally suppressed individuals on ART and the mechanisms responsible for HIV persistence.
Recent findings
Residual levels of viral replication during ART are associated with persistent low levels of immune activation, suggesting that unresolved inflammation can promote the replenishment of the HIV reservoir in tissues. In addition, the recent findings that the latent HIV reservoir is maintained by continuous proliferation of latently infected cells provide another mechanism by which residual inflammation could contribute to HIV persistence.
Summary
Residual inflammation during ART is likely to be a critical parameter contributing to HIV persistence. Therefore, reducing inflammation may be an efficient way to interfere with the maintenance of the HIV reservoir in virally suppressed individuals on ART.
Keywords: HIV Reservoir, Immune activation, Immune checkpoint blockers, Proliferation
Introduction
ART dramatically inhibits HIV replication but does not eradicate HIV. The virus persists in cellular and anatomical reservoirs for the lifetime of the individuals receiving ART [1–3]. Two major mechanisms contribute to HIV persistence during ART. First, HIV may still replicate at low levels in anatomical reservoirs in which antiretroviral drugs (ARVs) may not diffuse [4]. Second, the establishment of a small pool of long-lived latently infected cells early in infection, provides the virus with a cellular niche that ensures its maintenance for decades during ART [5, 6].
Several studies have clearly shown that the magnitude of the viral reservoir is strongly associated with the residual levels of immune activation that persists during ART, suggesting that HIV persistence and residual inflammation are interdependent [6–8]. The unresolved inflammation observed in the majority of individuals on ART may contribute to the maintenance of a stable reservoir for HIV by promoting the two aforementioned mechanisms of persistence. Interfering with these mechanisms is now proposed as a strategy to reduce the size of the viral reservoir, a pre-requisite to HIV remission in individuals receiving ART.
Inflammation and the active viral reservoir
Residual levels of viral replication may continuously replenish the latent reservoir during ART [9–13]. Several anatomical compartments such as the gut mucosa, genital tract, lymph nodes and the central nervous system have been shown to be enriched in HIV-infected cells [14–21]. Indeed, the concentrations of ARVs in some of these tissues were found to be lower compared to their levels in peripheral blood, even in suppressed individuals [4, 22, 23], which may allow persistent replication in lymphatic tissues. A recent study of mass spectrometry imaging for quantifying ARVs distribution into tissues showed that the drug tissue distribution was heterogeneous and could vary greatly between tissues within an individual [24]. In this setting, the high CD4+ T cell density in these anatomical sanctuaries combined with lower sensitivity to ART inhibition may favor cell-to-cell HIV transmission [25]. Moreover, residual HIV replication may induce activation of resident cells, which could become more permissive to HIV infection [26], contributing to slow, but continuous, replenishment of viral reservoirs. Despite this conceivable local tissue inflammation, HIV infects abortively resting non-permissive cells that represent >95% of the CD4+ T cell population in lymphoid tissues [27]. Subsequently they die due to an innate immune response against cytosolic viral DNA culminating with pyroptosis, a highly inflammatory form of programmed cell death [28, 29]. Therefore, dying CD4+ T cells release inflammatory signals that could re-activate more latently infected cells and attract more uninfected cells to die and to sustain inflammation creating a pathogenic vicious cycle.
Persistent immune activation and inflammation lead to collagen deposition in the lymphoid tissue and to a progressive disruption of the lymph node architecture [30, 31]. This restricts T cell access to the survival factor IL-7 on the fibroblastic reticular cell network and results in apoptosis and depletion of T cells [32]. Despite this fact, significant accumulation of follicular helper CD4+ T cells (Tfh) has been described in the germinal centrals during HIV [33] or SIV-pathogenic infection [34, 35]. Indeed, Tfh, are 30–40 times more likely to be productively infected than extra follicular CD4+ T cells in untreated individuals [34, 36–39], and significant differences still exist after ART-initiation [37]. The paucity of SIV-specific CD8+ T cells in follicles may protect infected Tfh cells from being killed [35] as shown in elite controllers rhesus monkey. This suggests that potent SIV-specific CD8+ T cells can effectively clear productive infection from extra-follicular sites, but their relative exclusion from B cell follicles limits elimination of infected Tfh [40]. Therefore, these cells may constitute privileged latent reservoirs in ART-treated individuals.
Different ART intensification strategies could reduce residual viral replication through the addition of ARVs with higher tissue penetration (i.e raltegravir) or an anti-inflammatory activity (i.e. Maraviroc). Several raltegravir intensification studies showed changes in markers of persistent viral replication (2-LTR circles, unspliced RNA in CD4+ T cells or replication competent viruses), concomitantly with a reduction in immune activation [11, 12, 41–44]. However, other studies (with different sample size, intensification periods, methodology and sampling frequencies) did not confirm these results [45–48]. Interestingly, CD8+ T-cell activation, specifically CD38 expression on memory cells, is modulated by raltegravir intensification [11, 12, 41, 42, 44, 49–51] and discontinuation [42]. Conversely, the effects on CD4+ T-cell activation are more limited, although systemic redistribution of CD45RA− CD4+ T cells in raltegravir intensified subjects reflects resolution of local tissue inflammation [42, 49]. Both observations suggest that persistent immune activation in CD8+ T cells during suppressive-ART is driven, at least in part, by residual viral infection as a result of incompletely suppressive ART. Furthermore, raltegravir reduces immune activation by increasing the level of viral suppression in a reversible manner. Elite controllers display higher levels of systemic immune activation than uninfected subjects [52], which is decreased by ART [53]. These findings provide indirect, but compelling, evidence of residual viral replication in these individuals.
