Abstract
Background & Aims
Liver-related complications such as hepatocellular carcinoma (HCC) are a major cause of morbidity and mortality in individuals infected with HIV, particularly among those also infected with hepatitis B or hepatitis C viruses. There is a lack of consensus regarding the clinical presentation, treatment options, and outcomes in HIV-infected patients with HCC. We compared the clinical presentation, treatment, and survival of patients with HCC, with and without HIV infection.
Methods
We conducted a retrospective cohort study of cirrhotic patients diagnosed with HCC at a large safety net hospital between January 2005 and December 2010. Patients without known HIV serologic status were excluded. Demographic features, tumor characteristics, treatment regimens, and survival were compared between patients (n=26) with and without HIV infection (n=164). Survival curves were generated using Kaplan-Meier plots and compared using the log rank test.
Results
A higher percentage of HIV-infected patients presented with compensated liver disease (Child-Turcotte-Pugh stage A) than those without HIV infection (62% vs 32%; P=.01), as well with early-stage tumors (Barcelona Clinic Liver Cancer stage A: 39% vs 17%; P =.04 and Okuda stage I: 50% vs 21%; P<.01). HIV-infected patients were more likely to be cured of HCC than uninfected patients (27% vs 4%; P=.01), but median overall survival times were similar between groups (9.6 months vs 5.2 months; P=0.85). The 1-year rates of survival for HIV-infected and uninfected patients were 40% and 38%, respectively.
Conclusions
HIV-infected patients present with earlier-stage HCC and more preserved liver function than uninfected patients, resulting in more curative treatment options. Despite this difference, overall survival was similar between patients with HCC with and without HIV infection.
Keywords: co-infection, prognosis, HBV, HCV, liver cancer
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and is responsible for more than 500,000 deaths annually. (1) The incidence and mortality of HCC have increased three-fold over the past two decades, and it is the fastest growing cause of cancer-related deaths in the United States. (2)
Since the widespread availability of highly active antiretroviral therapy (HAART) in 1996, more than 60% of deaths in HIV-infected patients are from causes other than HIV/AIDS. (5) Given that HIV-infected patients are now living longer and the high prevalence of co-infection with either hepatitis B virus (HBV) and/or hepatitis C virus (HCV), it is not surprising that sequela of chronic liver disease is an increasing factor of morbidity and mortality. (4,6) The risk for HCC is seven-fold higher in HIV-infected than in HIV-uninfected patients and is increasing in incidence. (3) The updated French Mortalite 2005 study demonstrated that the incidence of HCC-related deaths in HIV-infected patients increased from 15% in 2000 to 25% in 2005 with underlying HCV co-infection in the majority of deaths. (3)
Early reports comparing presentation, treatment and outcome parameters in HCC patients with known HIV serostatus suggested that HIV-infected and -uninfected patients have a similar stage of tumor presentation and median survival, but HIV-infected patients present at a younger age with a higher incidence of HBV co-infection. (7–9) More recent reports have suggested that HIV-infected patients fare worse than their HIV-uninfected counterparts despite presenting with less advanced tumors and receiving similar rates of potentially curative treatment. (10) Due to the relative paucity of HCC cases with HIV-infection, prior reports have relied on multi-center collaborations between academic centers, and have primarily only included patients with well-controlled HIV infection; furthermore, these cohorts have lacked socioeconomic, racial and ethnic diversity. The conflicting reports between these studies have led to a lack of clear understanding regarding the presentation, treatment options and outcomes in HCC patients with HIV-infection. The purpose of this study was to better characterize and compare the features of HIV-infected with HIV-uninfected patients with HCC at a single institution with detailed information on rates of screening, clinical presentation, treatment modalities and outcome.
Materials and Methods
Study Population
Using a prospectively maintained HCC database at Parkland Memorial Health and Hospital System (PHHS) at UT Southwestern Medical Center, all patients with known HIV serology and HCC diagnosed between January 2005 and December 2010 were identified. As the safety-net hospital system for Dallas County, Texas, Parkland serves a large population of patients with cirrhosis and cares for approximately 50% of HCC patients in the area.
HCC diagnosis was based on American Association for the Study of Liver Disease (AASLD) criteria. (11) Any patients without imaging studies were excluded given that tumor characteristics could not be adequately determined. We also excluded patients who had a liver mass without characteristic imaging (arterial enhancement with delayed washout) or histologic confirmation. The Institutional Review Board of the UT Southwestern Medical Center approved this study.
