Abstract
Background
Theory of mind (ToM) refers to the ability to understand and make inferences about other people’s intentions, feelings, and beliefs. Although children with fetal alcohol spectrum disorders (FASD) are known to have deficits in social-cognitive function, little is known about ToM in FASD.
Methods
ToM ability was assessed using a developmentally sensitive ToM battery, including the Reading the Mind in the Eyes (RME) test, a measure of mental inferential ability that has been found to be impaired in other clinical populations. IQ and executive function (EF) were assessed as potential mediating variables. The battery was administered to 63 children (aged 9–11 years) from Cape Town, South Africa, whose mothers had been prospectively recruited during pregnancy. Children with fetal alcohol syndrome (FAS; n=8) and partial FAS (PFAS; n=19), as well as nonsyndromal heavily exposed children (HE; n=17), were compared to children born to abstaining or light drinkers (n=19) from the same community.
Results
No FASD group differences were found on the less challenging ToM tasks. By contrast, children with FAS and PFAS performed more poorly than controls on a more challenging ToM task, the RME test. A continuous measure of prenatal alcohol exposure was more sensitive than FASD diagnosis in that it was related to four higher-order ToM measures, particularly the ability to attribute mental states assessed on RME. IQ only partially mediated the effect of exposure on RME performance, and these effects were not mediated by EF. Hence, the data suggest that these ToM measures tap into a specific alcohol-related social-cognitive deficit that does not merely reflect poorer EF. FASD diagnosis and prenatal alcohol exposure were each also related to RME after control for Attention Deficit/Hyperactivity Disorder.
Conclusions
These findings suggest that deficits in higher-order ToM function may play a significant role in the social-cognitive behavioural impairment in FASD.
Keywords: Theory of mind, fetal alcohol spectrum disorders, fetal alcohol syndrome, social cognition, affect recognition, Reading the Mind in the Eyes, prenatal alcohol exposure
INTRODUCTION
Research examining detrimental effects of alcohol on the developing brain has documented a broad range of cognitive and behavioral deficits in children with fetal alcohol spectrum disorders (FASD), including IQ and attention deficits; poorer learning and memory, and impaired executive function (EF; Mattson et al., 2011). Social and behavioral problems have also been reported in children with FASD. In one study adaptive behaviour scores in children with fetal alcohol syndrome (FAS) exceeded what could be explained by low IQ scores (Thomas et al., 1998). In another study, parents reported more social, delinquent, and aggressive behaviours in alcohol-exposed children than controls (Mattson and Riley, 2000). Similarly, teachers reported more problems in these domains in children with prenatal alcohol exposure (PAE) at 7.5 and 14 years (Jacobson et al., 2006; Dodge et al., 2014).
Social cognition is based on observation and interpretation of social stimuli, including affective expressions. Emotions of the self and others play an essential role in interpreting social situations that ultimately shape social knowledge and interactions (Halberstadt et al., 2001). Although deficits in social information processing and social problem-solving skills have frequently been described by clinicians, teachers, and caregivers (Roebuck et al., 1999), social cognitive impairment in FASD has been documented in only a few recent studies, and specific aspects of social cognition impaired in children with FASD are not well-understood. In a study using video vignettes of children in problematic social situations, alcohol-exposed children exhibited more dysfunctional social information processing than controls (McGee et al., 2009), and adolescents with heavy PAE showed impairment in everyday social problem solving, deficits that were related to poorer EF (McGee et al., 2008). A deficit in Theory of Mind (ToM), one domain of social cognitive ability related to social behaviour, may play a role in the development of these children’s poor social skills (Thomas et al., 1998).
ToM is defined as the cognitive capacity to recognize and understand mental states of others and to predict their behaviour (Baron-Cohen et al., 1994). Because social interaction requires complex and flexible behavioral initiations and responses, ToM is considered a crucial component of effective social communication (Baron-Cohen et al., 2001b). The processes underlying ToM can be characterized by (a) epistemic inferences, which are made about others’ knowledge and beliefs, and (b) affective inferences, which allow the interpretation and understanding of others’ emotional states (Stone et al., 1998). Some research suggests that ToM exists as a separate cognitive module independent of general intelligence (Baron-Cohen et al., 1994) and can be selectively impaired even when other aspects of cognition, such as language, remain intact (Stone et al. 1998).