Several intensification studies using maraviroc, an antagonist of the HIV co-receptor CCR5, have been executed with contradictory results [54–56]. Since maraviroc may exert an anti-inflammatory activity through its ability to inhibit CCR5-mediated chemotaxis and by lowering the threshold for cellular activation, it could reduce the number of activated cells in which residual replication could occur (i.e. gut). Despite this, several authors did not observe a decrease of immune activation [55, 56], but rather an increase, specially in the rectal mucosa [54]. Moreover, it had limited effects on reducing the replication competent reservoir [55]. Likewise, autologous CD4 T cells from HIV-infected subjects were rendered permanently CCR5-dysfunctional by a zinc-finger nuclease (SB-728-T) ex vivo to induce acquired-genetic resistance to HIV infection. The infusion of modified-cell increased durably the levels of circulating CD4 T cells concomitantly with a decrease in HIV DNA [57]. However it is still unclear if the beneficial effect observed is related to the CCR5-genetic modification or to the sustained expansion of HIV-resistant T cell with a memory stem cell-like phenotype [58]
HIV replication disrupts the intestinal epithelial barrier, causing persistent depletion of mucosal CD4+ T cell [15]. This loss of CD4+ T cells is marked by a significant depletion of T helper 17 (Th17) cells [59], which are involved in intestinal epithelial barrier homeostasis as well as in mucosal defense. The disruption of the mucosal integrity promotes the leakage of luminal microbial products to the circulation, stimulating innate immune cells through the TLR pathways, thus contributing to the pro-inflammatory cytokine milieu and systemic immune activation even during suppressive ART [60]. Despite the restoration of Th17 during ART, the functionality of these cells might still be impaired, delaying the normalization of immune activation and microbial translocation [61, 62]. In addition, the sigmoid provirus reservoir in ART-treated subjects is associated with persistently elevated microbial translocation and with impaired restoration of Th17 populations [61]. Early initiation of ART is able to preserve Th17 number and function and fully reversed any initial HIV-related immune activation [63]. Recently, Micci et al. demonstrated the beneficial role of IL-21 in the restoration of CD4+ T cells in the rectal mucosa of virally suppressed SIV infected macaques, which was associated with reduced markers of viral persistence [64].
Many co-infections non-specifically activate the host immune system and several organisms can directly facilitate HIV replication [65, 66]. Persistent and intermittent CMV replication causes bystander activation [67], thereby potentially generating more activated CD4+ T cells for HIV replication. Even asymptomatic shedding of CMV in the genital tract from HIV-infected individuals has been associated with increased T-cell activation and proliferation in blood and increased levels of total HIV DNA in both ART-naïve [68, 69] and ART-suppressed individuals [70]. Importantly, valganciclovir, an agent with potent activity against CMV and most other herpesviruses, in combination with ART has been shown to significantly reduce CD8+ T-cell activation and this effect persisted even after stopping the drug [71]. Larger studies will be needed to determine the clinical benefit of treatment of CMV and its effect on HIV persistence.
Inflammation and the latent reservoir
As previously mentioned, inflammation is associated with T-cell activation and proliferation during HIV infection, even during ART. The frequency of CD4+ T cells harbouring integrated HIV DNA is associated with the frequency of proliferating CD4+ T cells (measured by Ki67) in individual receiving ART [6]. The direct relationship between T-cell proliferation and HIV persistence has recently been reported in two seminal studies. Maldarelli et al. and Wagner et al. identified specific HIV integration sites linked to clonal expansion of HIV-infected cells [72, 73]. Integrations of proviruses were frequently found into genes associated with cancers or cell cycle regulation, promoting the persistence of HIV-infected cells through proliferation and increased survival. The limited number of subjects extensively analyzed in these two studies did not allow assessing the contribution of ongoing T-cell activation, homeostatic proliferation or antigen-induced expansion to HIV persistence. The replication capacity of the clonally expanded provirus is still under debate. In their study combining integration sites analysis with viral sequencing, Cohn et al. did not identify functional viral sequences in the clonally expanded proviruses [74].
