Abstract
Soluble morphogen gradients have long been studied in the context of heart specification and patterning. However, recent data have begun to challenge the notion that long-standing in vivo observations are driven solely by these gradients alone. Evidence from multiple biological models, from stem cells to ex vivo biophysical assays, now supports a role for mechanical forces in not only modulating cell behavior but also inducing it de novo in a process termed mechanotransduction. Structural proteins that connect the cell to its niche, for example, integrins and cadherins, and that couple to other growth factor receptors, either directly or indirectly, seem to mediate these changes, although specific mechanistic details are still being elucidated. In this review, we summarize how the wingless (Wnt), transforming growth factor-β, and bone morphogenetic protein signaling pathways affect cardiomyogenesis and then highlight the interplay between each pathway and mechanical forces. In addition, we will outline the role of integrins and cadherins during cardiac development. For each, we will describe how the interplay could change multiple processes during cardiomyogenesis, including the specification of undifferentiated cells, the establishment of heart patterns to accomplish tube and chamber formation, or the maturation of myocytes in the fully formed heart.
Keywords: cadherins, heart, integrins, stem cells, transforming growth factor
Development from single fertilized cells into the diversity we enjoy in the animal kingdom is a highly conserved process driven by robust signaling gradients. Remarkably, similar gradients create simple patterns like the body axis1 as well as complex ones such as fruit fly wing development2 by controlling an equally diverse set of cell behaviors, for example, migration, proliferation, and specialization.2 These behaviors, in turn, are affected by spatiotemporal changes in ligand composition, gradient strength and magnitude, and combinations of agonists and antagonists (which has been more completely reviewed elsewhere3). For myocardium specifically, many highly specialized cardiac lineages arise from a set of common progenitors but specify based on differences in the factors to which they were exposed.4–6 These descriptions primarily rely on soluble cues, but a growing body of literature suggests that specification and subsequent tissue patterns can also arise from physical changes. Whether examined in vitro or in vivo, these cues come in many forms; generally they can be categorized as passive stimuli that occur within the microenvironment surrounding each lineage7–9 or as active forces that are directly applied to cells by other cells or fluids.10–13 For the former case, cells must directly probe the environment through myosin-mediated contractions to sense changes in their surroundings and use the mechanical feedback it provides to elicit changes in cell behavior.14,15 Conversely the latter case requires the cell to remodel internally or migrate to transmit the load,16 a process that could activate similar molecular mechanisms.15 Regardless of their type and mechanism, these cues can be studied in model organisms10 or built into protocols to direct stem cell specification or to test their influence more generally as a cue on cell behavior.17 Although significant evidence from developmental biology exists on soluble gradients, there is growing evidence using unique physical assays ex vivo and engineered microenvironments in vitro that mechanical cues and their interplay with chemical signals influence specification, which we will focus on here.
Although we will summarize what is currently known about the influence that forces and their transduction have on cardiomyogenesis, it would be naive to presume that these pathways are distinct, nonintersecting, or orthogonal to each other, whether or not you limit yourself to physical stimuli only. So whether used separately or in combination with the plethora of soluble morphogen gradients, data described here will suggest a more complex integration of signals such that stimuli are not additive but rather can be compensatory or antagonistic.18,19 We will provide further examples where physical cues, as with their molecular biology counterparts, can differentially regulate a process depending on the specific lineage or stage of the cell. As an example at the outset of this discussion, changes in wall stiffness can influence tube looping when presented during tube formation11 but in adulthood could lead to fibrosis.20 Just as with soluble factors, physical stimuli can vary with space and time9 and must be transmitted between myocytes and transduced to the nucleus to be interpreted.15,21 So in the following sections, we will address how mechanical forces drive lineage specification of early progenitors and their subsequent differentiation, specifically focusing on the interaction of forces and established signaling pathways.
Mechanical Cues in Early Myocardial Specification: Gastrulation and Mesoderm Formation
Cardiomyogenesis in vivo is governed by spatiotemporally defined signaling pathways that regulate transcription factor (TCF) expression to drive myocardial specification, differentiation, and maturation.5,6 In concert with these chemical signals, mechanical cues act on the developing embryo throughout the process of cardiac development. In this first section, we will focus on the earliest specification decisions in model organisms and how mechanics regulates these decisions. Murine models indicate cardiac specification as early as embryonic day 6.5 (E6.5) where a population of cardiac precursor cells residing in the lateral posterior epiblast develops before primitive streak (PS) formation22,23 and gastrulation. As epiblast cells migrate through the PS, they undergo epithelial–mesenchymal transition (EMT) to form early mesoderm, which will undergo a series of morphological changes, beginning with formation of the cardiac crescent, to develop into the heart. After EMT and formation of nascent mesoderm, irreversible myocardial commitment occurs,22,24 indicating that cues during this transition, either chemical or physical, drive cardiac determination. To better orient this discussion, Figure 1 highlights developmental stages of the heart and when in time signaling pathways and mechanics are prevalent. Mechanical signals play a vital role throughout the entirety of the process, beginning in early cardiomyogenesis by initiating gastrulation and contributing to the establishment of chemical gradients that drive early axis development and subsequent cell specification, commitment, and determination. Two such examples of the mechanical influence in early myocardial specification are cell tension–induced tissue invagination and mechanically generated fluid flow–driven chemical gradients. Both are described in the following section and summarized in Figure 2.
Figure 1. Cardiomyogenesis during stages of development.
Overview of the stages of cardiomyogenesis from the first appearance of cardiac precursor cells in the pregastrulation epiblast through the emergence of the 4-chambered heart. The embryonic (E) days noted at each stage reflect mouse development. Both biochemical and biophysical influences on cardiomyogenesis are summarized at each developmental stage. BMP, bone morphogenetic protein; ECM, extracellular matrix; EV, early ventricle; IFT, inflow tract; LA, left aorta; LV, left ventricle; OFT, outflow tract; PLA, primitive left aorta; PRA, primitive right aorta; RA, right aorta; RV, right ventricle; and TGFβ, transforming growth factor-β.
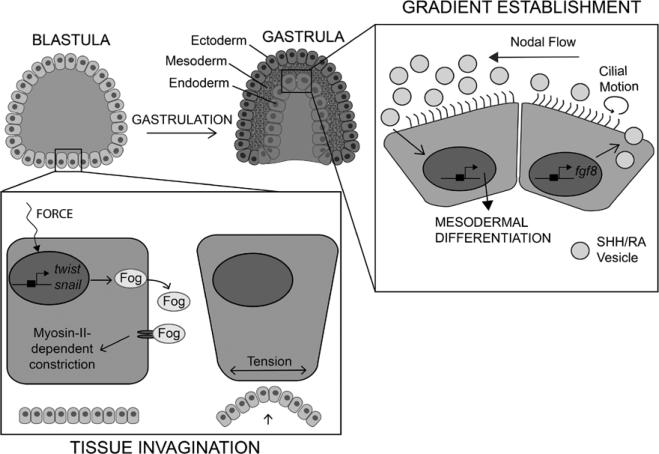
Figure 2. Influence of mechanical forces during gastrulation and early mesoderm formation.
Schematic illustration depicting the role of mechanical forces in initiation of tissue invagination during gastrulation (left) and establishment of chemical gradients during mesoderm development (right). Mechanical force induces expression of twist and snail genes that mediate folded gastrulation (Fog) secretion and receptor binding to activate myosin II–dependent cell constriction. This generates tension at the cell surface that drives invagination at the onset of gastrulation. Asymmetrical gradients of sonic hedgehog (SHH) and retinoic acid (RA) are created as nodal cilia rotate to generate nodal flow to drive SHH/RA-containing vesicles to the left region of the primitive streak node.
In Drosophila melanogaster morphogenesis, gastrulation begins after the apical constriction of a population of cells at the ventral midline.25 The apical surface of the cells shrinks in size because the basal surface increases, creating tension at the cell surface that triggers tissue invagination. Expression of TCFs Twist and Snail induce apical constriction during which myosin II localizes to the apical surface of the cells to generate contractile pulses necessary for cell shape remodeling.26,27 Snail triggers contraction, whereas Twist prevents relaxation, creating a ratchet-like process to achieve constriction, and embryos mutant in either snail or twist do not gastrulate properly. It has been proposed that Twist expression during gastrulation is maintained not by direct biochemical signals, such as snail expression, but instead by the mechanical cues that occur during PS formation.28 Ectopic expression of Twist can be induced by mechanically deforming Drosophila embryo, demonstrating its mechanosensitivity.29 In addition, in Drosophila mutants lacking Snail expression that consequently exhibit abnormal gastrulation, mechanical indentation rescues mesoderm invagination via the Twist signaling pathway and gastrulation proceeds.30 This mechanically induced rescue indicates that it is the contractile pulses generated by Snail and not a Snail-mediated intracellular signaling mechanism that enables gastrulation to proceed. Twist induces the expression and secretion of folded gastrulation, a critical protein leading to myosin II apical localization and constriction.31,32 In the absence of mechanical force, folded gastrulation is endocytosed, its signaling pathway is not activated and myosin II localization does not occur.30 However, in the presence of mechanical force applied by either mechanical indentation or Snail-induced pulsatile contraction, folded gastrulation endocytosis is inhibited and it remains in the extracellular space.