ToM consists of a set of interrelated skills that emerge sequentially as the child matures. For example, as shown in Table 1, typically developing children understand First- and Second-order False Beliefs by 4–6 years (Wellman, 2012). Thus, when examining ToM ability in children with FASD, a developmentally sequenced ToM test battery needs to be used (Steele et al., 2003). Only three previous studies have examined ToM in FASD. Rasmussen et al. (2009) found that children (4–8 years) with FASD performed more poorly on false belief tasks than controls. Because poorer response inhibition by children with FASD predicted their ToM performance, the authors concluded that the ToM deficit was attributable to difficulties with inhibitory control. Greenbaum et al. (2009) found that children (6–13 years) with FASD were poorer at identifying facial emotions and predicting a protagonist’s behaviour and corresponding facial expression compared to children with Attention Deficit/Hyperactivity Disorder (ADHD) and healthy controls. These social skills were related to performance on tasks of emotion processing but not to performance on a ToM second-order false-belief task. A recent study comparing children with FASD to controls (aged 6–16) detected no between-group differences on the NEPSY-II Social Perception subtest (Rasmussen et al. 2013). Although these studies focused on specific aspects of ToM, none employed a comprehensive developmentally sensitive ToM battery.
Table 1.
Theory of Mind battery
| Theory of Mind measures | Description |
|---|---|
| False Belief | Assesses the ability to infer epistemic mental states. Develops by 4–6 years of age. Administered as a set of picture stories. |
| 1st-order False Beliefa | Assesses the ability to infer that others can have mental states that differ from reality. Two picture stories are presented to the participant during which an object is moved while the main character is not present. The participant is asked whether the main character, who was previously absent, will know where the object is, where the character will look for the object and whether the character will look there. Example of a 1st-order False Belief story: One day, Emma is in the kitchen eating a chocolate bar. Then Emma’s mom comes in and says, “Emma, put away that chocolate. It is time to do your chores.” “Okay,” says Emma, “but when I come back I’m going to finish eating my chocolate.” Emma puts her chocolate in the drawer and leaves to go do her chores. It is a very hot day. Mom is afraid that the chocolate will melt. Mom takes the chocolate form the drawer and puts it in the fridge. Later on, Emma comes back into the kitchen and wants to eat her chocolate. |
| 2nd-order False Beliefa | Assesses the ability to conceptualize what another person thinks a second person thinks. Two picture stories are presented to the participant and are followed by three questions: (1) an ignorance question, e.g., does the character think/know what the second character thinks/knows; (2) a belief question, e.g., what does the character think/know about what the second character thinks/knows; and (3) a justification question. Example of a 2nd-order false belief story: John and Mary are in the park when they see an ice-cream truck. Mary would like to buy an ice-cream but has no money with her. The ice-cream man tells her to go home and get her money because he will be staying in the park all day. Mary goes home, and John stays in the park. Then the ice-cream man John tells he is moving to the church. He drives off, and John goes home. On his way to the church, the ice-cream man meets Mary and tells her where he is going. They arrange to meet at the church, so Mary can buy her ice-cream. Later John goes to Mary’s house. Her sister says she has gone to buy ice-cream. |
| Strange Storiesa | Assesses the ability to infer the motivation behind responses that are not literally true. Administered as a set of 18 stories (12 test and 6 control stories); each test story assesses the understanding of a different concept, namely: lie, white lie, joke, pretend, double-bluff, persuasion, forgetting, misunderstanding, figure of speech, appearance-reality, irony and contrary emotions. This test assesses the participant’s ability to consider why the protagonist behaved in a certain way. Example of a strange story test story: Katie and Emma are playing in the house. Emma picks up a banana from the fruit bowl and holds it up to her ear. She says to Katie, “Look This banana is a telephone”! |
| Faux-Pasa | Assesses the ability to recognize a socially awkward situation. Develops by 7–11 years of age. Administered as a set of 10 stories (5 control and 5 test stories), with either a normal social event (control story) or an awkward or embarrassing event (test story). This task yields 2 variables: Faux-Pas detection (i.e., the ability to recognise when a faux-pas is present, e.g., did someone say something they should not have said?) and Faux-Pas comprehension (i.e., the ability to understand/explain a faux-pas, e.g., why should they not have said it?). Example of a faux-pas test story: Kim helped her mom make an apple pie for her uncle when he came to visit. She carried it out of the kitchen. “I made it just for you”, said Kim. “Mmm”, replied Uncle Tom, “That looks lovely. I love pies, except fo apple, of course”! |
| NEPSY-IIb | Standardized measure that provides reliable and flexible assessment of neuropsychological functioning in children aged 3–16 years. |
| Contextual task | Assesses the ability to understand the relation between emotions and the appropriate affect that would follow that emotion in various social contexts. Administration of a set of pictorial social scenes where one of four photographs needs to be chosen that best shows what a person in the scene is feeling |
| Affect Recognition | Assesses the ability to recognize emotions from facial expressions. Administration of a set of photographs of children displaying a certain emotion need to be matched to photographs with children with the same affect. |
| Reading the Mind in the Eyes Child Versionc | Assesses the ability to infer complex mental states of others from their eye expressions. Administration of 28 photographs of the eye region of males and females and one of four (1 target, 3 foil) words presented needs to be chosen that four best depict what the person may be feeling or thinking. Both cognitive and affective words are presented. |
EF contributes to social cognitive functioning and ToM ability (Fahie and Symons, 2003). Since PAE adversely impacts EF over and above low IQ (Rasmussen, 2005) and predicts social skills deficits (Schonfeld et al., 2006), it is important to determine the degree to which alcohol-related ToM deficits are due to EF impairment. Similarly, since ADHD is commonly diagnosed in children with FASD (Jacobson et al., 2011) and has an impact on ToM (Uekermann et al., 2010), we examined the extent to which these deficits are attributable to ADHD.