Interestingly, two studies reinforced the concept of proliferation as a mechanism of viral persistence in memory CD4+ T cells in blood and lymphoid tissues during ART [75, 76]. In-depth longitudinal phylogenetic analysis of plasma and cell-associated viruses revealed that a majority of viral sequences are identical, suggesting that HIV persists through cell proliferation rather than through ongoing replication in these individuals. Further investigations will be needed to establish how inflammation would favor one or the other mechanism. Additionally, effector memory CD4+ T cells more frequently harbor proviruses with identical sequences than less differentiated CD4+ T-cell subsets, indicating that clonal expansion of HIV-infected cells is a characteristic of differentiated cells. Higher frequencies of these differentiated cells were observed in virally suppressed individuals with persistent lymphopenia [77] suggesting that homeostatic proliferation may drive HIV persistence in this group of subjects. IL-7 is a major contributor to CD4+ T cell proliferation in vivo [78]. IL-7 administration has been reported to increase the size HIV reservoir pool by induction of CD4+ T-cell proliferation without increasing genetic diversity of the proviral population [79]. This study clearly demonstrates the contribution of IL-7-induced proliferation to the maintenance of latently infected cells during ART.
Overall, it remains difficult to determine whether the clonally expanded reservoir is the result of homeostatic or antigen-induced proliferation. While, the restricted number of highly expanded clone supports antigenic expansions of a restricted number of clonotypes, the association between CD4+ T-cell depletion, IL-7 levels and reservoir persistence strongly support a role for homeostatic proliferation in that process.
The inflammatory environment not only promotes T-cell proliferation but also stimulates a compensatory response aimed at breaking the inflammatory vicious circle. Immune checkpoint molecules (ICs) are key players in this arsenal of regulatory factors by modulating the duration and magnitude of immune responses. PD-1, the archetype immune checkpoint, is highly expressed on CD4+ and CD8+ T cells during HIV infection and its expression is not fully normalized by ART [80]. During ART, PD-1 expression is related to CD4+ T cell homeostasis as suggested by its association with CD4+ T cell count [80] and its up regulation by γ-c-cytokines such as IL-7 [81]. In addition, the expression of PD-1 on CD4+ T cells is associated with virological markers of HIV persistence suggesting a connection between PD-1 and HIV persistence [6, 7]. Therefore, PD-1 may regulate the interplay between T cell proliferation and HIV persistence. PD-1 and two additional ICs, LAG-3 and TIGIT, were identified as markers of HIV infected CD4+ T cells during ART, suggesting a direct role for ICs in HIV persistence [82]. Future studies will be needed to better characterize the relationship between ICs and HIV persistence in tissues, as the expression of these molecules is tightly regulated by the microenvironment. For instance, CTLA-4 is mainly expressed by CD4+ T cells in gastrointestinal tissues and remains elevated during ART [83]. It is currently difficult to determine if the association between ICs and HIV persistence reflects a cause or a consequence. The impact of the blockade of these pathways by IC blockers (ICBs) on HIV persistence will certainly be informative.
T regulatory CD4+ T cells (Tregs) are a key modulator of the inflammatory pathways. The suppressive capacity of Tregs is influenced by several factors, such as IL-2, inhibitory cytokines (IL-10, TGF-β, or IL-35) and ICs such as CTLA-4 and LAG-3 [84, 85]. In lymphopenic ART individuals, the frequency of Tregs is associated with markers of activation [86] and Tregs may act as a preferential reservoir for HIV during ART [87].
A complex balance between pro-inflammatory cytokines and anti-inflammatory cytokines determine the magnitude and the duration of inflammation. Recently, IL-37, an anti-inflammatory cytokine, has been found to be up regulated during HIV infection but not normalized by ART [88]. An association between IL-37 transcripts and HIV DNA suggests a relationship between HIV persistence and the anti-inflammatory response. Further investigations are needed to fully characterize the role of anti-inflammatory cytokines such as IL-10 and TGF-β in HIV persistence during ART.
Clinical interventions: breaking the vicious circle
Since residual inflammation during ART is likely to be a critical parameter contributing to HIV persistence, its reduction may efficiently interfere with the maintenance of the HIV reservoir.
During ART, persistent low levels of viral transcription occurring in latently infected cells can generate transcripts [89, 90] and maybe proteins [91] that are likely to contribute to the unresolved inflammation observed in suppressed individuals. Current ARVs do not inhibit viral transcription from an integrated viral genome and do not prevent reactivation from latency nor the inflammation that results from this phenomenon. Recently, Mousseau et al. identified a novel Tat-inhibitor (didehydro-cortistatin, dCA) that has the ability to prevent reactivation of viral transcription after potent cell stimulation [92]. In addition to its direct beneficial effect through inhibition of residual viral replication, treatment with dCA may also decrease the production of incomplete viral products that stimulate the innate and adaptive immune responses, thereby reducing inflammation, which would in turn decrease the proliferation of latently infected cells. Therefore, one can hypothesize that dCA may have beneficial effects on both the active and latent reservoirs. This promising novel approach is still at its development stage and the clinical potential and toxicity of dCA are currently unknown. Nonetheless, this innovative approach appears to be unique as it may significantly impact inflammation by targeting the virus, a possible cause of inflammation, rather than its consequence.