After EMT markers, the earliest myocardial regulators, for example, Mesp1/2 and Fgf8, are expressed by the gastrulating mesoderm and mediate cardiac precursor cell migration through the PS33,34; interruption in expression of either of these genes results in abnormal heart development.35,36 In the developing chick embryo, Fgf8 expression is symmetrical across the PS but becomes asymmetrical by Hamburger Hamilton stage 5, residing primarily in the right side of the PS node as the mesodermal layer forms.37 The origin of embryonic asymmetries are debated with some evidence indicating that early lineage bias drive later asymmetries, whereas other evidence argues for a stochastic, environmentally driven model.38,39 In addition to the chemical cues known to regulate PS node asymmetry, mechanical forces can also influence the patterning of developmental gradients. For example, during mouse development, Fgf8 signaling induces secretion of sonic hedgehog and retinoic acid containing vesicles; both are factors that initiate embryonic tissue patterning required for proper mesodermal development.40 Leftward sonic hedgehog and retinoic acid gradients across the node are then created by fluid flow, termed nodal flow, that is generated by cilia forces on nodal cells (Figure 2).41 Disruption of nodal flow in knockout mice was embryonic lethal and created a variety of developmental abnormalities, including pericardial sac ballooning and reversed heart tube looping.42 In chick PS where nodal cells do not express cilia and there is no extraembryonic space through which nodal flow could occur, asymmetry is established by cell migration across the node rather than by transport of secreted factors.43 Cells exhibit a brief leftward myosin II–dependent migration at Hamburger Hamilton stage 4 that is required for the lateralization of Fgf8- and sonic hedgehog–expressing cells at Hamburger Hamilton stage 5.44 Thus, regardless of the system and mechanism, it would seem that mechanical signals play a role in the establishment of early Fgf8 and sonic hedgehog signaling gradients that drive pattern formation in the embryonic heart, and in some cases, for example, Fgf8, morphogens can feed back to initiate mechanically controlled pattern formation.
Because these niche are dominated by cell–cell contact, a discussion of early events must include cadherins, a homo-philic class of cell adhesion receptors that bind to neighboring cells and create stable mechanical linkages. Cadherins have been implicated in regulating early expression of Fgf1 and Mesp1/2 in the gastrulating embryo to control epiblast cell migration when they ingress into the PS and begin to form the primitive heart tube.33,34 In addition, cadherins act as mechanosensors by initiating intracellular signaling cascades in response to changes in tension to transduce mechanical information about the surrounding environment.45 Cadherin-mediated cell adhesion and its associated signaling pathways play a variety of vital roles in tissue morphogenesis and development, cardiomyogenesis, and cardiac disease.46–48 These functions will be described further in the following sections, but taken together, these data form the basis for how mechanical forces could reshape cardiomyogenesis.
Mechanical Regulation of Signaling Pathways During Cardiomyogenesis
Multiple defined signaling pathways interact during development in exceedingly complex temporal and spatial patterns to drive cardiomyogenesis. These pathways include those that are initiated by soluble cues such as cytokines and growth factors, and those initiated by cell adhesive contacts with the extracellular matrix (ECM) and other cells. Foremost among the soluble signaling pathways are the Wingless (Wnt) and transforming growth factor β (TGFβ) superfamily pathways, whereas the major adhesive pathways include integrin- and cadherin-mediated signaling. Each of these act on cardiomyogenesis at multiple time points and in multiple capacities to orchestrate the process of mesoderm formation, cardiac specification, and differentiation. In the following sections, we will describe the influence of these pathways on cardiomyogenesis and provide examples of an additional layer of complexity in the process in which mechanical forces play a role in modulating the signaling pathways.
Wnt Signaling During Cardiac Development
There are 12 different Wnt growth factor family members, each acting on a variety of receptors at precise points in developmental time and space.49 Wnts modulate numerous steps during cardiomyogenesis and their actions can be biphasic, for example, activation before gastrulation promotes mesoderm formation, early expression postgastrulation decreases cardiac progenitor cell production, and later activation improves expansion.50–52 During early cardiac mesoderm specification, Wnt inhibition in the endodermal layer induces diffusible factor production that promotes expression of markers of cardiac lineage, such as Nkx2.5 and Tbx5, in the nascent mesoderm.53 After mesoderm specification, activation of the Wnt/β–catenin pathway in cardiovascular progenitors located in the secondary heart field leads to their accumulation and inhibits cardiomyocyte differentiation, indicating an important role for the pathway in cardiac progenitor cell maintenance.54 Wnt3a inhibits terminal differentiation of cardiomyocytes and in order for cardiac progenitor cells to proceed down the cardiomyocyte lineage, the Wnt/β–catenin pathway must be inhibited by extracellular signaling such as regulatory Notch1 or insulin-like growth factors–binding protein-4.50,55–57
Wnt acts through several signaling pathways summarized in Figure 3A. Signaling is initiated by binding to Frizzled family receptors. In canonical Wnt signaling, the expression of Wnt-associated genes is mediated by cytosolic β-catenin accumulation, which initiates its translocation into the nucleus where it binds the TCF/lymphoid enhancer–binding factor family of TCFs to alter gene expression (Figure 3A; nucleus). Without Wnt activation of Frizzled, β-catenin binds its destruction complex consisting of axin, adenomatous polyposis coli, and glycogen synthase kinase 3 β. Once associated with its destruction complex, β-catenin is phosphorylated and targeted for degradation, and cytosolic accumulation is inhibited. However, Wnt binding to Frizzled induces the disassembly of the axin/adenomatous polyposis coli/glycogen synthase kinase 3 β destruction complex and cytosolic β-catenin is stabilized, allowing for translocation into the nucleus. Once localized, it alters transcription of genes such as Brachyury and Mesp1 during mesoderm induction and Isl1, Flk1, and Nkx2.5 during cardiac progenitor specification and differentiation.49 In noncanonical Wnt signaling, Frizzled binding activates GTPases RhoA and Rac that mediate contractility and cytoskeletal reorganization, and alter gene expression via c-Jun N-terminal kinase activation of transcriptional regulator, activator protein 1.58 In myocardial development, noncanonical Wnt and cytoskeletal reorganization have been implicated in migration and polarization of cardiac progenitor cells during gastrulation and cardiac morphogenesis.59,60 In addition, noncanonical Wnt ligands, Wnt2 and Wnt11, act through c-Jun N-terminal kinase/activator protein 1 to positively influence cardiac differentiation.61,62
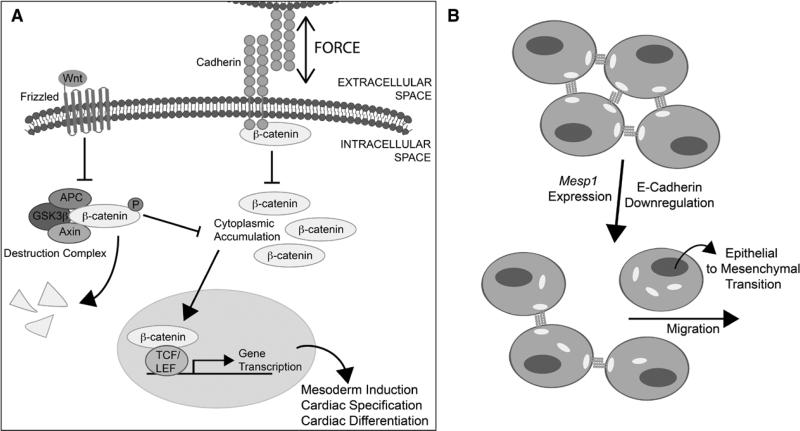
Figure 3. Force-induced modulation of Wnt signaling during cardiomyogenesis.
A, Schematic representation of Wnt signaling cascade and cadherin-based mechanical interactions between adjacent cells. Wnt ligand signaling through its Frizzled receptor inhibits the proteolytic degradation of β-catenin by its destruction complex, enabling cytoplasmic accumulation. Increased cytoplasmic β-catenin drives nuclear translocation and initiates expression of Wnt-related genes to modulate cardiomyogenesis. Cadherins interact with the Wnt pathway by binding β-catenin to block its cytoplasmic accumulation and subsequent nuclear translocation. B, Illustration of epithelial–mesenchymal transition (EMT) driving myocyte specification and migration. The loss of E-cadherin–based mechanical connections between cells enables cell migration and increases intracellular β-catenin pools to drive expression of EMT-related genes. APC indicates adenomatous polyposis coli; GSK3β, glycogen synthase kinase 3 β; LEF, lymphoid enhancer–binding factor; and TCF, transcription factor.
This complex Wnt signaling that orchestrates cardiomyogenesis is influenced by cell adhesions and cadherin expression, which play a role in mesoderm specification and cardiac differentiation.49,63 The canonical Wnt signaling pathway interacts bidirectionally with cadherin-mediated signaling to regulate cell migration and cell adhesion during development and cardiac morphogenesis.49,64 Mechanical forces may modulate expression of adhesions and the Wnt/β-catenin signaling cascade (Figure 3A; orange), which in turn can influence cell mechanics by altering cytoskeletal organization and contractility.65–68
Mechanical Forces Modulate the Wnt Pathway During Gastrulation
Adherens junctions, composed of homophilic cadherin binding between 2 adjacent cells, are sites of mechanical linkage between the cells and modulate tissue surface tension, which linearly increases with cadherin expression.69,70 Cadherins are sensitive to mechanical signals and in response to increased mechanical tension, will remodel cortical F-actin to increase junctional stiffness.71 Cadherins are linked to F-actin through interactions with β-catenin, which associates with α-catenin and vinculin, 2 mechanosensitive proteins that bind F-actin. Dynamic reorganization of the actin cytoskeleton and modulation of tissue surface tension are essential during development and morphogenesis, implying a crucial role for cadherins and adherens junctions.66,69,72 For example, as a requirement of cardiac precursor cell migration out of the PS during gastrulation, cells must downregulate expression of E-cadherin to break or remodel cell–cell contacts and allow motility through the PS to form nascent mesoderm (Figure 3B). Fgf8 and Mesp1 signaling generates a reduction in E-cadherin expression by blocking its transcription via repressors, such as Snail.73,74 Snail knockout embryos exhibit abnormal mesoderm morphology and fail to undergo complete EMT, likely because E-cadherin downregulation does not occur.75
Studies examining the differentiation of embryonic stem cells (ESCs) have verified the important role of E-cadherin–mediated cell–cell contacts in the formation of mesoderm and cardiac differentiation, and indicate that Wnt signaling, via β-catenin, plays a key role in the process.76–79 To initiate ESC differentiation, proliferating cells can be forced to aggregate into embryoid bodies using various methods, such as hanging drop, microwell aggregation, or rotary suspension techniques. In the hanging drop method, small volumes of media containing cells are placed on a petri dish lid, the lid is inverted, and the cells aggregrate within the droplet because of forces created by the liquid surface tension. Alternately, in microwell aggregation, cell suspensions are placed into microwells of defined size and forced to aggregate because of constrictions of the size of the well. The rotary suspension culture technique drives embryoid body formation of ESCs in suspension by submitting them to orbital motion. Each of these forced aggregation techniques generates more homogenous embryoid bodies more efficiently than passive embryoid body formation using liquid suspension culture.78,80–82 Interestingly, forced aggregation techniques improve differentiation into the cardiac lineage as evidenced by a higher number of spontaneously contracting embryoid bodies within days of formation, increased early expression of mesoderm marker, Brachyury T, and a later increase in gene expression of cardiac markers Nkx2.5, α-MHC, MLC-2v, TNNT2, MYL7, and Mef2c.79,82 In both rotary suspension and microwell cultures, there is an accompanying increase in expression of E-cadherin, indicative of the improved cell–cell contacts created between the aggregated cells.76,77 Forced aggregation increased early nuclear translocation of β-catenin and increased TCF/lymphoid enhancer–binding factor activity, suggesting that the cellular adhesion dynamics altered the Wnt/β-catenin pathway and downstream transcription of cardiac genes, and plays a role in coordinating EMT and cardiac differentiation.