This study is the first to investigate ToM in children with FASD using a developmentally-sensitive ToM test battery. We administered this battery, IQ and EF tests, to a sample of school-age children with and without heavy PAE. The aims were to examine the degree to which (1) FASD diagnosis and a continuous measure of PAE obtained during pregnancy are related to poorer ToM, (2) prenatal alcohol-related effects on ToM are attributable to lower IQ or are mediated by alcohol-related deficits in EF, and (3) observed ToM effects are attributable to ADHD.
MATERIALS AND METHODS
Participants
The study was conducted in Cape Town, South Africa, where the prevalence of FASD in the Cape Coloured (mixed ancestry) community is among the highest in the world (13.6–20.9%; May et al., 2013). The Cape Coloured population comprised of descendants of European settlers, Malaysian slaves, Khoi-San aboriginals, and black Africans, historically constituted the majority of workers in the wine-producing region of the Western Cape. Weekend binge drinking continues to be a major source of recreation for many in urban and rural Cape Coloured communities (May et al. 2013).
The sample consisted of 63 Cape Coloured children (aged 9–11) born to women recruited into the Cape Town Longitudinal Cohort between 1999–2002 at the antenatal clinic of a midwife obstetric unit serving an economically disadvantaged, predominantly Cape Coloured population (Jacobson et al. 2008). Our research nurse interviewed each mother at her first antenatal visit (M=18.6 weeks gestation, SD=6.5), using a timeline follow-back procedure (Jacobson et al., 2002), regarding her alcohol consumption both at time of recruitment and conception. Volume was recorded for each type of beverage consumed each day and converted to ounces (oz) of absolute alcohol (AA; 1 oz AA≈2 standard drinks). Any mother who reported drinking at least 14 drinks/week or engaging in binge drinking (≥5 drinks/occasion) was invited to participate. Women who abstained or drank only minimally (≤ 0.5 oz AA/day and did not binge drink) were recruited as controls.
Timeline follow-back interviews were administered again in mid-pregnancy and at 1-month postpartum focusing on the latter part of pregnancy. Data from the three interviews were averaged to provide a summary measure of PAE (oz AA/day). Women <18 years of age and those with diabetes, epilepsy, or cardiac problems requiring treatment were excluded, as were observant Muslim women whose religious practices prohibit alcohol consumption. Infant exclusionary criteria were major chromosomal anomalies, neural tube defects, multiple births, and seizures.
In September 2005, we organized a clinic in which each child was examined for alcohol-related anomalies and growth by two U.S.-based expert dysmorphologists (H.E. Hoyme and L.K. Robinson) using a standard diagnostic protocol (Hoyme et al. 2005). FAS, the most severe of the fetal alcohol-related disorders, is characterized by distinctive craniofacial dysmorphology, small head circumference, and growth retardation. PFAS is diagnosed when at least two of the facial anomalies, as well as small head circumference, growth retardation, or cognitive/behavioural abnormalities, are present. Nonsyndromal heavily exposed (HE) children lack the distinctive alcohol-related facial features but still demonstrate significant neurobehavioral and cognitive deficits (Jacobson et al., 2004; Suttie et al., 2013). There was substantial inter-examiner agreement on the assessment of the principal fetal alcohol-related dysmorphic features among the two dysmorphologists and a Cape Town-based FAS dysmorphologist (N. Khaole), who examined the two children who could not attend the clinic (Jacobson et al., 2008). Case conferences including the two U.S.-based dysmorphologists, SWJ, JLJ, and CDM were held to reach consensus regarding diagnosis of FAS and PFAS.