While anti-inflammatory therapeutic strategies have been used with the aim of restoring the CD4 compartment or reducing HIV co-morbidities [93], their impact on HIV persistence is a relatively novel area of research. Whether a decrease in the levels of residual inflammation and fibrosis can significantly reduce HIV persistence is currently explored in vivo by administering agents with specific anti-inflammatory and anti-fibrotic actions such as the angiotensin II blocker losartan to ART-suppressed individuals (NCT01852942). Telmisartan, which has a similar mechanism of action, is currently used in a phase I clinical study in acute HIV infection to determine if biological markers of immune activation in the blood and cerebrospinal fluid can be reduced to limit the spread of HIV reservoirs early in infection (NCT02170246).
Immunosuppressant are also considered as adjunct therapy to ART to reduce inflammation, which may impede HIV persistence. Sirolimus (rapamycin) inhibits a key regulatory kinase that controls cell-cycle progression (mTOR). Stock et al. explored the impact of sirolimus following kidney transplantation [94]. Sirolimus use was associated with lower post-transplant HIV DNA levels, suggesting that immunosuppressant may affect the level of HIV persistence during effective therapy. Of note, this effect was not observed in virally suppressed individuals who received cyclosporine [95]. The precise mechanism by which sirolimus exerts this beneficial effect is still unknown and will be evaluated in an ongoing clinical trial (NCT02440789). Everolimus, another mTOR inhibitor, will also be evaluated for its capacity to interfere with HIV persistence in virally suppressed individuals after kidney or liver transplant (NCT02429869).
Janus kinase (JAK) inhibitors, another class of anti-inflammatory molecules indicated for the treatment rheumatoid arthritis, myelofibrosis and other type of cancers, may also have an impact on inflammation and HIV persistence during ART. Gavegnano et al. reported that ruxolitinib and tofacitinib, two inhibitors with selectivity for subtypes JAK1/2 can potently inhibit viral reactivation in vitro [96]. This suggests that in addition to their direct anti-inflammatory effects, these molecules may also directly act on the virus life cycle, and like dCA, may prevent residual levels of viral production from the reservoir. The effect of ruxolitinib on residual inflammation and HIV persistence is currently evaluated in vivo (NCT02475655).
The aforementioned anti-inflammatory strategies were designed to reduce viral persistence during ART. However, a transient increase in the level of immune activation may also reflect an efficient reactivation of the latent reservoir, as desired outcome in shock and kill curative strategies. Indeed, recent data from a clinical trial using the HDACi panobinostat indicate that innate immune factors, such as natural killer cells, plasmacytoid dendritic cells, and the expression patterns of interferon-stimulated genes, are associated with a decline in the HIV reservoir measured by viral DNA [97]. This suggests that innate immunity may play an important role in reducing the residual reservoir of HIV-infected cells or that innate immune functions are a sensitive sensor of viral production induced by latency reversing agents during ART.
Another example of a transient increase in the level of immune activation that may result in a decrease in the level of viral persistence is given by the manipulation of ICs. Several ICBs have been successfully used in the treatment of cancer, particularly those targeting the PD-1 and CTLA-4 receptors [98–100]. This has led to the idea of using these antibodies to restore HIV-specific function in HIV infection, which will likely be a key component of a successful eradication strategy [101]. A single case report study has evaluated the in vivo effects of CTLA-4 blockade (ipilimumab) on the HIV reservoir in an HIV-infected individual with metastatic melanoma [102]. Treatment with ipilimumab induced a marked increase in cell-associated unspliced HIV RNA and was associated with a subsequent decline in plasma HIV RNA. Concomitantly, increases in CD4+ T-cell activation as measured by HLA-DR, CD38 and CCR5 expression were noted. Whether these increases reflect a direct enhancement of T-cell function following CTLA-4 blockade or antigen-induced T-cell responses following viral reactivation remains unclear. Nonetheless, this study provides the first evidence that ICBs can be used to perturb the HIV reservoir and modify immune activation levels.
Conclusion
Viral persistence and residual inflammation are interdependent and fuel each other in a “vicious circle” that seems difficult to interrupt (Figure 1). Therapeutic strategies that will tackle residual levels of viral production will certainly help in assessing the impact of residual HIV on inflammation. Conversely, the results from several ongoing clinical studies will be critical to understand the role played by inflammation on HIV persistence. Most likely, breaking the vicious circle will require targeting both phenomena concomitantly. If the panel of anti-inflammatory molecules available is relatively large, there is currently no drug available to prevent continuous production of viral products from the reservoir, a likely cause of residual inflammation. The development of such novel antivirals will certainly be a major weapon of the therapeutic arsenal aimed at reducing inflammation during ART.