The intersecting cadherin/β-catenin and Wnt/β-catenin pathways demonstrate the interplay of mechanotransduction and traditional autocrine/paracrine signaling that occurs during development. Before gastrulation, epiblast tissue exhibits a compact, tightly packed cell morphology, formed as a result of proliferation and migration occurring during morphogenesis that is thought to be heavily influenced by mechanical forces, inducing cells to create a large number of E-cadherin–mediated mechanical cell linkages.65,66 Wnt signaling from the underlying endoderm tissue initiates E-cadherin downregulation during EMT by turning on transcription of Mesp1, which activates the repressor Snail to reduce E-cadherin transcription.74,83 Consequently, as membrane-localized E-cadherin decreases, its bound β-catenin is released and added to the existing pool of intracellular β-catenin, increasing translocation into the nucleus to alter gene expression (Figure 3). This signal feeds into the Wnt/β-catenin cascade, increasing transcription of Wnt-induced genes, including Mesp1 among others, which can also activate expression of Dkk1, a Wnt pathway inhibitor that promotes cardiac differentiation.84,85 Thus, mechanical interactions during epiblast development prime the tissue to respond to endoderm-originating Wnt signals during EMT by enriching the membrane-bound pool of β-catenin. Furthermore, loss of cadherin-based mechanical linkages induced by Wnt signaling creates a cascade that feeds forward into the canonical Wnt pathway to enhance cardiac differentiation by Wnt inhibition. This pathway, illustrated in Figure 3B, highlights the exceptional complexity of the coordinated signals that occur during cardiac specification and differentiation that rely on both opposing types of signals (physical versus chemical) and modes of application (spatial versus temporal).
Mechanical Forces Modulate the Wnt Pathway During Tube Formation
After gastrulation, cardiac precursor cells migrate and fuse to form a linear heart tube, which later loops into a C- then S-shape that produces the 4 chambers of the heart (Figure 1). During cardiac morphogenesis, N-cadherin cell adhesions are required for heart tube fusion,86 and noncanonical Wnt signaling can modulate the expression of cadherins to drive this process.87 Both noncanonical Wnt5a and Wnt11 signaling pathways have been shown to play a role in cardiac morpho-genesis, and it is likely because of the alteration of passive mechanical linkages via N-cadherin that these pathways act. Wnt11-null mice and primary cardiomyoblasts exhibit abnormal N-cadherin and β-catenin expression characterized by a delocalization from the cell membrane and loss of colocalization between the 2 proteins.88 These cell adhesion abnormalities result in defects in the cardiac outflow tract and altered cell polarity in mouse Wnt11-null mutants,89 and linear heart tube defects in Xenopus Wnt11 loss of function experiments.62 Similarly, Wnt5a-null mice exhibit abnormalities in the cardiac outflow tract and do not survive postnatally because of gross organ defects.90
These examples describing the importance and role of Wnt signaling, especially as it relates to modulating cadherins, suggest that traditional views of development in which the process is primarily driven by chemical signaling, should be amended to include a role for mechanical influences and interactions. Conversely, passive mechanical forces transduced to cells through their adhesion proteins can both modulate and be modulated by Wnt signaling cascades to shape the fate of cardiomyogenesis at the earliest stages of gastrulation through cardiac fate specification, differentiation, and maturation.
TGFβ Superfamily Signaling During Cardiac Development
During development, the TGFβ growth factor superfamily (consisting of >30 family members) generates an assortment of spatial gradients necessary to drive axis formation and patterning of the embryo.91 In this section, we will briefly outline this pathway and in a subsequent section describe its mechanical sensitivities as described in Figure 4.
Figure 4. Role of mechanical forces in regulation of transforming growth factor-β(TGFβ) superfamily signaling.
Biophysical forces regulate TGFβ superfamily signaling at multiple points in the pathway. A, Both passive and active mechanical cues alter gene expression of bone morphogenetic protein (BMP) ligands and mothers against decapentaplegic homolog (SMAD) effector proteins. B, Membrane tension generated by mechanical interactions with the extracellular matrix (ECM) drives TGFβ receptor endocytosis. C, TGFβ ligands are sequestered by the ECM through interactions with latency-associated protein (LAP) and latent TGFβ-binding protein (LTBP). Cell contraction initiates ligand release by unfolding mechanosensitive LAP to interfere with LAP–TGFβ binding.
In mouse models, TGFβ family signaling begins in the epi-blast to establish the first embryonic axis and continues to play a role throughout organogenesis and onwards, with continuing roles in adult tissue homeostasis. This diversity of function relies on the variety of family members, which include TGFβs, bone morphogenetic proteins (BMPs), and Nodal, and each of these 3 influence cardiomyogenesis. Mesoderm induction in mouse begins with the initiation of Nodal expression in the epiblast at E5 that activates BMP4 expression in the extra-embryonic ectoderm, which in turn induces Wnt3 expression in the epiblast.92,93 Wnt3 then activates canonical Wnt signaling to drive EMT and later cardiomyogenesis.49,92,93 Knockout mouse models demonstrate the importance of TGFβ super-family signaling in early cardiomyogenesis, with Nodal and BMP4 homozygous deletions leading to lethality between E7.5 and E9.5 with a failure to undergo normal gastrulation, PS formation, and mesoderm induction.94,95 Although Nodal seems to primarily mediate early mesoderm formation, BMP signaling continues play a critical role in cardiac specification and differentiation after gastrulation. Mice with homozygous BMP2 deletions survive past gastrulation, but die by E10.5 and before that time point exhibit delayed cardiac morphogenesis and ectopic localization of the heart tube, which never develops past the linear tube stage.96 In addition, forced expression of BMP2 in chick embryos resulted in ectopic expression of cardiac markers Nkx2.5 and GATA-4, whereas inhibition of BMP signaling with antagonist noggin blocked cardiac mesoderm differentiation.97,98 Although BMP signaling acts as a positive cue for cardiac differentiation, an early transient inhibition with noggin is necessary to induce cardiomyocyte differentiation in ESCs, indicating a biphasic regulatory role in cardiogenesis.99 After cardiac progenitors migrate and fuse to form the linear heart tube, BMP signaling continues to influence cardiac morphogenesis, with BMP6, BMP7, and BMP10 orchestrating heart tube looping and chamber formation, evidenced by the absence or hypoplasticity of ventricles in transgenic mice models lacking expression of these BMPs.100,101
Similarly to the BMP branch of the TGFβ superfamily, the TGFβ branch also mediates cardiomyogenesis, but in some instances it inhibits cardiomyocyte differentiation while pushing cardiac progenitors toward smooth muscle and endothelial lineages, and in other instances it promotes differentiation to the cardiomyocyte lineage. Mouse models of TGFβ1 and TGFβ2 gene knockout both exhibit early lethality and cardiac defects.102 Like BMP, TGFβ2 creates a biphasic influence on cardiomyogenesis, with early expression acting as a positive cue for cardiac mesoderm formation and later expression acting as an inhibitor of cardiac differentiation. Early during cardiac specification, Nodal activates TGFβ2 and the 2 signals act in concert to promote formation of cardiac mesoderm.103 Although Nodal undergoes autoinhibition after its early expression, TGFβ2 expression persists and continues on to mediate cardiomyogenesis. Inhibition of TGFβ2 with RNA interference enhances expression of cardiac marker Myh2, demonstrating its role in blocking cardiomyocyte differentiation, whereas treatment with recombinant TGFβ2 upregulates expression of Pecam1 and Myh11, markers of endothelial and smooth muscle cells. However, in cell models of cardiomyocyte differentiation, TGFβ1 promotes cardiac differentiation by increasing expression of cardiac markers and contractile proteins, further illustrating the biphasic control of TGFβ over cardiomyocyte differentiation.104,105
The importance of spatial and temporal modulation of TGFβ superfamily signaling during cardiomyogenesis and morphogenesis indicates that a complex regulatory system must be established to orchestrate the diverse effects of the pathways. Modulation of BMP occurs on multiple levels, including post-translational processing, extracellular antagonism, and intracellular inhibition. After translation, enzymes cleave secreted, inactive BMP precursors to produce the active form that can bind its receptor and initiate downstream effects.106,107 Many extracellular proteins, such as noggin, can bind BMP to impede or promote receptor binding, and modulation of receptor availability via internalization or degradation can alter transduction.108–111 Along with soluble regulators of the TGFβ superfamily, components of the ECM modulate signaling by sequestering TGFβ family members. ECM proteins can interact with both BMPs and TGFβs via their heparin-binding sites or prodomains, and these interactions regulate their availability and consequent downstream signaling.112,113 For example, in Drosophila development, the gradient of BMP analog decapentaplegic is maintained through interactions with type-IV collagen.114 Decapentaplegic secreted from cells in the dorsal ectoderm binds type-IV collagen and limits its active range, where it maintains germline stem cell populations and regulates dorsoventral axis patterning. In addition, the BMP4 signaling range increases when its ECM-binding prodomain is cleaved, demonstrating the importance of ECM sequestration in establishing BMP gradients.115 Release of these ligands from the ECM can be initiated by both chemical or mechanical stimulation, which will be discussed in detail in the following section.116
Mechanical Regulation of TGFβ and BMP Signaling During Cardiac Development
In addition to the chemical regulation of TGFβ superfamily signaling, mechanical forces also modulate the pathways by extracellular and intracellular mechanisms, summarized in Figure 4, and may play a role in driving cardiomyogenesis. BMP signaling is mediated by mechanical forces at multiple stages during the signaling pathway, including BMP receptor availability and transcriptional regulation of BMP ligands and antagonists.117 Receptor internalization via endocytosis or degradation inhibits BMP signaling,118 and cardiomyocyte differentiation requires transient periods of BMP inhibition, indicating that receptor internalization may mediate this process.99 In addition, stem cell models of cardiomyogenesis demonstrate that small molecule degradation of TGFβ receptors promote cardiomyocyte differentiation.108 Endocytosis can be mediated by a variety of mechanical stimuli including cell membrane tension and mechanical deformation and thus, it may be proposed that mechanical forces occurring during morphogenesis may mediate TGFβ and BMP receptor endocytosis to mediate progenitor differentiation (Figure 4B).119 In fact, passive mechanical forces generated by ECM elasticity modulate BMP signaling through BMP receptor endocytosis and alter lineage specification of stem cells; however, it should be noted that mechanically induced endocytosis is not specific to the BMP signaling pathway and modulates cell membrane trafficking generally.120 At the transcriptional level, mechanical forces can alter several members of the BMP pathway (Figure 4A). Changes in BMP ligand expression and their mothers against decapentaplegic homolog (SMAD) family effectors on compliant ECM are myosin-dependent, indicating that they are mechanosensitive.121 Dynamic mechanical stretch applied to cultured neonatal rat cardiomyocytes upregulates BMP2 expression while transiently downregulating BMP4 expression.122 These changes in BMP expression were accompanied by an increase in cardiac differentiation marker GATA-4, suggesting that the BMP pathway may transduce forces to mediate cardiomyocyte differentiation.