Measures
Theory of Mind
The UCT ToM battery (Hoogenhout and Malcolm-Smith, 2014) was administered at 11.0 years. It consists of four measures: First- and Second-order False Belief, Strange Stories, and Faux-Pas (Faux-Pas Detection and Faux-Pas Comprehension), which follow a developmental trajectory and have been adapted for the South African context (Table 1). Hoogenhout and Malcolm-Smith found that Cape Town children with high-functioning autism performed more poorly than controls on this battery, whereas a higher proportion of children with Aspergers passed Faux-Pas and Strange Stories than controls. The Contextual Task and the Affect Recognition (AR) subtest, both from the NEPSY-II Social Perception subtest were also administered.
The children’s version of the Reading the Mind in the Eyes test (RME; Baron-Cohen et al., 2001a) was used to assess the child’s ability to recognize emotional expressions depicted in photographs of the eye region, including simple emotional expressions (e.g., sad), subtle emotions (e.g., kind), and complex mental states (e.g., coercion; Fig. 1). RME has been validated as an advanced test of ToM, which can identify subtle impairments in social cognition and the only test with no ceiling effects in healthy adults (Baron-Cohen et al., 2001a). Although most epistemic inference tasks place demands on other cognitive abilities, such as memory or problem-solving abilities (Stone et al., 1998), RME provides a purer measure of mental inferential ability since it does not include a naming, memory, or problem-solving component. RME investigates a fundamental aspect of social cognition that is not assessed in any of the other ToM tasks outlined above. To minimize memory and linguistic demands, the ToM stories and accompanying pictures were printed out and placed in front of the child during the tasks.
Figure 1.
Examples of the stimuli presented in the child version of the “Reading the Mind in the Eyes” task (from Baron-Cohen et al., 2001). The word choices include both basic and more complex emotions and mental state words.
Cognitive ability
IQ was assessed using the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV; Wechsler, 2003). The WISC-IV scores obtained for this sample were strongly correlated (r=0.76, p<.001) with IQ scores obtained at 5 years on the Junior South African Individual Scale (Madge et al., 1981), which is normed for South African children. Four domains of EF were assessed: attentional control by the Stop Task (Rubia et al., 1998); cognitive flexibility by the Children’s Color Trails Test (CCTT) (Llorente et al., 2003) and the Verbal Fluency and Stroop (shift and interference) subtests of the Delis-Kaplan Executive Functioning System (DKEFS; Delis et al., 2001); goal-setting by the Tower of London, 2nd Edition (TOL; Culbertson and Zillmer, 2001); and working memory (WM) by the WISC-IV Digit Span Backwards. 1- and 2-back WM data (using letters) were available for a subset of the children (Diwadkar et al., 2013).
ADHD assessment
Each child was also assessed for ADHD following research criteria developed in collaboration with two experts in ADHD research (Joel Nigg, Ph.D., and Rafael Klorman, Ph.D.) This diagnosis was based on a maternal interview administered by CDM using the Schedule for Affective Disorders and Schizophrenia for School Aged Children (K-SADS; Kaufman et al., 2005) and ratings by the child’s classroom teacher on the Disruptive Behavior Disorders Scale (DBD; Pelham et al. 1992). An ADHD classification was assigned if (a) at least 6 of the 9 inattention and/or 6 of the 9 hyperactivity/impulsivity symptoms were endorsed (“pretty much” or “very much true”) by one or more informants, and (b) endorsement of at least 2 of these symptoms was reported by 7 years, in two or more settings.
Procedure
The mother and child were transported to our UCT laboratory. Each child was tested individually on measures of ToM, IQ, and EF, while the mother was interviewed in a nearby room regarding demographic background and the child’s school and health history. Testing took place over 2 days to avoid possible fatigue effects. Breakfast, a snack, and lunch were provided, and the child was encouraged to take breaks, as needed. Except in the most severely affected FAS cases, examiners were blind regarding FASD diagnosis and alcohol exposure history.
Testing was conducted in Afrikaans or English, depending on the primary language of instruction in the child’s school by two experienced Master’s-level graduate research assistants. The ToM, WISC-IV, and EF tests were translated by a native Afrikaans-speaking Master’s-level child psychologist with extensive experience working with the children in this cohort, and back-translated by CDM, a fluent Afrikaans speaker.
Human subjects approval was obtained from the Wayne State University and University of Cape Town ethics committees. Informed consent was obtained from mothers at recruitment and child assessment visits; assent, from the children. Children received a small gift, and mothers received compensation consistent with guidelines from the ethics committees.