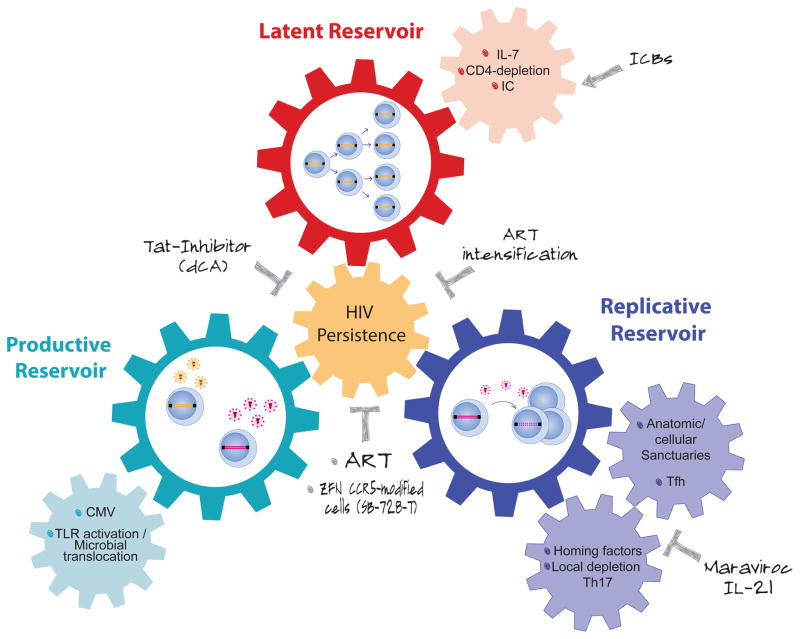
Figure 1.
In HIV-infected, ART-suppressed subjects, the latent reservoir (red wheel) is maintained by continuous proliferation of latently infected cells, which can produce viral particles upon activation constituting the productive reservoir (turquoise wheel). When reactivated cells are in anatomical/cellular sanctuaries, where ART cannot diffuse efficiently, residuals levels of viral replication can occur (blue wheel), fueling continuously the latent reservoir. Increased levels of immune activation and/or inflammation promote the three mechanisms of HIV persistence (small wheels). Several therapeutic strategies designed to act directly on viral production/replication or to modulate the immune cells could be used to interrupt this vicious circle.
Key points.
HIV persistence and residual inflammation are interdependent mechanisms.
Anatomical and cellular sanctuaries (low ARV penetration in tissues and Tfh, respectively) may favor residual levels of replication in tissues during ART. The local inflammation could re-activate more latently infected cells and attract more uninfected cells into the lymphoid tissue to sustain inflammation and residual replication.
Homeostatic and antigen-induced proliferation of latently infected cells contribute to HIV persistence during ART.
Interventions aiming to block viral production/replication or to modulate immune cells could be used to break this vicious-cycle
Acknowledgments
Financial support
This work was supported by NIH grant 1R21AI113096, by the Delaney AIDS Research Enterprise (DARE) to Find a Cure 1U19AI096109 and by the Foundation for AIDS Research (amfAR Research Consortium on HIV Eradication (108928-56-RGRL). MM and RF are supported by Beatriu de Pinos (AGAUR) and amfAR (108264-51-RFRL) post-doctoral fellowships, respectively.
None
Footnotes
Conflict of interest
MM, RF and NC do not have any commercial or other associations that might pose a conflict of interest.
References
- 1.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 2.Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proceedings of the National Academy of Sciences. 2014;111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 6.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature Publishing Group. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatano H, Jain V, Hunt PW, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208:50–56. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockerham LR, Siliciano JD, Sinclair E, et al. CD4+ and CD8+ T Cell Activation Are Associated with HIV DNA in Resting CD4+ T Cells. Plos One. 2014;9:e110731. doi: 10.1371/journal.pone.0110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun T-W, Justement JS, Moir S, Hallahan CW, Maenza J, Mullins JI, Collier AC, Corey L, Fauci AS. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–1764. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 10.Sigal A, Baltimore D. As Good As It Gets? The Problem of HIV Persistence despite Antiretroviral Drugs. Cell Host Microbe. 2012;12:132–138. doi: 10.1016/j.chom.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Buzon MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 12.Yukl SA, Shergill AK, Mcquaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS (London, England) 2010;24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun T-W, Nickle DC, Justement JS, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. Journal of Clinical Investigation. 2005;115:3250–3255. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yukl SA, Gianella S, Sinclair E, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–1561. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deleage C, Moreau M, Rioux-Leclercq N, Ruffault A, Jégou B, Dejucq-Rainsford N. Human Immunodeficiency Virus Infects Human Seminal Vesicles in Vitro and in Vivo. Am J Pathol. 2011;179:2397–2408. doi: 10.1016/j.ajpath.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, Orenstein JM, Kotler DP, Fauci AS. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 18.North TW, Higgins J, Deere JD, Hayes TL, Villalobos A, Adamson L, Shacklett BL, Schinazi RF, Luciw PA. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. Journal of virology. 2010;84:2913–2922. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheth PM, Chege D, Shin LYY, et al. Immune reconstitution in the sigmoid colon after long-term HIV therapy. Mucosal immunology. 2008;1:382–388. doi: 10.1038/mi.2008.23. [DOI] [PubMed] [Google Scholar]
- 20.Chun T-W, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 21.Rothenberger MK, Keele BF, Wietgrefe SW, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proceedings of the National Academy of Sciences. 2015;112:E1126–34. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. Journal of Clinical Investigation. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the fetal compartment (placenta and amniotic fluid) Antivir Ther (Lond) 2011;16:1139–1147. doi: 10.3851/IMP1918. [DOI] [PubMed] [Google Scholar]