Studies of ECM sequestration and release of TGFβ1 have revealed complex mechanisms that include both chemical and mechanical control of liberation.116,123 TGFβ1 is synthesized in its inactive form, in which it is associated with a latency-associated protein. During post-translational processing, the complex is covalently liked via disulfide bonding to a latent TGFβ-binding protein before secretion into the extracellular space. Once there, the latent TGFβ-binding protein interacts with deposited ECM proteins fibronectin, fibrillin and vitronectin to maintain an inactive pool of TGFβ1 that can later be liberated in its active form to induce various actions on the surrounding cells.124,125 Common methods of activation rely on release of TGFβ1 from its latency-associated protein and latent TGFβ-binding protein through proteolytic/enzymatic cleavage via matrix metalloprotinases, proprotein convertases, or glycosidases, and work in the past decade has also revealed a role for integrin-dependent activation of latent TGFβ1.116 Integrins are transmembrane proteins that associate extracellularly with components of the ECM and intracellularly with components of the cytoskeleton. They are considered to be mediators of force transduction between the inside and outside of the cell, and they are vital players in a variety of processes during development, including migration, differentiation, and morphogenesis.126,127 Integrins can mediate both proteolytic and mechanically induced release of TGFβ1. Both matrix metalloprotinases and latent TGFβ-binding proteins bind integrins, indicating that integrins may catalyze proteolytic cleavage of TGFβ1 by bringing it into close association with its cleavage enzyme.128–130 However, integrin-mediated liberation of TGFβ1 can also occur nonenzymatically, with mechanical forces initiating release. In this model, latency-associated protein is a mechanosensitive protein that, in response to contractile forces transmitted through integrins, unfolds into a conformation that does not support binding with TGFβ1, thus releasing it into the extracellular space and enabling receptor binding (Figure 4C).131–133 Force-induced release of TGFβ1 has been well characterized in the context of myofibroblast differentiation in wound healing and cardiac fibrosis.131,134,135 In the later example, contraction of cardiac fibroblasts activates latent TGFβ1 from the ECM and induces differentiation of myofibroblasts that promotes fibrosis in damaged cardiac tissue. However, similarly to the biphasic effects of TGFβ1 discussed above, mechanically induced TGFβ signaling also enacts differing effects depending on the target cell population and the temporal window of application. For example, cyclic strain applied to ESCs induces SMAD2/3 activation and plays a role in maintaining pluripotency.136,137 To date, the influence of mechanical forces on TGFβ1 signaling has not been studied in cardiac progenitor cell differentiation; however, because TGFβ1 plays a role in cardiomyocyte, vascular smooth muscle, and endothelial cell differentiation during development, it is possible that the contractile forces occurring during cardiac morphogenesis may also regulate TGFβ1 release and action during cardiomyogenesis in a similar fashion.
Integrin Regulation of Cardiac Development
Integrins are transmembrane receptors that link ECM and cytoskeleton to mediate tissue structure and function. Composed of heterodimers of α and β subunits that act as receptors for ECM proteins, integrins transduce mechanical information from the cellular environment into the cell (outside–in) and from inside the cell back out (inside–out).138 Transduction begins with ECM ligand binding and subsequent initiation of the accumulation of several proteins on the cytoplasmic region of integrin dimers to form focal adhesion (FA) complexes. These proteins include adaptor proteins that structurally link integrins to the cytoskeleton by binding F-actin, scaffolding proteins that bind additional FA proteins, and signaling molecules that propagate intracellular signaling cascades. In the myocardium, many of the integrin-associated FA proteins and downstream signaling result from chemical changes that have been associated with cardiac development and disease.139 However, most are also influenced by mechanics; the impact of both are highlighted in Figure 5 and described here for their chemical changes and in the next section for their mechanical changes.
Figure 5. Interplay of chemical and mechanical signals that underlie cardiac specification and development.
Schematic representation of integrin-mediated signaling specifically detailing how chemical signals (left), for example, integrin ligation, drive focal adhesion formation, and downstream events leading to transcriptional changes. Mechanical signals (right) are mediated by actomyosin contractions. Both influence transcriptional changes via common mechanisms diagrammed at the bottom of the illustration, including chromatin remodeling and changes in nuclear pore complex diffusion among others. Dash and solid lines indicate the direction of biophysical and biochemical interactions, respectively. AKT indicates protein kinase B; ECM, extracellular matrix; FAK, focal adhesion kinase; GSK, glycogen synthase kinase; ILK, integrin-linked kinase; and PI3K, phosphoinositide 3-kinase.