Statistical Analyses
The continuous alcohol and drug measures collected during pregnancy underwent log(x+1) transformation if they were skewed (skew>3.0) to reduce the effects of outliers. Four control variables were assessed—maternal education and smoking during pregnancy; child sex and age at testing. The four EF tests assessing cognitive flexibility were averaged after transformation to z-scores. Correlations were run between the control variables and the ToM measures. Any control variable even weakly related to each ToM outcome (p<0.10) was adjusted statistically in multivariate analyses of effects on that outcome. The relation of FASD diagnosis to the ToM outcome measures was examined using analysis of variance. Multiple regression was used to examine the relation between a continuous measure of PAE (AA/day during pregnancy) and each of the ToM outcome measures after control for potential confounders. Mediation by IQ and EF of the effects of prenatal alcohol on ToM was assessed using the Sobel (1982) test. The relation of FASD and AA/day to ADHD was examined using ANOVA and Pearson correlation, respectively. The association of ADHD to ToM was examined using Pearson correlation.
RESULTS
Sample characteristics
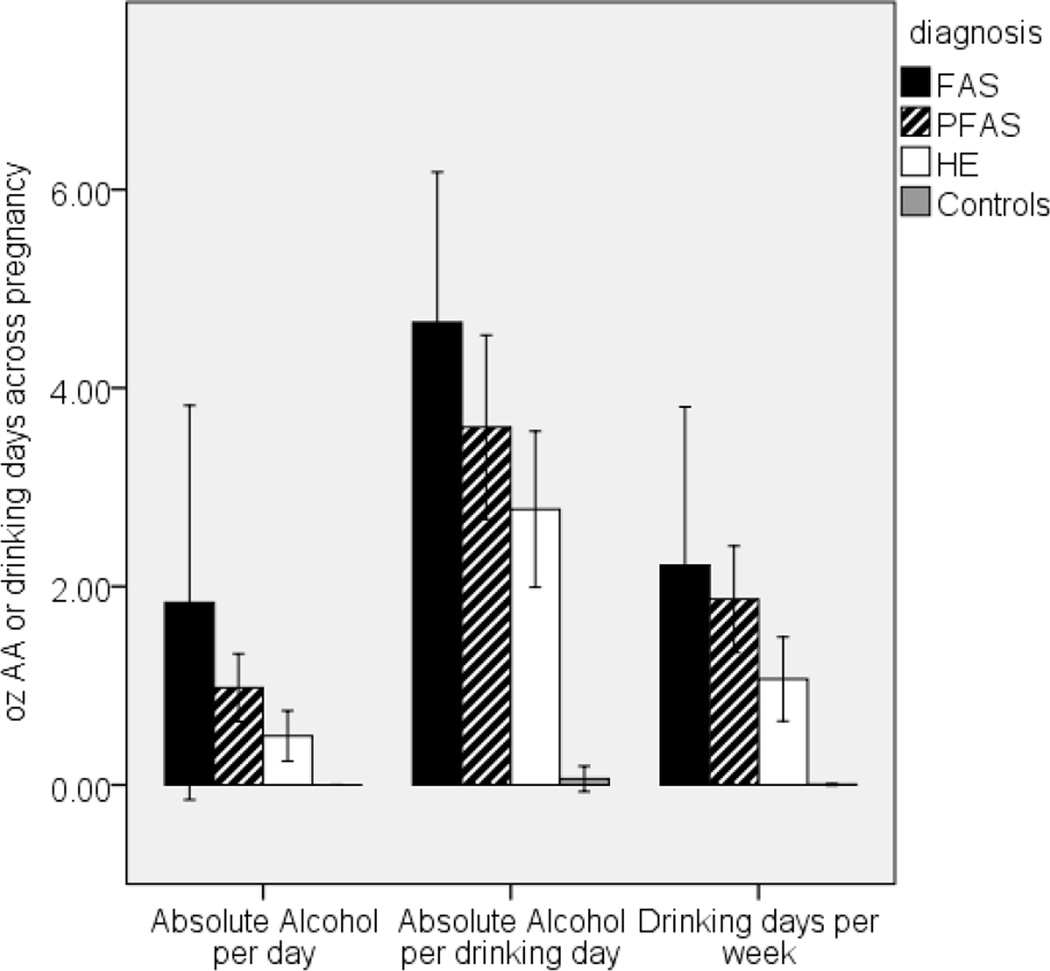
Table 2 summarizes the demographic and background characteristics of the sample. The children came from a poorly educated, socioeconomically disadvantaged population. Mothers in the FAS/PFAS group were somewhat more economically disadvantaged (mean=Level V—Unskilled Laborers, lowest of five levels) than those in the HE and control groups (mean=Level IV—Semiskilled Workers) on the Hollingshead (2011) scale. As in previous studies (Jacobson et al., 2004; May and Gossage, 2011), mothers of children with FAS were older than those of other exposed children and controls. There was a significant between-group difference for both AA/day across pregnancy (p<.001) and frequency (days/wk) (p<.001), suggesting a dose-dependent relation between maternal drinking pattern and FASD diagnosis (FAS > HE, p<.01; FAS > controls, p<.001; PFAS > HE, p<.05; PFAS > controls, p<.001; HE > HC, p<0.01; Fig. 2), but no difference in drinking pattern between the FAS and PFAS groups, p=0.43). For AA/occasion there was a similar pattern but on this measure, the FAS group did not differ significantly from the HE group (p>.05). It should be noted, however, that mothers of exposed children concentrated their drinking, resulting in consumption of an average of 3.0–4.8 oz AA/occasion (≈ 6.0–9.6 standard drinks/occasion) on 1–2 days/week. All but one control mother (98.4%) abstained from drinking during pregnancy; the one control drank 2 drinks on three occasions. Mothers of exposed children also reported smoking more than controls during pregnancy. Drug use other than alcohol and smoking during pregnancy was rare: three women (4.8%) reported using marijuana (1–3 days/month), one used cocaine, and none used methaqualone (“mandrax”). Because prenatal exposure to these drugs was too rare for statistical adjustment, associations with prenatal alcohol use were rerun omitting the children exposed to these drugs.