Data Collection
A retrospective review of each patient’s medical record was performed to obtain patient demographics, clinical history, laboratory data, and imaging results. Clinical history of interest included HIV, HCV and HBV serostatus, alfa-fetoprotein (AFP), alcohol abuse (defined as intake >60 g/day for males and >40 g/day for females), treatment delivery, and long-term outcome. Insurance status at HCC diagnosis was determined and categorized as Medicare, Medicaid, Parkland health plus, private insurance, or no insurance. Parkland health plus insurance is a payment program for Dallas County residents based on income and family size that subsidizes medical care. Laboratory data included total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, platelet count, and international normalized ratio (INR). Tumor characteristics of interest included the number of lesions, maximum tumor diameter, lymph node involvement, portal vein invasion, presence of extrahepatic metastases, and stage of the tumor at diagnosis. The Barcelona Clinic Liver Cancer (BCLC) and Okuda systems were used for tumor staging. (11,12)
Screening for HCC was based on the 2005 AASLD guidelines that pertained to the time period of the study, which recommended screening every 6–12 months. (11) A patient was considered to have received HCC screening if an ultrasound of the abdomen was done in the 12-month period prior to HCC diagnosis. Treatment for HCC was considered curative if it consisted of local ablative techniques (radiofrequency ablation (RFA) or ethanol injection), surgical resection, or liver transplantation. Treatment was considered locoregional if it consisted of any embolization techniques, including bland embolization, transarterial chemoembolization (TACE) or drug-eluting bead embolization, or stereotactic body radiation. Treatment was considered systemic if chemotherapy was given. Patients given no treatment were treated with best supportive care. If patients received multiple treatments, they were classified as having received the most curative therapy. For example if a patient received TACE prior to liver transplantation, they were classified as having liver transplantation, i.e. a curative treatment.
Statistical Analysis
Fisher exact and Chi-square tests were used to analyze differences between the two groups of patients. Survival analysis was performed by the Kaplan-Meier method with log rank univariate analysis. All variables significant in the univariate analysis (log rank p<0.05) were placed in the multivariate model. The hazard ratios and p values for the multivariate model were obtained using Cox proportional hazard regression. All tests were two-sided and performed at the 5% significance level. Statistical analysis was performed using the SPSS statistical software package (Version 17.0 for Macintosh, SPSS Inc, Chicago, IL).
Results
Patient Characteristics
A total of 190 patients with known HIV serostatus and a diagnosis of HCC between January 2005 and December 2010 were identified (Table 1). Twenty-six patients had a positive HIV immunofluorescent assay (IFA) with subsequent confirmatory testing, and 164 patients had negative HIV testing by IFA. Seventeen of 26 (73%) HIV-infected patients were on HAART in the year prior to HCC diagnosis, and 15 of the 22 (68%) patients with recorded CD4 counts had CD4 counts ≥ 200/mm3 at the time of HCC diagnosis. Seven (32%) of the 22 patients had HIV RNA levels < 50 copies/ml at time of HCC diagnosis.
Table 1.
Differences in Patient Characteristics by HIV Serostatus
| Variables | HIV Positive n=26 |
HIV Negative n=164 |
p value |
|---|---|---|---|
| Gender | |||
| Male | 23 (89%) | 134 (82%) | 0.40 |
| Female | 3 (19) | 30 (18) | |
| Age (years) at diagnosis (mean) | 55.4 +/− 9.5 | 56.0 +/−7.6 | 0.73 |
| Body Mass Index at diagnosis (kg/m2) | 22.3 +/−4.8 | 26.1 +/−6.2 | 0.01 |
| Race/Ethnicity | |||
| White | 10 (39) | 34 (21) | 0.02 |
| Black | 14 (54) | 68 (42) | |
| Hispanic | 2 (8) | 52 (32) | |
| Asian | 0 | 10 (6) | |
| Insurance Status | |||
| Medicare | 11 (42) | 29 (18) | 0.01 |