Integrins and their ECM adhesions are vital for organism formation and function, and they coordinate many events during development, such as proliferation, differentiation, and migration. In mammals, >18 α subunits and 8 β subunits have been identified, with the myocardium expressing a small subset of these; however, integrin expression patterns are dynamic, adding to their diversity of function, and turnover can be mediated by mechanical forces.139–142 Numerous knockout studies illustrate the importance of integrins during cardiomyogenesis, particularly the α4, α5, and β1 subunits. Animals null for α4 integrin die by E15.5 and exhibit cardiac defects including absence of epicardium and coronary vessels, cardiac hemorrhage, and abnormal epicardial progenitor migration.143 Homozygous knockout of α5 expression is embryonic lethal by E11, interferes with mesodermal axis patterning, and embryos develop abnormal heart morphologies, including dys-functional cardiac looping and outflow tract formation.144,145 In the heart, β1 integrin is the main β subunit that dimerizes with all of the expressed α subunits. Global homozygous knockout of β1 integrin is embryonic lethal by E5.5, whereas cardiac-specific homozygous knockout leads to a variety of cardiac defects.146–148 In addition, ESCs lacking β1 integrin exhibit a delayed expression of cardiac markers MHC, MLC-2V, and ANF after the onset of cardiac differentiation, aberrant sarcomeric architecture, and abnormal specification to atrial, ventricular, and sinusnodal type cells.149
A large body of work demonstrates that integrin-mediated signaling mediates cardiac specification and differentiation. Studies using cell models of cardiomyogenesis indicate that ECM composition and the activation of integrins play a major role in the process. For example, ESCs cultured in 3-dimensional (3D) ECM constructs based on laminin or vitronectin differentiate more efficiently into cardiomyocytes than ESCs cultured on 2D ECM substrates.150,151 In 3D culture, there are more points of contact between the cell and ECM, more FAs and possibly enhanced integrin signaling, supporting the important role that integrins play in development. Furthermore, ESCs differentiate more efficiently into cardiomyocytes when cultured on native heart ECM than on commercial ECM mixtures or isolated ECM components.152,153 Even in the absence of supplemental growth factors commonly used to drive cardiomyogenesis, native ECM is able to induce cardiac differentiation, indicating that cell signaling pathways initiated by integrin activation via ECM binding are stronger differentiation cues than soluble signals.153
In ESCs deficient in β1 integrin expression, early cardiomyocyte differentiation was delayed, but ultimately proceeded to result in beating cardiomyocytes, albeit with impaired functional properties.149,154,155 These mutant cells exhibited an accompanying upregulation of β3 integrin, suggesting a compensatory role that enabled early cardiac specification and differentiation.156 However, these cell lines exhibit abnormalities in terminal differentiation, indicating the importance of β1 integrin-mediated signaling in the differentiation process. Integrin-linked kinase, an intracellular signaling molecule bound to β integrin subunits, is directly activated by integrins and initiates downstream responses that regulate cell survival, proliferation, and differentiation.157 Fetal myocar-dial cells overexpressing integrin-linked kinase produce more cardioblast aggregates than wild-type cells, and knockdown of integrin-linked kinase expression with RNA interference reduces cardioblast generation, verifying the importance of integrin signaling in cardiomyogenesis.158 In addition to β integrins, multiple α integrin subunits are expressed in ESC-derived cardiomyocytes, for example, α3, α5, α6, α7, α11, and αψ, depending on the matrix on which they are cultured, with expression levels of some subunits such as α6 correlating to cardiac marker expression, indicating a role in cardiac differentiation.159,160
Integrin-Mediated Mechanotransduction in Cardiac Development
During myocardial development, several mechanical forces, both active and passive, are generated. ECM secreted and assembled by progenitor cells gives the developing tissues structural integrity and results in changes in tissue stiffness. Because myocardial tissue begins to fold during morphogenesis and with the onset of chamber pumping, cardiac progenitors, and later cardiomyocytes are submitted to stretching forces. Both of these active and passive forces are mediated by integrin-based cell adhesions. Integrins act as mechanotransductors to pass these signals to the inside of the cell and alter cell function. In addition to the soluble signaling pathways initiated by integrin–ECM interactions and FA complex assembly, direct mechanical linkages exist between the ECM and the nucleus that are thought to alter gene expression by propagating mechanical waves to the nuclear membrane to directly remodel chromatin or open nuclear pores and modify nuclear transport (Figure 5).161 In addition, the soluble signaling pathways themselves can be activated based on force applied through integrins and the FA complexes. Several mechanosensitive adaptor proteins exist that contain cryptic-binding sites for kinases that are uncovered in the presence of force to allow phosphorylation and downstream signaling to proceed.138 In this fashion, cyclic stretch activates multiple pathways in cardiomyocytes to mediate processes, such as cell survival, cell–cell communication, structural reorganization, and electrophysiological functioning.162
During development, cardiac progenitors respond to both passive and active mechanical cues, demonstrating the importance of these forces in cardiomyogenesis. Because the myocardium develops, it transitions from a soft mesoderm tissue (≈500 kPa) to an intermediate stiffness (≈11 kPa). Embryonic cardiomyocytes cultured on soft ECM substrates that mimic the mechanical properties of mature heart tissue develop more organized sarcomeres and exhibit more mature functional behavior than cells on softer or stiffer matrices.7,8 However, other work using human ESCs indicated that cells are sensitive to passive mechanical cues only during early cardiac specification, with substrate stiffness affecting mesoderm induction, but not cardiomyocyte differentiation after that time point.163 Culture on dynamically stiffening hydrogels that mimic in vivo cardiac tissue stiffening further improves cardiomyocyte differentiation and structural organization over culture on static myogenic hydrogels.9 This slow stiffening occurs during the course of several hours, similar to the time course observed in vivo; however, additional in vitro studies have demonstrated that more rapid mechanical perturbations also affect cardiomyocyte differentiation and maturation. In some cases, cyclic mechanical strain improved cell survival and cardiomyogenesis,13,164 but impaired cardiomyocyte differentiation in others.165 These conflicting results may arise artificially based on experimental design (time course, stimulation frequency, etc.) but may also reflect differences in cell type (mouse versus human) or maturation stage (pluripotent versus specified).
Mechanistic studies have implicated cell adhesive contacts as mediators of force transduction. Several intracellular signaling pathways associated with integrins such as the phosphoinositide 3-kinase/protein kinase B and p38 mitogen-activated protein kinase pathways were found to be upregulated in response to dynamic stiffening, suggesting that integrins may be mediators of the external mechanical cues leading to improved cardiomyocyte differentiation and maturation.14 Integrin internalization via endocytosis is enhanced on softer substrates; thus, as cardiac tissue stiffens during development, integrin signaling may be enhanced because of a greater number of receptors present at the cell surface.120 In addition, integrin expression is upregulated in response to mechanical stimulation, indicating that forces improve cell adhesion and FA assembly, potentially increasing downstream FA-mediated signaling to modify cardiomyogenesis.140,164 However, future work to clarify the impact of both passive and dynamic forces remains to be done to gain a full understanding of the role of mechanics in cardiomyogenesis.
Perspectives: Implications for Disease and Tissue Regeneration
Continued work in clarifying the role of mechanical signaling in myocardial development has the potential to largely broaden our understanding of the origin of congenital and adult heart disease and dysfunction, as well as to affect future therapies aimed at treating these disorders. Abnormal mechanical cues during development and reduced expression of mechanosensitive receptors or proteins can lead to heart deformations or the emergence of cardiomyopathies. For example, as described above, knockout of various integrin receptors and consequently, cell–ECM interactions that maintain cell shape and tension cues, can create several cardiac defects. In addition, mutations in force-sensitive cytoskeletal proteins such as vinculin, titan, integrin-linked kinase, dystrophin, or sarcoglycan are associated with the development of dilated or hypertrophic cardiomyopathies.166 Cytoskeletal maintenance of cell shape drives the healthy function of cardiomyocytes, but when cytoskeletal remodeling occurs in response to changing mechanical forces (ie, pressure or volume overload), pathological states develop.21 To develop efficacious treatments for these diseases, we must consider not only the biophysical or biochemical contributions to the disease state in isolation but also examine how both of these pathways feed into each other to create the complex network of signaling that occurs physiologically.
Because the adult heart holds a limited capacity for regeneration, efforts to generate myocardial cells and tissue from human pluripotent stem cells have garnered significant attention in recent years. These engineered cells and tissues have potential uses as regenerative therapies or as improved in vitro platforms for drug discovery and testing. Ongoing work has established highly efficient cardiomyocyte differentiation protocols based on modulation of FGF, Wnt, TGFβ, or BMP pathways (reviewed in Burridge et al167), but these methods produce cardiomyocyte populations that contain large heterogeneity in structural and functional maturation. For this reason, novel techniques to engineer cardiomyocytes and cardiac tissue based on a variety of physical signals are being investigated in an attempt to generate mature cells and tissues that more faithfully recapitulate the in vivo myocardium.168,169 As described in this review and elsewhere,65,170 mechanical forces play a significant role in in vivo development and cardiomyo-genesis, which suggests that similar physical cues may aid in the ex vivo production of cardiomyocytes and promote their organization into artificial cardiac tissues and organoids. In fact, development of tissue culture platforms that investigate the effects of substrate elasticity,163,171 stretching, and shearing13,172,173 has indicated that these systems have potential to improve the efficiency of human pluripotent stem cell differentiation into mature cardiomyocytes. In addition, mechanical stimulation of engineered cardiac tissues can improve their function and these grafts have the capacity to repair damaged heart tissue in vivo.174 Thus, by leveraging the important roles that mechanical cues play at multiple stages during cardiomyogenesis, we can engineer grafts from the cellular to the tissue level and create more effective regenerative strategies for cardiac disease and dysfunction.
Conclusions and Future Directions
Multicellular organisms use a wide array of signaling cues to govern their elaborate patterning and organization from the most basic level of a single cell up to the complex interworking of organ systems. Beginning with the appearance of the first cardiac precursors in the pregastrulation epiblast and continuing through the morphological organization of the beating 4-chambered heart, both soluble cues and biophysical signals regulate cardiomyogenesis. In this review, we have summarized several major chemical pathways contributing to cardiac development and proposed multiple points at which mechanical cues intersect these well-studied pathways to mediate the process. Although the study of mechanotransduction is a relatively new one, great strides have been made in uncovering the function of biophysical influences on cardiac development, function, and disease. However, many of the biochemical–biophysical interactions discussed in this review have yet to be thoroughly characterized and require future investigation to fully unravel their role in cardiomyogenesis. An example of this discussed above is that although cyclic stretch has been shown to release latent TGFβ ligands from the ECM to influence myofibroblast differentiation, its influence on cardiac progenitor differentiation has not been investigated. Multiple TGFβ pathways influence cardiomyocyte differentiation, so although it has not been shown yet, it can be assumed that cyclic stretch might act in the same way to regulate differentiation. Although cyclic stretch improves cardiomyocyte differentiation, its actions are not limited to TGFβ signaling. Other instances of TGFβ's mechanical influence include integrin or cadherin signaling; further studies should also help to clarify the complex role of biophysical interactions with these signaling pathways. Beyond TGFβ, the other signaling pathways described here are equally likely to have complex biochemical–biophysical interactions, both during development and in an adult context. Yet by exploiting knowledge gained about the mechanotransduction pathways activated during development specifically, we have the opportunity to generate novel regenerative strategies for disease intervention. For example, by using applied cyclic stretch to populations of cultured cardiomyocytes and modulating mechotransduction pathways in the cell, more mature cardiomyocytes may be produced that can then be used for cell transplantation therapies, in vitro modeling or drug discovery. Ultimately, by uncovering the ways in which mechanical forces influence development and adding this knowledge to the prevailing chemical gradient-based views, we will create a more complete picture of mammalian cardiomyogenesis. Advances in this direction will aid future work toward clarifying the healthy development and function of myocardial tissue and assist in creating an understanding of pathological and disease states.
Acknowledgments
Sources of Funding
We thank the National Institutes of Health and American Heart Association for funding support (R01AG045428 to A.J. Engler and 15POST25720070 to C.L. Happe).
Nonstandard Abbreviations and Acronyms
- BMP
bone morphogenetic protein
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- ESCs
embryonic stem cells
- FA
focal adhesion
- PS
primitive streak
- TCF
transcription factor
- TGFβ
transforming growth factor β
- Wnt
wingless
Footnotes
Disclosures
None.