Table 2.
Sample characteristics
| FAS (n=8) |
PFAS (n=19) |
HE (n=17) |
Controls (n=19) |
F or χ2 | p | |
|---|---|---|---|---|---|---|
| Age at delivery | 32.6 (6.9) | 27.5 (7.2) | 25.7 (4.9) | 25.9 (3.4) | 3.19 | 0.030 |
| Education (years) | 9.1 (2.2) | 6.7 (2.6) | 9.5 (2.0) | 10.3 (1.3) | 10.39 | <0.001 |
| Socioeconomic statusa | 16.2 (8.2) | 15.2 (6.7) | 23.7 (9.1) | 27.4 (6.7) | 9.7 | <0.001 |
| Alcohol use during pregnancy | ||||||
| AA/day (oz) | 1.8 (2.4) | 1.0 (0.7) | 0.5 (0.5) | 0.0 (0.0) | 8.49 | <0.001 |
| AA/occasion (oz) | 4.7 (1.8) | 3.8 (1.8) | 3.0 (1.4) | 0.1 (0.0) | 34.17 | <0.001 |
| Frequency (days/week) | 2.2 (1.9) | 2.0 (1.0) | 1.1 (0.8) | 0.1 (0.0) | 16.96 | <0.001 |
| Smoking during pregnancy (cigarettes/day) | 8.7 (5.3) | 7.7 (6.0) | 7.5 (6.9) | 1.9 (4.9) | 4.63 | 0.006 |
| Child characteristics | ||||||
| Age at ToM and EF testing | 10.6 (0.4) | 11.1 (0.4) | 11.1 (0.4) | 11.0 (0.3) | 3.60 | 0.018 |
| Sex (% male) | 37.5 | 57.9 | 58.8 | 47.4 | 1.42 | 0.702 |
| WISC-IV IQb | 65.7 (8.2) | 64.1 (10.2) | 72.8 (7.6) | 78.1 (10.5) | 8.03 | <0.001 |
| ADHDc (number of cases) | 1 | 7 | 2 | 3 |
Values are means (SD) or %. AA=absolute alcohol; 1 oz AA/day ≈ 2 standard drinks.
Socioeconomic status (SES) at time of follow-up based on Hollingshead (2011) scale; mean for the FAS and PFAS = Level V (lowest of five social strata: Unskilled labor), while mean for the HE and control group = Level IV (semi-skilled workers).
Wechsler Intelligence Scale for Children, Fourth edition.
Met criteria for ADHD according to DSM-IV if (a) at least 6 of the 9 inattention and/or 6 of the 9 hyperactivity-impulsivity symptoms were endorsed (“pretty much” or “very much true”) by one or more informants, and (b) some impairment was reported by 7 years in two or more settings.
Figure 2.
Relation of prenatal alcohol exposure to FASD diagnosis
Children with FAS were slightly younger than those in the other groups (p=.018), and there were no between-group sex differences (Table 2). However, as in previous studies (see Vellante et al., 2013) typically developing control girls performed more optimally than boys on the RME test (M for girls=17.6, SD=1.9, M for boys=16.7, SD=4.8, p=0.001), a difference not seen in the alcohol-exposed children (p=0.77). IQ differed significantly across the four groups: children with FAS had a lower IQ than controls (p<0.01); children with PFAS, lower than both HE (p<.01) and controls (p<.001). IQ did not differ between the FAS and PFAS groups (p>.20) or between the HE and control groups (p>.05).