| Medicaid | 1 (4) | 26 (16) | |
| Parkland health plus | 12 (46) | 69 (42) | |
| Private | 2 (8) | 12 (7) | |
| None | 0 (0) | 28 (17) | |
| Etiology of chronic liver disease | |||
| Hepatitis C Alone | 7 (26) | 32 (20) | <0.01 |
| Hepatitis B Alone | 8 (31) | 5 (3) | |
| Alcohol Alone | 1 (4) | 16 (10) | |
| Hepatitis C and Alcohol | 7 (27) | 92 (56) | |
| Hepatitis B and Alcohol | 3 (12) | 5 (3) | |
| Non-alcoholic steatohepatitis | 0 | 7 (4) | |
| Other/Unknown | 0 | 7 (4) | |
| Symptoms at diagnosis | |||
| Yes | 13 (50) | 98 (60) | 0.35 |
| ECOG functional status | |||
| 0–1 | 21 (81) | 138 (84) | 0.18 |
| ≥2 | 5 (19) | 26 (16) | |
| Total serum bilirubin (mg/dl) (median) | 0.9 (0.2–6.5) | 1.4 (0.2–37) | 0.01 |
| Serum albumin (g/dl) (median) | 3.4 (2.0–4.7) | 2.9 (1.3–4.6) | 0.03 |
| Serum platelets (k/ul) (median) | 137 (17–426) | 121 (6–569) | 0.41 |
| Serum AST (u/l) (median) | 139 (17–422) | 132 (6–1089) | 0.52 |
| Serum ALT (u/l) (median) | 46 (15–245) | 56 (11–601) | 0.51 |
| MELD (median) | 10 (6–43) | 10 (6–43) | 0.12 |
| Child-Pugh-Turcotte | |||
| A | 16 (62) | 52 (32) | 0.01 |
| B | 7 (27) | 67 (41) | |
| C | 3 (12) | 45 (27) | |
| HIV RNA (copies/ml) (median) | 400 (48–34,200) | ||
| CD 4+ cells (per mm3) (median) | 247 (17–813) | ||
| HAART therapy (Yes/No) | 19/26 | ||
| Visits within 2 years of HCC diagnosis | |||
| Primary care physician (median) | 6 (0–22) | 1 (0–39) | 0.02 |
HIV-infected patients had a lower body mass index (BMI) than HIV-uninfected patients (p=0.01) but had a similar age at presentation and gender composition. They were also more likely to be classified as non-Hispanic compared to their HIV-uninfected counterparts (93% vs. 63%, p=0.02). HIV-infected patients also had a higher number of visits to a primary care physician in the two years prior to HCC diagnosis (median 6 (range, 0–22) vs. 1 (range, 0–39), p=0.02).
In both HIV-infected and –uninfected patients, chronic HCV infection, either alone or with associated heavy alcohol use, was the main etiology of chronic liver disease. HCV genotypes were available in 8 of the 14 HIV-infected patients with concomitant HCV infection. Genotype 1 was seen in 7 patients and genotype 4 in 1 patient. HCV genotype information was available in 47 of the 164 HIV-uninfected patients with HCV infection with the following distribution: genotype 1, 77%; genotype 2, 15%; genotype 3, 4%; and genotype 4, 4%. HBV infection, either alone or in conjunction with heavy alcohol use, was present in 43% of HIV-infected patients, compared to only 6% in HIV-uninfected patients (p<0.01). Serum HBV DNA was known in 9 of the 11 HIV-infected patients with HBV infection. 6 of the 9 had undetectable serum HBV DNA levels. Only one of the 11 patients had none delta antigen status.
All patients had underlying cirrhosis, except two – one in the HIV-infected group and one in the HIV-uninfected group. HIV-infected patients had more preserved liver function, demonstrated by 62% of patients presenting with CTP A cirrhosis compared to only 32% in the HIV-uninfected group (p=0.01). There were no diferences in patient performance status (p=0.18) or the presence of symptoms at presentation (p=0.35).
Clinical Presentation
Table 2 demonstrates the differences in HCC tumor presentation associated with HIV serostatus. HIV-infected patients had more screening ultrasounds in the year prior to HCC diagnosis (31% vs. 17%, p=0.01). HIV-infected patients presented more frequently with solitary tumors (65% vs. 40%, p=0.02) and small tumor size (median size 2.6 cm vs. 4.6 cm, p=0.02) compared to HIV-uninfected patients. They also presented with tumors at an earlier stage, with 39% of HIV-infected patients presenting at BCLC stage A compared to only 17% of the HIV-uninfected patients (p=0.04). Interestingly, despite an earlier stage of presentation, HIV-infected patients had a significantly higher level of serum alfa-fetoprotein than HIV-uninfected patients, with median levels of 325 ng/mL (4–191, 200) and 95 ng/mL (1–910, 900), respectively (p<0.01).
Table 2.