- 24.Thompson CG, Bokhart MT, Sykes C, Adamson L, Fedoriw Y, Luciw PA, Muddiman DC, Kashuba ADM, Rosen EP. Mass spectrometry imaging reveals heterogeneous efavirenz distribution within putative HIV reservoirs. Antimicrob Agents Chemother. 2015;59:2944–2948. doi: 10.1128/AAC.04952-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011:1–5. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 26.Biancotto A, Iglehart SJ, Vanpouille C, Condack CE, Lisco A, Ruecker E, Hirsch I, Margolis LB, Grivel J-C. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111:699–704. doi: 10.1182/blood-2007-05-088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doitsh G, Galloway NLK, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monroe KM, Yang Z, Johnson JR, Geng X, Doitsh G, Krogan NJ, Greene WC. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343:428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, Haase AT. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. Journal of Clinical Investigation. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, Skarda D, Larson M, Douek DC, Haase AT. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clinical and Vaccine Immunology. 2006;13:556–560. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindqvist M, van Lunzen J, Soghoian DZ, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrovas C, Yamamoto T, Gerner MY, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial Alterations between CD4+ T Follicular Helper, B, and CD8+ T Cells during Simian Immunodeficiency Virus Infection: T/B Cell Homeostasis, Activation, and Potential Mechanism for Viral Escape. The Journal of Immunology. 2012 doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connick E, Mattila T, Folkvord JM, et al. CTL Fail to Accumulate at Sites of HIV-1 Replication in Lymphoid Tissue. The Journal of Immunology. 2007;178:6975–6983. doi: 10.4049/jimmunol.178.11.6975. [DOI] [PubMed] [Google Scholar]
- 37.Perreau M, Savoye A-L, De Crignis E, Corpataux J-M, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. Journal of Experimental Medicine. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu GJ, Kula T, Xu Q, et al. Comprehensive serological profiling of human populations using a synthetic human virome. Science. 2015;348:aaa0698–aaa0698. doi: 10.1126/science.aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folkvord JM, Armon C, Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS Res Hum Retroviruses. 2005;21:363–370. doi: 10.1089/aid.2005.21.363. [DOI] [PubMed] [Google Scholar]
- 40.Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nature Publishing Group. 2015;21:132–139. doi: 10.1038/nm.3781. This study reveals the relative exclusion of CD8+ effector T cells from B cell follicles and the ability of SIV to persist and preferentially replicate within CD4+ Tfh cells, thereby escaping highly potent CD8+ T cell responses. Therapies intended to eradicate HIV infection will almost certainly need to overcome the B follicular sanctuary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llibre JM, Buzon MJ, Massanella M, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther (Lond) 2012;17:355–364. doi: 10.3851/IMP1917. [DOI] [PubMed] [Google Scholar]
- 42.Massanella M, Esteve A, Buzon MJ, et al. Dynamics of CD8 T-Cell Activation After Discontinuation of HIV Treatment Intensification. J Acquir Immune Defic Syndr. 2013;63:152–160. doi: 10.1097/QAI.0b013e318289439a. [DOI] [PubMed] [Google Scholar]
- 43.Hatano H, Strain MC, Scherzer R, et al. Increase in 2-Long Terminal Repeat Circles and Decrease in D-dimer After Raltegravir Intensification in Patients With Treated HIV Infection: A Randomized, Placebo-Controlled Trial. J Infect Dis. 2013;208:1436–1442. doi: 10.1093/infdis/jit453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallejo A, Gutiérrez C, Hernández-Novoa B, et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS. 2012;26:1885–1894. doi: 10.1097/QAD.0b013e3283584521. [DOI] [PubMed] [Google Scholar]
- 45.Gandhi RT, Coombs RW, Chan ES, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59:229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gandhi RT, Bosch RJ, Aga E, et al. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J Infect Dis. 2010;201:293–296. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010 doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203:960–968. doi: 10.1093/infdis/jiq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Massanella M, Negredo E, Puig J, Puertas MC, Buzon MJ, Pérez-Álvarez N, Carrillo J, Clotet B, Martinez-Picado J, Blanco J. Raltegravir intensification shows differing effects on CD8 and CD4 T cells in HIV-infected HAART-suppressed individuals with poor CD4 T-cell recovery. AIDS. 2012;26:2285–2293. doi: 10.1097/QAD.0b013e328359f20f. [DOI] [PubMed] [Google Scholar]
- 50.Negredo E, Massanella M, Puertas MC, et al. Early but limited effects of raltegravir intensification on CD4 T cell reconstitution in HIV-infected patients with an immunodiscordant response to antiretroviral therapy. J Antimicrob Chemother. 2013;68:2358–2362. doi: 10.1093/jac/dkt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichtenstein KA, Armon C, Nagabhushanam V, Efaw BJ, Frazer-Abel A, Hiserote ME, Alam R. A pilot study to assess inflammatory biomarker changes when raltegravir is added to a virologically suppressive HAART regimen in HIV-1-infected patients with limited immunological responses. Antivir Ther (Lond) 2012;17:1301–1309. doi: 10.3851/IMP2350. [DOI] [PubMed] [Google Scholar]