ToM Findings
Potential confounders
Higher maternal education was associated with better performance on the First- and Second-order False Belief, Faux-Pas Detection and RME tasks (rs ranged from 0.30 – 0.47, all ps<.05). Socioeconomic status (SES) at recruitment and at the current follow-up visit, measured using the Hollingshead (2011) scale, was associated with the NEPSY-II Affect Recognition and RME tasks (rs ranged from 0.23 – 0.53, all ps<.05). Maternal smoking during pregnancy was not related to any of the ToM measures. Boys performed better than girls on NEPSY-II Contextual (p<.01). Child age at testing was not related to ToM performance, all ps>.10.
Relation of FASD to ToM
No group differences were found for First- and Second-order False Belief, Strange Stories, and NEPSY-II tests (Table 3). Although the groups did not differ in their ability to detect a faux pas, group differences in the ability to explain a faux-pas scenario (Faux-Pas Comprehension) fell just short of statistical significance. When the data for the three exposed groups were pooled, the exposed children’s scores on Faux-Pas Comprehension were significantly lower than those of the controls, t(52)=2.68, p<.01). Analysis of the RME task showed a highly significant between-group difference even after controlling for maternal education, the only potential confounder, F(3,57)=7.54, p<.0001 (Table 3). Children in the FAS and PFAS groups performed more poorly than those in the HE and control groups (FAS and PFAS<HE, both ps<.01; FAS and PFAS<controls, both ps<.001).
Table 3.
Relation of FASD diagnosis to Theory of Mind measures
| FASD diagnostic group | FAS | PFAS | HE | Controls | F | p |
|---|---|---|---|---|---|---|
| 1st-order False Belief | 11.4 (1.5) | 11.3 (2.4) | 12.0 (0.0) | 11.0 (2.9) | 0.67 | .574 |
| 2nd-order False Belief | 2.0 (2.9) | 2.9 (2.0) | 3.8 (2.1) | 3.9 (2.0) | 2.02 | .121 |
| Strange Stories | 25.6 (6.3) | 29.5 (5.9) | 30.2 (5.3) | 29.9 (6.1) | 1.11 | .354 |
| Faux-Pas Detection | 2.1 (1.4) | 2.0 (1.5) | 2.7 (1.7) | 2.8 (1.9) | 2.57 | .506 |
| Faux-Pas Comprehension | 1.4 (1.6) | 1.7 (2.4) | 2.4 (2.6) | 4.2 (4.0) | 2.57 | .065 |
| NEPSY-II Contextual | 3.5 (1.2) | 4.1 (1.2) | 4.3 (1.3) | 4.4 (0.9) | 1.24 | .303 |
| NEPSY-II Affect Recognition | 22.6 (2.7) | 22.9 (5.5) | 24.1 (2.6) | 24.2 (3.2) | 1.07 | .370 |
| Reading the Mind in the Eyes | 10.0 (2.4) | 12.2 (2.8) | 15.4 (4.6) | 17.2 (3.5) | 10.98 | <.001 |
Values are means (SD) of raw scores.
Relation of AA/day to ToM
AA/day was related to four of the ToM measures (Faux-Pas Comprehension, NEPSY-II Contextual, NEPSY-II Affect Recognition, and RME) after control for potential confounders (Table 4), with the magnitude of the effect on the RME task clearly the strongest. The associations of PAE with Second-order False Belief and Faux-Pas Detection were attributable to higher maternal education. By contrast, the effect on Faux-Pas Comprehension remained significant after control for maternal education, as did the effect on RME. Similarly, the alcohol effect on NEPSY-II Contextual remained significant after adjustment for child sex. Although the relation of alcohol on the NEPSY-II Affect Recognition was no longer significant after adjustment for SES at recruitment and follow-up, the relation of alcohol exposure to RME remained significant after control for SES.
Table 4.
Effects of prenatal alcohol exposure and potential confounders on ToM measures
| Absolute alcohol/day |
Potential confounder |
||||
|---|---|---|---|---|---|
| Measures | N | r | βa | r | βb |
| Controlling for maternal education | |||||
| 1st-order False Belief | 61 | −0.10 | 0.12 | 0.30** | 0.37* |
| 2nd-order False Belief | 62 | −0.24* | −0.07 | 0.34** | 0.30* |
| Faux-Pas Detection | 54 | −0.31** | −0.15 | 0.36** | 0.27† |
| Faux-Pas Comprehension | 54 | −0.36** | −0.34* | 0.23* | 0.04 |
| Reading the Mind in the Eyes | 63 | −0.55*** | −0.42** | −0.47*** | −0.22† |
| Controlling for child sex | |||||
| NEPSY-II Contextual | 62 | −0.31** | −0.32** | 0.23* | 0.25* |
| Controlling for SES at recruitment | |||||
| NEPSY-II Affect Recognition | 62 | −0.30* | −0.18 | 0.34* | 0.24 |
| Reading the Mind in the Eyes | 63 | −0.53*** | −0.45*** | 0.40*** | 0.17 |
| Controlling for SES at follow-up | |||||
| NEPSY-II Affect Recognition | 62 | −0.33** | −0.15 | 0.37** | 0.27† |
| Reading the Mind in the Eyes | 63 | −0.53*** | −0.33* | 0.53*** | 0.31* |
p < 0.10;
p < 0.05;
p < 0.01;
p < 0.001.