Differences in Tumor Characteristics by HIV Serostatus
| Variables | HIV Positive n=26 |
HIV Negative n=164 |
p value |
|---|---|---|---|
| Number of tumors | |||
| Solitary | 17 (65%) | 66 (40%) | 0.02 |
| Multiple | 9 (35) | 88 (60) | |
| Tumor size (cm) (median) | 2.6 (1.4–17.5) | 4.6 (0.9–17.5) | 0.02 |
| Portal vein thrombus | |||
| None | 19 (73) | 108 (66) | 0.81 |
| Bland | 2 (9) | 22 (13) | |
| Tumor | 5 (19) | 34 (21) | |
| Presence of distant metastases | |||
| Yes | 6 (23) | 34 (21) | 0.89 |
| BCLC Staging | |||
| A | 10 (39) | 28 (17) | 0.04 |
| B | 0 | 16 (10) | |
| C | 10 (39) | 72 (44) | |
| D | 6 (23) | 48 (29) | |
| TNM Staging | |||
| I | 3 (12) | 5 (3) | 0.19 |
| II | 13 (50) | 84 (51) | |
| III | 4 (14) | 41 (25) | |
| IV | 6 (24) | 34 (21) | |
| Okuda Staging | |||
| I | 13 (50) | 35 (21) | 0.01 |
| II | 9 (36) | 89 (54) | |
| III | 4 (14) | 40 (24) | |
| Screening ultrasound in year prior to HCC diagnosis | |||
| Completed | 8 (31) | 27 (17) | 0.01 |
| Not done | 18 (69) | 137 (83) | |
| AFP at diagnosis (ng/ml) (median) | 325 (4–191200) | 95 (1–910900) | <0.01 |
Treatment Modalities
The most common treatment modality in both HIV-infected and –uninfected patients was best supportive care (Table 3). HIV-infected patients were more likely to receive curative treatment compared to HIV-uninfected patients (27% vs. 7%, p=0.01), with surgical resection being the most common curative therapy in both groups. There was no difference in the number of patients receiving locoregional treatment or systemic chemotherapy by HIV serostatus; however, HIV-uninfected patients who were treated with TACE underwent more sessions than the HIV-infected patients (median number of TACE sessions 2 vs. 1, respectively, p=0.03).
Table 3.
Treatment Characteristics by HIV Serostatus
| Treatment | HIV Positive n=26 |
HIV Negative n=164 |
p value |
|---|---|---|---|
| Curative | |||
| Surgical resection | 7 (27%) | 6 (4%) | |
| Liver transplantation | 0 | 3 (2) | |
| Radiofrequency ablation | 0 | 1 (1) | |
| Locoregional therapy | |||
| TACE | 4 (15) | 44 (26) | |
| SBRT | 1 (4) | 1 (1) | |
| Systemic chemotherapy | 1 (4) | 10 (6) | |
| No therapy/Best supportive care | 13 (50) | 100 (61) | |
| Curative | 7 (27) | 10 (6) | 0.007 |
| Locoregional therapy | 5 (20) | 45 (27) | |
| Systemic therapy | 1 (4) | 10 (6) | |
| No therapy/Best supportive care | 13 (50) | 100 (61) |
Clinical Outcomes and Predictors of Survival
Table 4 demonstrates the clinicopathological variables associated with outcome following HCC presentation. On multivariate analysis, age ≤ 60 years, absence of symptoms at presentation, CTP status, and curative treatment were independently associated with better overall survival.
Table 4.