References
- 1.De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–181. doi: 10.1038/35042039. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 3.Rogers KW, Schier AF. Morphogen gradients: from generation to interpretation. Annu Rev Cell Dev Biol. 2011;27:377–407. doi: 10.1146/annurev-cellbio-092910-154148. doi: 10.1146/annurev-cellbio-092910-154148. [DOI] [PubMed] [Google Scholar]
- 4.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 6.Van Vliet P, Wu SM, Zaffran S, Pucéat M. Early cardiac development: a view from stem cells to embryos. Cardiovasc Res. 2012;96:352–362. doi: 10.1093/cvr/cvs270. doi: 10.1093/cvr/cvs270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Pal S, Maiti S, Davidson LA. Force production and mechanical accommodation during convergent extension. Development. 2015;142:692–701. doi: 10.1242/dev.116533. doi: 10.1242/dev.116533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamir EA, Srinivasan V, Perucchio R, Taber LA. Mechanical asymmetry in the embryonic chick heart during looping. Ann Biomed Eng. 2003;31:1327–1336. doi: 10.1114/1.1623487. [DOI] [PubMed] [Google Scholar]
- 12.Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 13.Gwak SJ, Bhang SH, Kim IK, Kim SS, Cho SW, Jeon O, Yoo KJ, Putnam AJ, Kim BS. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials. 2008;29:844–856. doi: 10.1016/j.biomaterials.2007.10.050. doi: 10.1016/j.biomaterials.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 14.Young JL, Kretchmer K, Ondeck MG, Zambon AC, Engler AJ. Mechanosensitive kinases regulate stiffness-induced cardiomyocyte maturation. Sci Rep. 2014;4:6425. doi: 10.1038/srep06425. doi: 10.1038/srep06425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy WL, McDevitt TC, Engler AJ. Materials as stem cell regulators. Nat Mater. 2014;13:547–557. doi: 10.1038/nmat3937. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shim J, Grosberg A, Nawroth JC, Parker KK, Bertoldi K. Modeling of cardiac muscle thin films: pre-stretch, passive and active behavior. J Biomech. 2012;45:832–841. doi: 10.1016/j.jbiomech.2011.11.024. doi: 10.1016/j.jbiomech.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCain ML, Sheehy SP, Grosberg A, Goss JA, Parker KK. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc Natl Acad Sci U S A. 2013;110:9770–9775. doi: 10.1073/pnas.1304913110. doi: 10.1073/pnas.1304913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Throm Quinlan AM, Sierad LN, Capulli AK, Firstenberg LE, Billiar KL. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PLoS One. 2011;6:e23272. doi: 10.1371/journal.pone.0023272. doi: 10.1371/journal.pone.0023272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 21.McCain ML, Parker KK. Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch. 2011;462:89–104. doi: 10.1007/s00424-011-0951-4. doi: 10.1007/s00424-011-0951-4. [DOI] [PubMed] [Google Scholar]
- 22.Auda-Boucher G, Bernard B, Fontaine-Pérus J, Rouaud T, Mericksay M, Gardahaut MF. Staging of the commitment of murine cardiac cell progenitors. Dev Biol. 2000;225:214–225. doi: 10.1006/dbio.2000.9817. doi: 10.1006/dbio.2000.9817. [DOI] [PubMed] [Google Scholar]
- 23.Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development. 1997;124:1631–1642. doi: 10.1242/dev.124.9.1631. [DOI] [PubMed] [Google Scholar]
- 24.Schoenwolf GC, Garcia-Martinez V. Primitive-streak origin and state of commitment of cells of the cardiovascular system in avian and mammalian embryos. Cell Mol Biol Res. 1995;41:233–240. [PubMed] [Google Scholar]
- 25.Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568–1576. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- 27.Mason FM, Tworoger M, Martin AC. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat Cell Biol. 2013;15:926–936. doi: 10.1038/ncb2796. doi: 10.1038/ncb2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouzés E, Supatto W, Farge E. Is mechano-sensitive expression of twist involved In mesoderm formation? Biol Cell. 2004;96:471–477. doi: 10.1016/j.biolcel.2004.04.009. doi: 10.1016/j.biolcel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomo-deal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 30.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 31.Costa M, Wilson ET, Wieschaus E. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell. 1994;76:1075–1089. doi: 10.1016/0092-8674(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 32.Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. Folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- 33.Bondue A, Blanpain C. Mesp1: a key regulator of cardiovascular lineage commitment. Circ Res. 2010;107:1414–1427. doi: 10.1161/CIRCRESAHA.110.227058. doi: 10.1161/CIRCRESAHA.110.227058. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 36.Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- 37.Boettger T, Wittler L, Kessel M. FGF8 functions in the specification of the right body side of the chick. Curr Biol. 1999;9:277–280. doi: 10.1016/s0960-9822(99)80119-5. [DOI] [PubMed] [Google Scholar]
- 38.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 39.Piotrowska-Nitsche K, Perea-Gomez A, Haraguchi S, Zernicka-Goetz M. Four-cell stage mouse blastomeres have different developmental properties. Development. 2005;132:479–490. doi: 10.1242/dev.01602. doi: 10.1242/dev.01602. [DOI] [PubMed] [Google Scholar]
- 40.Ribes V, Le Roux I, Rhinn M, Schuhbaur B, Dollé P. Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development. 2009;136:665–676. doi: 10.1242/dev.016204. doi: 10.1242/dev.016204. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 42.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 43.Dathe V, Gamel A, Männer J, Brand-Saberi B, Christ B. Morphological left-right asymmetry of Hensen's node precedes the asymmetric expression of Shh and Fgf8 in the chick embryo. Anat Embryol (Berl) 2002;205:343–354. doi: 10.1007/s00429-002-0269-2. doi: 10.1007/s00429-002-0269-2. [DOI] [PubMed] [Google Scholar]
- 44.Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen's node establish left/right asymmetric gene expression in the chick. Science. 2009;324:941–944. doi: 10.1126/science.1172478. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leckband DE, de Rooij J. Cadherin adhesion and mechanotransduction. Annu Rev Cell Dev Biol. 2014;30:291–315. doi: 10.1146/annurev-cellbio-100913-013212. doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- 46.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 47.Resink TJ, Philippova M, Joshi MB, Kyriakakis E, Erne P. Cadherins and cardiovascular disease. Swiss Med Wkly. 2009;139:122–134. doi: 10.4414/smw.2009.12429. doi: smw-12429. [DOI] [PubMed] [Google Scholar]
- 48.Piven OO, Kostetskii IE, Macewicz LL, Kolomiets YM, Radice GL, Lukash LL. Requirement for N-cadherin-catenin complex in heart development. Exp Biol Med (Maywood) 2011;236:816–822. doi: 10.1258/ebm.2011.010362. doi: 10.1258/ebm.2011.010362. [DOI] [PubMed] [Google Scholar]
- 49.Gessert S, Kühl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107:186–199. doi: 10.1161/CIRCRESAHA.110.221531. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- 50.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Want/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eisenberg LM, Eisenberg CA. Want signal transduction and the formation of the myocardium. Dev Biol. 2006;293:305–315. doi: 10.1016/j.ydbio.2006.02.014. doi: 10.1016/j.ydbio.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 52.Tzahor E. Want/beta-catenin signaling and cardiogenesis: timing does matter. Dev Cell. 2007;13:10–13. doi: 10.1016/j.devcel.2007.06.006. doi: 10.1016/j.devcel.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Foley AC, Mercola M. Heart induction by Want antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qyang Y, Martin-Puig S, Chiravuri M, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Want/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 55.Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol. 2009;11:951–957. doi: 10.1038/ncb1906. doi: 10.1038/ncb1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Want/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, Minamino T, Nagai T, Kikuchi A, Asashima M, Komuro I. IGFBP-4 is an inhibitor of canonical Want signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
- 58.Schlessinger K, Hall A, Tolwinski N. Want signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 59.Phillips HM, Murdoch JN, Chaudhry B, Copp AJ, Henderson DJ. Vangl2 acts via RhoA signaling to regulate polarized cell movements during development of the proximal outflow tract. Circ Res. 2005;96:292–299. doi: 10.1161/01.RES.0000154912.08695.88. doi: 10.1161/01.RES.0000154912.08695.88. [DOI] [PubMed] [Google Scholar]
- 60.Yue Q, Wagstaff L, Yang X, Weijer C, Münsterberg A. Wnt3a-mediated chemorepulsion controls movement patterns of cardiac progenitors and requires RhoA function. Development. 2008;135:1029–1037. doi: 10.1242/dev.015321. doi: 10.1242/dev.015321. [DOI] [PubMed] [Google Scholar]