- 52.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatano H, Yukl SA, Ferre AL, et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog. 2013;9:e1003691. doi: 10.1371/journal.ppat.1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunt PW, Shulman NS, Hayes TL, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood. 2013;121:4635–4646. doi: 10.1182/blood-2012-06-436345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gutiérrez C, Díaz L, Vallejo A, et al. Intensification of Antiretroviral Therapy with a CCR5 Antagonist in Patients with Chronic HIV-1 Infection: Effect on T Cells Latently Infected. Plos One. 2011;6:e27864. doi: 10.1371/journal.pone.0027864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beliakova-Bethell N, Jain S, Woelk CH, et al. Maraviroc intensification in patients with suppressed HIV viremia has limited effects on CD4+ T cell recovery and gene expression. Antiviral Res. 2014;107:42–49. doi: 10.1016/j.antiviral.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeidan J, Lee G, Fourati S, Wang S, Giedlin MA, Nichol GM, Tang W, Chomont N, Deeks SG, Sekaly RP. Decay of the HIV Reservoir Post Autologous Transfer of ZFN CCR5 Modified CD4 T Cells (SB-728-T) Correlates with Generation of a T Memory Stem Cell-Like Population and Enhanced HIV-Specific CD8 T Cell Polyfunctionality. Keystone Symposia. Mechanisms of HIV Persistence: Implications for a Cure 2015 [Google Scholar]
- 59.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 61.Chege D, Sheth PM, Kain T, et al. Sigmoid Th17 populations, the HIV latent reservoir, and microbial translocation in men on long-term antiretroviral therapy. AIDS (London, England) 2011;25:741–749. doi: 10.1097/QAD.0b013e328344cefb. [DOI] [PubMed] [Google Scholar]
- 62.Kim CJ, McKinnon LR, Kovacs C, et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. The Journal of Immunology. 2013;191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 63.Schuetz A, Deleage C, Sereti I, et al. PLOS Pathogens: Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PLoS Pathog. 2014;10:e1004543. doi: 10.1371/journal.ppat.1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Micci L, Ryan ES, Fromentin R, et al. Interleukin-21 decreases residual inflammation and reduces the virus reservoir in ART-treated, SIV-infected rhesus macaques. Journal of Clinical Investigation. 2015 doi: 10.1172/JCI81400. in press This study provides a proof of concept for supplementing ART with IL-21 to improve disease prognosis in infected individuals. IL-21 supplementation is associated with long-term benefits with a reduction of residual immune activation and the levels of viral reservoir in ART-treated SIV-infected rhesus macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gianella S, Massanella M, Wertheim JO, Smith DM. The Sordid Affair Between Human Herpesvirus and HIV. J Infect Dis. 2015 doi: 10.1093/infdis/jiv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. The Lancet Infectious Diseases. 2010;10:455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. Journal of virology. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gianella S, Strain MC, Rought SE, Vargas MV, Little SJ, Richman DD, Spina CA, Smith DM. Associations between Virologic and Immunologic Dynamics in Blood and in the Male Genital Tract. Journal of virology. 2012;86:1307–1315. doi: 10.1128/JVI.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gianella S, Anderson CM, Vargas MV, Richman DD, Little SJ, Morris SR, Smith DM. Cytomegalovirus DNA in Semen and Blood Is Associated With Higher Levels of Proviral HIV DNA. J Infect Dis. 2013;207:898–902. doi: 10.1093/infdis/jis777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gianella S, Massanella M, Richman DD, et al. Cytomegalovirus replication in semen is associated with higher levels of proviral HIV DNA and CD4+ T cell activation during antiretroviral treatment. Journal of virology. 2014;88:7818–7827. doi: 10.1128/JVI.00831-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, Tracy RP, Corey L, Deeks SG. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maldarelli F, Wu X, Su L, et al. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345:179–183. doi: 10.1126/science.1254194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wagner TA, McLaughlin S, Garg K, Cheung CYK, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, Frenkel LM. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345:570–573. doi: 10.1126/science.1256304. These two studies identify specific HIV integration sites linked to clonal expansion of HIV-infected cells demonstrating for the first time the direct relationship between T-cell proliferation and HIV persistence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohn LB, Silva IT, Oliveira TY, et al. HIV-1 Integration Landscape during Latent and Active Infection. Cell. 2015;160:420–432. doi: 10.1016/j.cell.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Josefsson L, Stockenstrom von S, Faria NR, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proceedings of the National Academy of Sciences. 2013;110:E4987–96. doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stockenstrom von S, Odevall L, Lee E, et al. Longitudinal Genetic Characterization Reveals That Cell Proliferation Maintains a Persistent HIV Type 1 DNA Pool During Effective HIV Therapy. J Infect Dis. 2015;212:596–607. doi: 10.1093/infdis/jiv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Massanella M, Gómez-Mora E, Carrillo J, Curriu M, Ouchi D, Puig J, Negredo E, Cabrera C, Clotet B, Blanco J. Increased ex vivo cell death of central memory CD4 T cells in treated HIV infected individuals with unsatisfactory immune recovery. Journal of Translational Medicine. 