Predictors of Overall Survival: Results of Multivariate Analysis
| Variables | N | Median Survival (mo,(95%CI)) |
One Year Overall Survival (%) |
p Univariate |
Multivariate* | Hazard Ratio (95%CI) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 157 | 6.1 (3,9) | 41 | 0.39 | ||
| Female | 33 | 4.9 (2,8) | 31 | |||
| Age (years) | ||||||
| ≤ 60 | 145 | 6.3 (2,10) | 43 | 0.02 | 0.01 | 1.7 (1,3) |
| > 60 | 45 | 3.8 (2,6) | 27 | |||
| Race/ethnicity | ||||||
| White | 44 | 11.4 (3.8,18) | 50 | 0.52 | ||
| Black | 82 | 4.7 (3,6) | 34 | |||
| Hispanic | 54 | 7.7 (3,12) | 41 | |||
| Asian | 10 | 2.8 (0,11) | 38 | |||
| Insurance | ||||||
| Yes | 162 | 7.4 (4.2,11) | 43 | <0.01 | ||
| None | 28 | 2.7 (2,4) | 14 | |||
| Etiology of chronic liver disease | ||||||
| HCV alone | 39 | 4.4 (2.5,6.1) | 32 | 0.18 | ||
| HBV alone | 13 | 9.6 (0,25) | 46 | |||
| Alcohol alone | 17 | 7.7 (3,13) | 39 | |||
| HCV/Alcohol | 99 | 6.0 (2,10) | 42 | |||
| HBV/Alcohol | 8 | 23.5 (0,58) | 13 | |||
| NASH | 7 | 5.3 (1,9) | 57 | |||
| Other | 7 | 5.3 (3,7) | 38 | |||
| Symptoms | ||||||
| Present | 111 | 2.8 (2,3) | 18 | |||
| Absent | 79 | 21.3 (13,29) | 67 | <0.01 | <0.01 | 2.1 (1,3) |
| Child-Pugh-Turcotte | ||||||
| A | 68 | 16.6 (7,26) | 57 | <0.01 | <0.01 | 1.6 (1,2) |
| B | 74 | 4.3 (1,8) | 36 | |||
| C | 48 | 1.8 (1,3) | 17 | |||
| Number of tumors | ||||||
| Solitary | 83 | 16.5 (3,30) | 57 | <0.01 | ||
| Multiple | 107 | 3.7 (2,9) | 26 | |||
| BCLC | ||||||
| A | 38 | 25.7 (25,39) | 75 | <0.01 | ||
| B | 16 | 19.6 (10,29) | 68 | |||
| C | 82 | 4.5 (3,6) | 31 | |||
| D | 54 | 1.9 (1,3) | 15 | |||
| Treatment | ||||||
| Curative | 17 | NR | 88 | <0.01 | <0.01 | 2.9 (2,4) |
| Locoregional | 50 | 24.6 (20,29) | 73 | |||
| Systemic | 10 | 5.9 (3,9) | 40 | |||
| None | 113 | 2.6 (2,3) | 16 | |||
| Screening US one year prior to HCC diagnosis | ||||||
| Completed | 35 | 12.5 (0,25) | 50 | 0.25 | ||
| Not done | 155 | 4.9 (4,6) | 37 | |||
| More than 2 visits to primary care physician within 2 years of HCC diagnosis | ||||||
| Yes | 97 | 12.1 (6,18) | 50 | 0.01 | ||
| No | 93 | 3.3 (2,5) | 27 | |||
| HIV serostatus | ||||||
| Positive | 26 | 9.6 (1,18) | 40 | 0.85 | ||
| Negative | 164 | 5.2 (4,7) | 38 | |||
Results of the multivariate analysis shown only for significant variables.
NR indicates not reached.
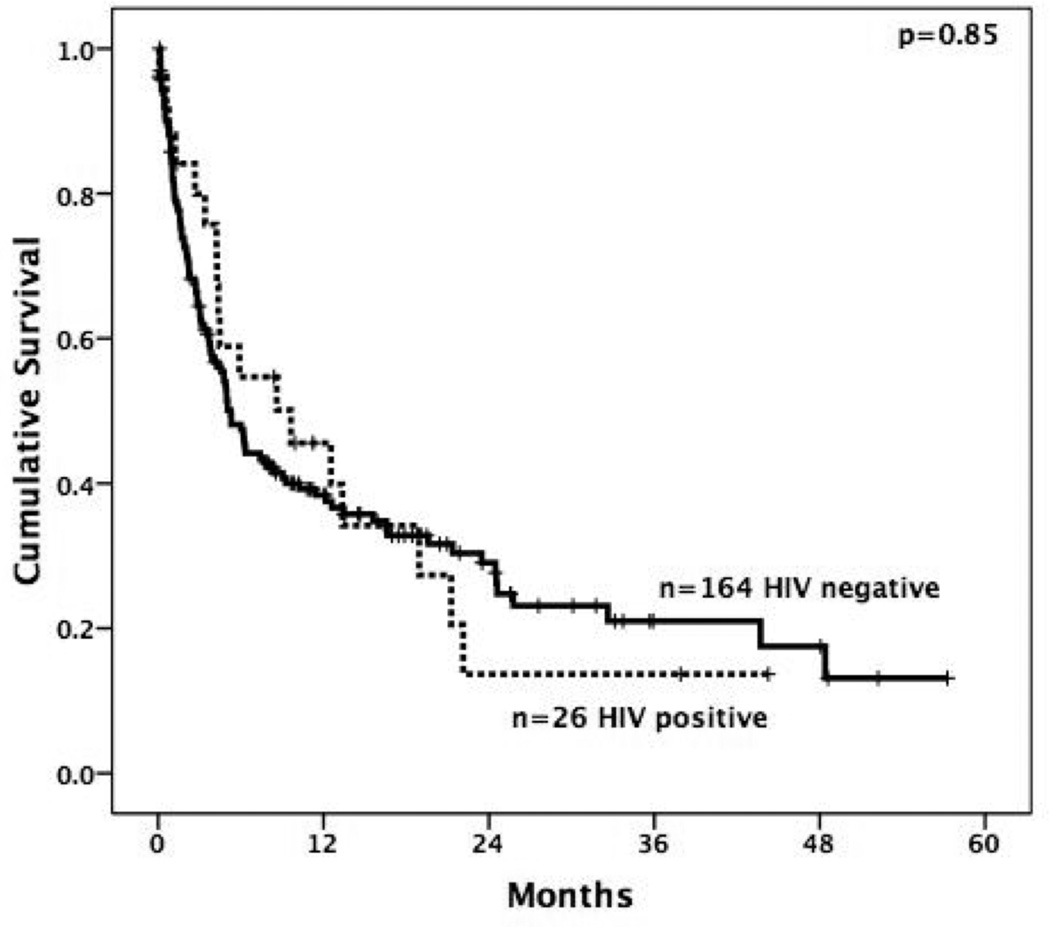
HIV serostatus was not associated with survival (Figure 1). HIV-infected patients had a median survival of 9.6 months compared to 5.2 months in HIV-uninfected patients (p=0.85). One-year overall survival rates following HCC diagnosis were 46% in HIV-infected patients and 38% in HIV-uninfected patients. Eight of the 26 (31%) HIV-infected patients and 52 of the 164 (32%) are still alive, with a median follow-up of 12 months and 20 months for the surviving patients, respectively. Most deaths were liver-related, including all 14 of the HIV-infected patients with known causes of death. Stratifying by underlying etiology of liver disease, there was no difference in median survival by HIV serostatus in those with underlying HCV infection (8.6 vs. 4.9 months respectively, p=0.88) or HBV infection (5.9 vs. 3.1 months respectively, p=0.32).
Figure 1.