- 61.Onizuka T, Yuasa S, Kusumoto D, et al. Wnt2 accelerates cardiac myocyte differentiation from ES-cell derived mesodermal cells via non-canonical pathway. J Mol Cell Cardiol. 2012;52:650–659. doi: 10.1016/j.yjmcc.2011.11.010. doi: 10.1016/j.yjmcc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 62.Pandur P, Läsche M, Eisenberg LM, Kühl M. Want-11 activation of a non-canonical Want signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 63.Baldwin HS, Buck CA. Integrins and other cell adhesion molecules in cardiac development. Trends Cardiovasc Med. 1994;4:178–187. doi: 10.1016/1050-1738(94)90055-8. doi: 10.1016/1050-1738(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 64.Nelson WJ, Nusse R. Convergence of Want, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009;10:34–43. doi: 10.1038/nrm2592. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang KS, Robling AG. New insights into Wnt-Lrp5/6-β-catenin signaling in mechanotransduction. Front Endocrinol (Lausanne) 2014;5:246. doi: 10.3389/fendo.2014.00246. doi: 10.3389/fendo.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JY, Marston DJ, Walston T, Hardin J, Halberstadt A, Goldstein B. Want/Frizzled signaling controls C. elegans gastrulation by activating actomyosin contractility. Curr Biol. 2006;16:1986–1997. doi: 10.1016/j.cub.2006.08.090. doi: 10.1016/j.cub.2006.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 70.Foty RA, Steinberg MS. The differential adhesion hypothesis: a direct evaluation. Dev Biol. 2005;278:255–263. doi: 10.1016/j.ydbio.2004.11.012. doi: 10.1016/j.ydbio.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Curr Opin Cell Biol. 2011;23:523–530. doi: 10.1016/j.ceb.2011.08.003. doi: 10.1016/j.ceb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Kasza KE, Zallen JA. Dynamics and regulation of contractile actin-myosin networks in morphogenesis. Curr Opin Cell Biol. 2011;23:30–38. doi: 10.1016/j.ceb.2010.10.014. doi: 10.1016/j.ceb.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 74.Lindsley RC, Gill JG, Murphy TL, Langer EM, Cai M, Mashayekhi M, Wang W, Niwa N, Nerbonne JM, Kyba M, Murphy KM. Mesp1 coordinately regulates cardiovascular fate restriction and epithelial-mesenchymal transition in differentiating ESCs. Cell Stem Cell. 2008;3:55–68. doi: 10.1016/j.stem.2008.04.004. doi: 10.1016/j.stem.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. Modulation of Want/β-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials. 2012;33:2041–2049. doi: 10.1016/j.biomaterials.2011.11.070. doi: 10.1016/j.biomaterials.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kinney MA, Sargent CY, McDevitt TC. Temporal modulation of β-catenin signaling by multicellular aggregation kinetics impacts embryonic stem cell cardiomyogenesis. Stem Cells Dev. 2013;22:2665–2677. doi: 10.1089/scd.2013.0007. doi: 10.1089/scd.2013.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sargent CY, Berguig GY, Kinney MA, Hiatt LA, Carpenedo RL, Berson RE, McDevitt TC. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol Bioeng. 2010;105:611–626. doi: 10.1002/bit.22578. doi: 10.1002/bit.22578. [DOI] [PubMed] [Google Scholar]
- 79.Sargent CY, Berguig GY, McDevitt TC. Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue Eng Part A. 2009;15:331–342. doi: 10.1089/ten.tea.2008.0145. doi: 10.1089/ten.tea.2008.0145. [DOI] [PubMed] [Google Scholar]
- 80.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25:2224–2234. doi: 10.1634/stemcells.2006-0523. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 81.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 82.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2010;31:1885–1893. doi: 10.1016/j.biomaterials.2009.11.033. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Y, Yu W, Cooney AJ, Schwartz RJ, Liu Y. Brief report: Oct4 and canonical Want signaling regulate the cardiac lineage factor Mesp1 through a TCF/LEF-OCT4 composite element. Stem Cells. 2013;31:1213–1217. doi: 10.1002/stem.1362. doi: 10.1002/stem.1362. [DOI] [PubMed] [Google Scholar]
- 84.David R, Brenner C, Stieber J, Schwarz F, Brunner S, Vollmer M, Mentele E, Müller-Höcker J, Kitajima S, Lickert H, Rupp R, Franz WM. MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol. 2008;10:338–345. doi: 10.1038/ncb1696. doi: 10.1038/ncb1696. [DOI] [PubMed] [Google Scholar]
- 85.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 86.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 87.Toyofuku T, Hong Z, Kuzuya T, Tada M, Hori M. Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-beta-catenin complex. J Cell Biol. 2000;150:225–241. doi: 10.1083/jcb.150.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nagy II, Railo A, Rapila R, Hast T, Sormunen R, Tavi P, Räsänen J, Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc Res. 2010;85:100–109. doi: 10.1093/cvr/cvp254. doi: 10.1093/cvr/cvp254. [DOI] [PubMed] [Google Scholar]
- 89.Zhou W, Lin L, Majumdar A, Li X, Zhang X, Liu W, Etheridge L, Shi Y, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat Genet. 2007;39:1225–1234. doi: 10.1038/ng2112. doi: 10.1038/ng2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schleiffarth JR, Person AD, Martinsen BJ, Sukovich DJ, Neumann A, Baker CV, Lohr JL, Cornfield DN, Ekker SC, Petryk A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr Res. 2007;61:386–391. doi: 10.1203/pdr.0b013e3180323810. doi: 10.1203/pdr.0b013e3180323810. [DOI] [PubMed] [Google Scholar]
- 91.Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 92.Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat Rev Mol Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 93.Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 94.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 95.Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 97.Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 98.Andrée B, Duprez D, Vorbusch B, Arnold HH, Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech Dev. 1998;70:119–131. doi: 10.1016/s0925-4773(97)00186-x. [DOI] [PubMed] [Google Scholar]
- 99.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 100.Chen H, Shi S, Acosta L, Li W, Lu J, Bao S, Chen Z, Yang Z, Schneider MD, Chien KR, Conway SJ, Yoder MC, Haneline LS, Franco D, Shou W. BMP10 is essential for maintaining cardiac growth during murine cardio-genesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- 102.Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44:253–265. [PubMed] [Google Scholar]
- 103.Cai W, Guzzo RM, Wei K, Willems E, Davidovics H, Mercola M. A Nodal-to-TGFβ cascade exerts biphasic control over cardiopoiesis. Circ Res. 2012;111:876–881. doi: 10.1161/CIRCRESAHA.112.270272. doi: 10.1161/CIRCRESAHA.112.270272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goumans MJ, de Boer TP, Smits AM, et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–149. doi: 10.1016/j.scr.2008.02.003. doi: 10.1016/j. scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 105.Abdel-Latif A, Zuba-Surma EK, Case J, Tiwari S, Hunt G, Ranjan S, Vincent RJ, Srour EF, Bolli R, Dawn B. TGF-beta1 enhances cardiomyogenic differentiation of skeletal muscle-derived adult primitive cells. Basic Res Cardiol. 2008;103:514–524. doi: 10.1007/s00395-008-0729-9. doi: 10.1007/s00395-008-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Degnin C, Jean F, Thomas G, Christian JL. Cleavages within the prodo-main direct intracellular trafficking and degradation of mature bone morphogenetic protein-4. Mol Biol Cell. 2004;15:5012–5020. doi: 10.1091/mbc.E04-08-0673. doi: 10.1091/mbc.E04-08-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sopory S, Nelsen SM, Degnin C, Wong C, Christian JL. Regulation of bone morphogenetic protein-4 activity by sequence elements within the prodomain. J Biol Chem. 2006;281:34021–34031. doi: 10.1074/jbc.M605330200. doi: 10.1074/jbc.M605330200. [DOI] [PubMed] [Google Scholar]
- 108.Willems E, Cabral-Teixeira J, Schade D, Cai W, Reeves P, Bushway PJ, Lanier M, Walsh C, Kirchhausen T, Izpisua Belmonte JC, Cashman J, Mercola M. Small molecule-mediated TGF-β type II receptor degradation promotes cardiomyogenesis in embryonic stem cells. Cell Stem Cell. 2012;11:242–252. doi: 10.1016/j.stem.2012.04.025. doi: 10.1016/j.stem.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walsh DW, Godson C, Brazil DP, Martin F. Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends Cell Biol. 2010;20:244–256. doi: 10.1016/j.tcb.2010.01.008. doi: 10.1016/j.tcb.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 110.Re'em-Kalma Y, Lamb T, Frank D. Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc Natl Acad Sci U S A. 1995;92:12141–12145. doi: 10.1073/pnas.92.26.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- 112.Gandhi NS, Mancera RL. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim Biophys Acta. 2012;1824:1374–1381. doi: 10.1016/j.bbapap.2012.07.002. doi: 10.1016/j.bbapap.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 113.Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang X, Harris RE, Bayston LJ, Ashe HL. Type IV collagens regulate BMP signalling in Drosophila. Nature. 2008;455:72–77. doi: 10.1038/nature07214. doi: 10.1038/ nature07214. [DOI] [PubMed] [Google Scholar]
- 115.Cui Y, Hackenmiller R, Berg L, Jean F, Nakayama T, Thomas G, Christian JL. The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev. 2001;15:2797–2802. doi: 10.1101/gad.940001. doi: 10.1101/gad.940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 117.Kopf J, Paarmann P, Hiepen C, Horbelt D, Knaus P. BMP growth factor signaling in a biomechanical context. Biofactors. 2014;40:171–187. doi: 10.1002/biof.1137. doi: 10.1002/biof.1137. [DOI] [PubMed] [Google Scholar]
- 118.Hartung A, Bitton-Worms K, Rechtman MM, Wenzel V, Boergermann JH, Hassel S, Henis YI, Knaus P. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26:7791–7805. doi: 10.1128/MCB.00022-06. doi: 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol. 2002;282:F179–F190. doi: 10.1152/ajprenal.2002.282.2.F179. [DOI] [PubMed] [Google Scholar]
- 120.Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng XQ, Chien S, Yang C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc Natl Acad Sci U S A. 2011;108:9466–9471. doi: 10.1073/pnas.1106467108. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 122.Tokola H, Rysä J, Pikkarainen S, Hautala N, Leskinen H, Kerkelä R, Ilves M, Aro J, Vuolteenaho O, Ritvos O, Ruskoaho H. Bone morpho-genetic protein-2–a potential autocrine/paracrine factor in mediating the stretch activated B-type and atrial natriuretic peptide expression in cardiac myocytes. Mol Cell Endocrinol. 2015;399:9–21. doi: 10.1016/j.mce.2014.09.003. doi: 10.1016/j.mce.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 123.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-β structure and activation. Nature. 2011;474:343–349. doi: 10.1038/nature10152. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.ten Dijke P, Arthur HM. Extracellular control of TGFbeta signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 125.Schoppet M, Chavakis T, Al-Fakhri N, Kanse SM, Preissner KT. Molecular interactions and functional interference between vitronectin and transforming growth factor-beta. Lab Invest. 2002;82:37–46. doi: 10.1038/labinvest.3780393. [DOI] [PubMed] [Google Scholar]