2015;13:141. doi: 10.1186/s12967-015-0601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camargo JF, Kulkarni H, Agan BK, et al. Responsiveness of T cells to interleukin-7 is associated with higher CD4+ T cell counts in HIV-1-positive individuals with highly active antiretroviral therapy-induced viral load suppression. J Infect Dis. 2009;199:1872–1882. doi: 10.1086/598858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vandergeeten C, Fromentin R, DaFonseca S, Lawani MB, Sereti I, Lederman MM, Ramgopal M, Routy J-P, Sékaly R-P, Chomont N. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. 2013;121:4321–4329. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cockerham LR, Jain V, Sinclair E, et al. Programmed death-1 expression on CD4+ and CD8+ T cells in treated and untreated HIV disease. AIDS (London, England) 2014;28:1749–1758. doi: 10.1097/QAD.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. The Journal of Immunology. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 82.Fromentin R, Bakeman W, Lawani MB, et al. The Immune Checkpoints PD-1, LAG-3 and TIGIT are Biomarkers of HIV Infected Cells During ART and Identify Distinct Cellular Reservoirs. Towards an HIV cure Pre-conference Symposium.2014. [Google Scholar]
- 83.Yukl SA, Shergill AK, Girling V, et al. Site-Specific Differences in T Cell Frequencies and Phenotypes in the Blood and Gut of HIV-Uninfected and ART-Treated HIV+ Adults. Plos One. 2015;10:e0121290. doi: 10.1371/journal.pone.0121290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang C-T, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 86.Saison J, Ferry T, Demaret J, et al. Association between discordant immunological response to highly active anti-retroviral therapy, regulatory T cell percentage, immune cell activation and very low-level viraemia in HIV-infected patients. Clin Exp Immunol. 2014;176:401–409. doi: 10.1111/cei.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran T-A, de Goër de Herve M-G, Hendel-Chavez H, et al. Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. Plos One. 2008;3:e3305. doi: 10.1371/journal.pone.0003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Højen JF, Rasmussen TA, Andersen KLD, et al. Interleukin-37 Expression Is Increased in Chronic HIV-1-Infected Individuals and Is Associated with Inflammation and the Size of the Total Viral Reservoir. Mol Med. 2015;21:337–345. doi: 10.2119/molmed.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmid A, Gianella S, Wyl von V, et al. Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. Plos One. 2010;5:e13310. doi: 10.1371/journal.pone.0013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pasternak AO, Adema KW, Bakker M, Jurriaans S, Berkhout B, Cornelissen M, Lukashov VV. Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol. 2008;46:2206–2211. doi: 10.1128/JCM.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Graf EH, Pace MJ, Peterson BA, et al. Gag-positive reservoir cells are susceptible to HIV-specific cytotoxic T lymphocyte mediated clearance in vitro and can be detected in vivo [corrected] Plos One. 2013;8:e71879. doi: 10.1371/journal.pone.0071879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mousseau G, Kessing CF, Fromentin R, Trautmann L, Chomont N, Valente ST. The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. MBio. 2015;6:e00465. doi: 10.1128/mBio.00465-15. This study reports that the Tat inhibitor didehydro-cortistatin A (dCA), unlike other antiretrovirals, reduces residual levels of viral transcription in several models of HIV latency. dCA combined with ART may inhibit ongoing viral replication, reactivation, and replenishment of the latent viral reservoir. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandler NG, Sereti I. Can early therapy reduce inflammation? Curr Opin HIV AIDS. 2014;9:72–79. doi: 10.1097/COH.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stock PG, Barin B, Hatano H, Rogers RL, Roland ME, Lee TH, Busch M, Deeks SG for Solid Organ Transplantation in HIV Study Investigators. Reduction of HIV persistence following transplantation in HIV-infected kidney transplant recipients. Am J Transplant. 2014;14:1136–1141. doi: 10.1111/ajt.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Markowitz M, Vaida F, Hare CB, et al. The virologic and immunologic effects of cyclosporine as an adjunct to antiretroviral therapy in patients treated during acute and early HIV-1 infection. J Infect Dis. 2010;201:1298–1302. doi: 10.1086/651664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gavegnano C, Detorio M, Montero C, Bosque A, Planelles V, Schinazi RF. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob Agents Chemother. 2014;58:1977–1986. doi: 10.1128/AAC.02496-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olesen R, Vigano S, Rasmussen TA, et al. Innate Immune Activity Correlates with CD4 T Cell-Associated HIV-1 DNA Decline during Latency-Reversing Treatment with Panobinostat. Journal of virology. 2015;89:10176–10189. doi: 10.1128/JVI.01484-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang H-C, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012:1–11. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, Palmer S, Mcneil C, Garsia R, Lewin SR. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS (London, England) 2015;29:504–506. doi: 10.1097/QAD.0000000000000562. This study is the first to show that that the administration of an ICB (anti-CTLA-4, ipilimumab) perturbs the latent HIV reservoir in a virally suppressed individuals with metastatic melanoma. Reactivation from the latent reservoir was associated with increased levels of T cell activation. [DOI] [PMC free article] [PubMed] [Google Scholar]