Kaplan-Meier estimated overall survival curves of HCC patients by HIV-infected (--------------) and HIV-uninfected (------------) serology.
There was no difference in survival by HIV serostatus when comparing patients undergoing curative treatment (median survival not reached in either group; 90% vs. 86% one-year survival, p=0.32), systemic therapy (median survival 5.9 months vs. 6.3 months, p=0.64) or best supportive care (median survival 4.2 months vs. 2.3 months, p=0.83). However, HIV-uninfected patients had a longer median survival following TACE than HIV-infected patients (25.8 months vs. 12.5 months, p=0.04).
Median survival was not different between HIV-infected and –uninfected patients when stratified by race/ethnicity, gender, BCLC, tumor staging, or CTP class. However, HIV-infected patients who had a CD4 cell count ≥ 200/mm3 (n=15) at HCC presentation had a significantly longer survival than those patients with CD4 count < 200 (n=7) (18.9 months vs. 4.2 months respectively, p=0.03). HIV RNA counts, including HIV RNA suppression rates, and HAART use were not associated with longer survival (data not shown).
Discussion
Despite an increasing number of HIV-infected patients developing HCC from chronic liver disease, the natural history of HCC in this cohort has not been fully delineated. To our knowledge, the current study represents the largest single institution experience with HCC patients and HIV co-infection. Previous studies have been small, retrospective studies drawing patients from multiple tertiary care academic centers with the inherent biases of collaborative group efforts with respect to patient selection and available treatment options. (6–10, 13–16)
While previous studies suggested that HIV-infected patients were likely to be younger than their uninfected counterparts, we found HIV-infected patients were of a similar age and gender compared to the HIV-uninfected cohort. In prior studies, the median age of the uninfected cohort was older than 65 years, compared to a median age of 56 years in our current study. (7,10,16) The discrepency between our study and prior studies is most likely a result of the study setting, from which the patients were drawn. Previous studies included patients from tertiary referal centers while the current study was from a safety net hospital. All of the patients in our study come from a similar socioeconomic background and thus were likely to have similar exposure to risk.
In the only other published report examining race/ethnicity as a demographic variable, Brau et al. demonstrated that HCC in HIV-infected patients occurred predominantly in a black population with no difference by HIV serostatus within Hispanic patients. (7) This contradicts our study, where we found that only 8% of the HIV-infected group were Hispanic compared to 32% in the HIV-uninfected cohort. The lack of HIV-seropositivity in the Hispanic group is undoubtedly related to the underlying cause of chronic liver dysfunction, as Hispanics account for nearly 18% of all newly diagnosed cases of HIV in the United States and at PHHS. (17) In the current study, 19 of the 54 (35%) Hispanic patients had a non-viral hepatitis etiology of chronic liver dysfunction, compared to 2.2% and 2.4% in whites and blacks, respectively.