- 126.Inger D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 127.Bökel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- 128.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT. Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 130.Ludbrook SB, Barry ST, Delves CJ, Horgan CM. The integrin alphav-beta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem J. 2003;369:311–318. doi: 10.1042/BJ20020809. doi: 10.1042/BJ20020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 133.McMahon GA, Dignam JD, Gentry LE. Structural characterization of the latent complex between transforming growth factor beta 1 and beta 1-latency-associated peptide. Biochem J. 1996;313(pt 1):343–351. doi: 10.1042/bj3130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Klingberg F, Chow ML, Koehler A, Boo S, Buscemi L, Quinn TM, Costell M, Alman BA, Genot E, Hinz B. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J Cell Biol. 2014;207:283–297. doi: 10.1083/jcb.201402006. doi: 10.1083/jcb.201402006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B. Integrins αvβ5 and αvβ3 promote latent TGF-β1 activation by human cardiac fibroblast contraction. Cardiovasc Res. 2014;102:407–417. doi: 10.1093/cvr/cvu053. doi: 10.1093/cvr/cvu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saha S, Ji L, de Pablo JJ, Palecek SP. Inhibition of human embryonic stem cell differentiation by mechanical strain. J Cell Physiol. 2006;206:126–137. doi: 10.1002/jcp.20441. doi: 10.1002/jcp.20441. [DOI] [PubMed] [Google Scholar]
- 137.Saha S, Ji L, de Pablo JJ, Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. doi: 10.1529/biophysj.107.119891. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Israeli-Rosenberg S, Manso AM, Okada H, Ross RS. Integrins and integrin-associated proteins in the cardiac myocyte. Circ Res. 2014;114:572–586. doi: 10.1161/CIRCRESAHA.114.301275. doi: 10.1161/CIRCRESAHA.114.301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pines M, Das R, Ellis SJ, Morin A, Czerniecki S, Yuan L, Klose M, Coombs D, Tanentzapf G. Mechanical force regulates integrin turnover in Drosophila in vivo. Nat Cell Biol. 2012;14:935–943. doi: 10.1038/ncb2555. doi: 10.1038/ ncb2555. [DOI] [PubMed] [Google Scholar]
- 141.Sharp WW, Simpson DG, Borg TK, Samarel AM, Terracio L. Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am J Physiol. 1997;273:H546–H556. doi: 10.1152/ajpheart.1997.273.2.H546. [DOI] [PubMed] [Google Scholar]
- 142.Meighan CM, Schwarzbauer JE. Temporal and spatial regulation of inte-grins during development. Curr Opin Cell Biol. 2008;20:520–524. doi: 10.1016/j.ceb.2008.05.010. doi: 10.1016/j.ceb.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 144.Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- 145.Mittal A, Pulina M, Hou SY, Astrof S. Fibronectin and integrin alpha 5 play requisite roles in cardiac morphogenesis. Dev Biol. 2013;381:73–82. doi: 10.1016/j.ydbio.2013.06.010. doi: 10.1016/j.ydbio.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 147.Baudoin C, Goumans MJ, Mummery C, Sonnenberg A. Knockout and knockin of the beta1 exon D define distinct roles for integrin splice variants in heart function and embryonic development. Genes Dev. 1998;12:1202–1216. doi: 10.1101/gad.12.8.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, Omura M, Leil TA, Becker KD, Jiang M, Smith DJ, Cherry SR, Loftus JC, Ross RS. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 149.Fässler R, Rohwedel J, Maltsev V, Bloch W, Lentini S, Guan K, Gullberg D, Hescheler J, Addicks K, Wobus AM. Differentiation and integrity of cardiac muscle cells are impaired in the absence of beta 1 integrin. J Cell Sci. 1996;109(pt 13):2989–2999. doi: 10.1242/jcs.109.13.2989. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J, Klos M, Wilson GF, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. doi: 10.1161/ CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heydarkhan-Hagvall S, Gluck JM, Delman C, Jung M, Ehsani N, Full S, Shemin RJ. The effect of vitronectin on the differentiation of embryonic stem cells in a 3D culture system. Biomaterials. 2012;33:2032–2040. doi: 10.1016/j.biomaterials.2011.11.065. doi: 10.1016/j.biomaterials.2011.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baharvand H, Azarnia M, Parivar K, Ashtiani SK. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol. 2005;38:495–503. doi: 10.1016/j.yjmcc.2004.12.011. doi: 10.1016/j.yjmcc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 153.Duan Y, Liu Z, O'Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rohwedel J, Guan K, Zuschratter W, Jin S, Ahnert-Hilger G, Fürst D, Fässler R, Wobus AM. Loss of beta1 integrin function results in a retardation of myogenic, but an acceleration of neuronal, differentiation of embryonic stem cells in vitro. Dev Biol. 1998;201:167–184. doi: 10.1006/dbio.1998.9002. doi: 10.1006/dbio.1998.9002. [DOI] [PubMed] [Google Scholar]
- 155.Zeng D, Ou DB, Wei T, Ding L, Liu XT, Hu XL, Li X, Zheng QS. Collagen/β(1) integrin interaction is required for embryoid body formation during cardiogenesis from murine induced pluripotent stem cells. BMC Cell Biol. 2013;14:5. doi: 10.1186/1471-2121-14-5. doi: 10.1186/1471-2121-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guan K, Czyz J, Fürst DO, Wobus AM. Expression and cellular distribution of alpha(v)integrins in beta(1)integrin-deficient embryonic stem cell-derived cardiac cells. J Mol Cell Cardiol. 2001;33:521–532. doi: 10.1006/jmcc.2000.1326. doi: 10.1006/jmcc.2000.1326. [DOI] [PubMed] [Google Scholar]
- 157.Legate KR, Wickström SA, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 158.Traister A, Aafaqi S, Masse S, Dai X, Li M, Hinek A, Nanthakumar K, Hannigan G, Coles JG. ILK induces cardiomyogenesis in the human heart. PLoS One. 2012;7:e37802. doi: 10.1371/journal.pone.0037802. doi: 10.1371/journal.pone.0037802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Thorsteinsdóttir S, Roelen BA, Goumans MJ, Ward-van Oostwaard D, Gaspar AC, Mummery CL. Expression of the alpha 6A integrin splice variant in developing mouse embryonic stem cell aggregates and correlation with cardiac muscle differentiation. Differentiation. 1999;64:173–184. doi: 10.1046/j.1432-0436.1999.6430173.x. doi: 10.1046/j.1432-0436.1999.6430173.x. [DOI] [PubMed] [Google Scholar]
- 160.van Laake LW, van Donselaar EG, Monshouwer-Kloots J, Schreurs C, Passier R, Humbel BM, Doevendans PA, Sonnenberg A, Verkleij AJ, Mummery CL. Extracellular matrix formation after transplantation of human embryonic stem cell-derived cardiomyocytes. Cell Mol Life Sci. 2010;67:277–290. doi: 10.1007/s00018-009-0179-z. doi: 10.1007/s00018-009-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 162.Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol. 2005;289:H2291–H2301. doi: 10.1152/ajpheart.00749.2005. doi: 10.1152/ajpheart.00749.2005. [DOI] [PubMed] [Google Scholar]
- 163.Hazeltine LB, Badur MG, Lian X, Das A, Han W, Palecek SP. Temporal impact of substrate mechanics on differentiation of human embryonic stem cells to cardiomyocytes. Acta Biomater. 2014;10:604–612. doi: 10.1016/j.actbio.2013.10.033. doi: 10.1016/j.actbio.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Heo JS, Lee JC. β-Catenin mediates cyclic strain-stimulated cardiomyo-genesis in mouse embryonic stem cells through ROS-dependent and integrin-mediated PI3K/Akt pathways. J Cell Biochem. 2011;112:1880–1889. doi: 10.1002/jcb.23108. doi: 10.1002/jcb.23108. [DOI] [PubMed] [Google Scholar]
- 165.Wan CR, Chung S, Kamm RD. Differentiation of embryonic stem cells into cardiomyocytes in a compliant microfluidic system. Ann Biomed Eng. 2011;39:1840–1847. doi: 10.1007/s10439-011-0275-8. doi: 10.1007/s10439-011-0275-8. [DOI] [PubMed] [Google Scholar]
- 166.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. doi: 10.1038/nrcardio.2013.105. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 167.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. doi: 10.1016/j. stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005;97:1220–1231. doi: 10.1161/01.RES.0000196562.73231.7d. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 169.Farouz Y, Chen Y, Terzic A, Menasché P. Concise review: growing hearts in the right place: on the design of biomimetic materials for cardiac stem cell differentiation. Stem Cells. 2015;33:1021–1035. doi: 10.1002/stem.1929. doi: 10.1002/stem.1929. [DOI] [PubMed] [Google Scholar]
- 170.Sheehy SP, Grosberg A, Parker KK. The contribution of cellular mechanotransduction to cardiomyocyte form and function. Biomech Model Mechanobiol. 2012;11:1227–1239. doi: 10.1007/s10237-012-0419-2. doi: 10.1007/s10237-012-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL, Palecek SP. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. doi: 10.1155/2012/508294. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Illi B, Scopece A, Nanni S, Farsetti A, Morgante L, Biglioli P, Capogrossi MC, Gaetano C. Epigenetic histone modification and cardiovascular lineage programming in mouse embryonic stem cells exposed to laminar shear stress. Circ Res. 2005;96:501–508. doi: 10.1161/01.RES.0000159181.06379.63. doi: 10.1161/01.RES.0000159181.06379.63. [DOI] [PubMed] [Google Scholar]
- 173.Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008;14:49–58. doi: 10.1089/ten.2007.0092. doi: 10.1089/ten.a.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]