We found that HIV-infected patients presented with a higher degree of preserved liver function and earlier tumor stage than their HIV-uninfected counterparts. This is in agreement with previous publications reporting on liver function and/or tumor staging. (7,10) Presentation with earlier tumor stage and more preserved liver function in our study is most likely related to more primary care physician visits and increased screening rates in the HIV-infected population than seen in the HIV-uninfected cohort. More frequent clinic visits has been associated wtih higher HCC screening rates, which likely leads to earlier detection and tumor stage. (18) Furthermore, some HIV providers in our center have hepatology subspecialty training, which has also been associated with higher HCC screening rates (19,20). In our cohort, HIV-infected patients were nearly twice as likely to have received a screening ultrasound in the year prior to their HCC diagnosis compared to HIV-uninfected patients. This finding differs from prior studies where HIV-infected patients were less likely to undergo screening for HCC; however this is likely related to these studies being conducted in tertiary care centers where most HIV-uninfected patients with cirrhosis are followed by subspecialists (7,10). Accordingtly HCC screening rates in these studies are substantialy higher than the 18% pooled screening rate among cirrhotic patients found in a recent meta analysis. (18)
Despite HIV-infected patients presenting with earlier stage tumors, preserved liver function and increased screening rates, there was no significant difference in overall survival compared to HIV-uninfected patients. Although it should be noted that there was a trend toward better survival among HIV-infected patients (median survival 9.6 vs 5.2 months), this was likely related to better preserved liver function and earlier tumor stage at diagnosis. This finding is in agreement with the report by Brau et al who compared 63 HIV-infected patients with 226 HIV-uninfected patients over the course of 14 years from 10 different centers from North America with a similar patient composition with the current study. (7) The median survival in their HIV-infected cohort (6.9 months) was similar to that seen in our study (9.6 months).
However, two series from Italy demonstrated that HIV-infected patients have a worse survival outcome than HIV-uninfected patients. (9,10,16) In the series from Puoti et al, HIV-infected patients had a median survival of 6 months but HIV-uninfected patients had a median survival of nearly 18 months. (9) One possible explanation for the difference in survival between our study and Puoti et al is HIV-uninfected cohort patients presented with more preserved liver function (42% vs. 32% CTP class A) and earlier tumor stage (5% vs. 21% with distant metastases) in our cohort.
A recent study from Berretta et al. also demonstrated that HIV-infected patients (35 months) have a worse survival outcome than HIV-uninfected patients (59 months). (10) The survival times in both cohorts are dramatically longer than seen in our series or other published series of HCC patients,which can possibly be explained by the high rate of screening seen in their study. Over 70% of all patients regardless of HIV serostatus underwent screening US at 6 month intervals prior to HCC diagnosis. The rate of screening is higher than any other published series in a Western center and resulted in a cohorts of patients that received some form of therapy in 86% percent of cases compared to less than 50% of patients in our study. In the study from Berretta et al, the collaborating centers were all university-based medical facilities unlike the current study and other studies from Western centers where the majority of patients are drawn from safety-net hospitals. (7,10) This undoubtedly led to higher screening rates and may have influenced treatment decisions and ultimately outcome. Another potential explanation for the discrepency is that we included all patients that presented with HCC, including those who succumbed to their disease in the first few months after diagnosis. Berretta et al only included patients with at least 3 months of follow-up time, leading to a potential selection bias. (10) In our institution, a high percentage of patients are unaware of their chronic liver disease and oftentimes presented at a late stage of disease. Inclusion of patients presenting at late tumor stages, and more importantly less preserved underlying liver function, undoubtedly skews the overall survival outcomes but unfortunately represents the reality of HCC in HIV-infected patients.
The retrospective nature and accrual of patients from a single insitution, safety-net hospital system were a limititation to our study as a potential bias from a single institution treatment philosophy exists. Similar to other retrospective studies the time from diagnosis of chronic liver disease to HCC was not easily obtained due to the vast majority of patients being unaware of their chronic disease with HCC presenting as the first manifestation. Similarly, we were unable to determine cause of death for all patients given the retropsective nature of this study and resultant missing data. The strengths of the current study lie in the racial and ethnic diversity seen in our patient population which differs from previous studies.
Summary
In summary, our series demonstrates that HCC patients who are HIV-infected have a similar outcome as HIV-uninfected patients despite presenting at an earlier tumor stage, having more preserved liver function, and undergoing higher rates of curative treatment. The vast majority of HCC patients, regardless of HIV serostatus, present with advanced stage disease and lack curative treatment options. These findings should be considered in implementing HCC surveillance programs in high-risk patients, including those with HIV infection.
Acknowledgments
Financial disclosures: This project was supported in part by grants KL2 RR024983-04 and UL1 RR024982
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None by contributing authors
Author Contributions:
Study concept and design: Yopp, Singal, Subramanian
Acquistion of data: Yopp, Singal
Analysis and interpretation of data: Yopp, Singal, Subramanian
Drafting of manuscript: Yopp, Singal, Subramanian
Critical revision of manuscript: Yopp, Singal, Subramanian, Balch, Jain, Schwarz, Mansour
Statistical analysis: Yopp, Singal
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001 Oct 15;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999 Mar 11;340(10):745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 3.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008 May 20;148(10):728–736. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal E, Pialoux G, Bernard N, Pradier C, Rey D, Bentata M, et al. Liver-related mortality in human-immunodeficiency-virus-infected patients between 1995 and 2003 in the French GERMIVIC Joint Study Group Network (MORTAVIC 2003 Study) J Viral Hepat. 2007 Mar;14(3):183–188. doi: 10.1111/j.1365-2893.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 5.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006 Aug 14–28;166(15):1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 6.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected patient. Clin Liver Dis. 2003 Feb;7(1):179–194. doi: 10.1016/s1089-3261(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 7.Brau N, Fox RK, Xiao P, Marks K, Naqvi Z, Taylor LE, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007 Oct;47(4):527–537. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Samaniego J, Rodriguez M, Berenguer J, Rodriguez-Rosado R, Carbo J, Asensi V, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001 Jan;96(1):179–183. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 9.Puoti M, Bruno R, Soriano V, Donato F, Gaeta GB, Quinzan GP, et al. Hepatocellular carcinoma in HIV-infected patients: epidemiological features, clinical presentation and outcome. AIDS. 2004 Nov 19;18(17):2285–2293. doi: 10.1097/00002030-200411190-00009. [DOI] [PubMed] [Google Scholar]
- 10.Berretta M, Garlassi E, Cacopardo B, Cappellani A, Guaraldi G, Cocchi S, et al. Hepatocellular Carcinoma in HIV-Infected Patients: Check Early, Treat Hard. Oncologist. 16(9):1258–1269. doi: 10.1634/theoncologist.2010-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2005 Nov;42(5):1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 12.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer. 1985;56:918–925. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Clifford GM, Rickenbach M, Polesel J, Dal Maso L, Steffen I, Ledergerber B, et al. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS. 2008 Oct 18;22(16):2135–2141. doi: 10.1097/QAD.0b013e32831103ad. [DOI] [PubMed] [Google Scholar]
- 14.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004 Nov 22;164(21):2349–2354. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 15.Bruno R, Sacchi P, Filice C, Puoti M, Filice G. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis: an emerging issue. J Acquir Immune Defic Syndr. 2002 Aug 15;30(5):535–536. doi: 10.1097/00126334-200208150-00011. [DOI] [PubMed] [Google Scholar]
- 16.Bourcier V, Winnock M, Ait Ahmed M, Sogni P, Pambrun E. Primary liver cancer is more aggressive in HIV-HCC coinfection than in HCV infection. A prospective study (ANRS CO13 Hepavih and CO12 Cirvir) Clin Res Hepatol Gastroenterol. 2012 Jun;36(3):214–221. doi: 10.1016/j.clinre.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 17.CDC. Estimated Lifetime Risk for Diagnosis of HIV Infection Among Hispanics/Latinos—37 States and Puerto Rico, 2007. MMWR. 2010;59:1297–1301. [PubMed] [Google Scholar]
- 18.Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro J. Utilization of hepatocellular carcinoma surveillance among American patients: a systemic review. J Gen Int Med. 2011 doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. Jan 18;154(2):85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 20.Singal AG, Volk ML, Rakoski MO, Fu S, Su GL, McCurdy H, et al. Patient involvement in healthcare is associated with higher rates of surveillance for hepatocellular carcinoma. J Clin Gastroenterol Sep. 45(8):727–732. doi: 10.1097/MCG.0b013e31820989d3. [DOI] [PubMed] [Google Scholar]