Values are Pearson rs and standardised regression coefficients (β).
Effects of AA/day after control for potential confounder;
Effects of potential confounder after control for AA/day
The NEPSY-II AR task uses two different formats of stimulus presentation: (1) facial photos for each problem presented simultaneously; (2) stimuli to be compared presented sequentially, thus, entailing an element of WM. Simple facial AR, measured by the simultaneous items, was not altered by alcohol at this age (r=-.09, p>.20). By contrast, the effect of alcohol on the more challenging sequential AR items (r=−.43, p=.001) remained significant after control for WM as measured by the 1-back (β=−0.47, p=.012) and 2-back (β=−0.42, p<.05) tasks. Thus, the alcohol effect on the sequential AR items was not attributable to WM.
Mediation by IQ and EF
Regression analyses relating AA/day and IQ to each of the ToM measures are shown in Table 5. Results of the Sobel tests indicate that the effects of PAE on Faux-Pas Comprehension and NEPSY-II Affect Recognition were significantly mediated by IQ and were no longer significant after adjustment for IQ, indicating that alcohol-related IQ deficits play a major role in the observed ToM impairment in these domains. By contrast, the effect of AA/day on RME was only partially mediated by IQ and continued to be significant after adjustment for IQ, indicating that PAE impacts RME over and above its effect on overall intellectual function. IQ did not significantly mediate the effect of prenatal alcohol on performance on the NEPSY Contextual task.
Table 5.
Mediation of the effects of prenatal alcohol exposure on ToM measures by IQ
| Measures | N | r | β | Sobel z |
|---|---|---|---|---|
| 1st-order False Belief | 61 | −0.10 | −0.04 | −0.76 |
| 2nd-order False Belief | 62 | −0.23† | −0.02 | −2.55** |
| Strange Stories | 50 | −0.17 | 0.04 | −3.16** |
| Faux-Pas Detection | 54 | −0.29* | −0.11 | −2.36* |
| Faux-Pas Comprehension | 54 | −0.32* | −0.14 | −2.45** |
| NEPSY-II Contextual | 62 | −0.31** | −0.21 | −1.29 |
| NEPSY-II Affect Recognition | 60 | −0.33** | −0.12 | −2.41* |
| Reading the Mind in the Eyes | 63 | −0.54*** | −0.29** | −3.18** |
p < 0.1;
p < 0.05;
p < 0.01;
p < 0.001.
r shows the relation between AA/day and each of the ToM measures.
β is the standardised regression coefficient relating AA/day to the ToM measure after statistical adjustment for WISC-IV IQ.
By contrast to IQ, there was little mediation of the effects of PAE on the ToM measures by EF (Table 6). The alcohol effect on Faux-Pas Comprehension was mediated by cognitive flexibility, Sobel z=−2.45, p=.014, and mediation of the effect on Faux-Pas Detection by WM fell short of statistical significance, Sobel z=1.89, p=.058. None of the EF measures mediated any of the other effects of PAE on ToM measures, all ps>.15. WM had a much stronger effect on Faux-Pas Detection than on Faux-Pas Comprehension, suggesting that it specifically impacts the child’s ability to hold the story in memory and then to manipulate it. The effect on RME remained significant even after controlling simultaneously for IQ and all four EF measures for both AA/day (β=−0.28, p<.05) and FASD diagnosis (F(3,53)=4.90, p<.01.).
Table 6.
Effect of prenatal alcohol exposure on ToM measures controlling for executive function
| Mediating variables | ||||||
|---|---|---|---|---|---|---|
| Working memory | Goal setting | Attentional controla | Cognitive flexibility | |||
| Measures | N | r | β | β | β | β |
| 1st-order False Belief | 61 | −0.10 | 0.01 | −0.10 | −0.18 | −0.06 |
| 2nd-order False Belief | 62 | −0.23† | −0.18 | −0.23† | −0.26† | −0.21 |
| Strange Stories | 50 | −0.17 | −0.11 | −0.16 | −0.12 | −0.15 |
| Faux-Pas Detection | 54 | −0.29* | −0.16† | −0.29* | −0.31* | −0.23 |
| Faux-Pas Comprehension | 54 | −0.32* | −0.24 | −0.32* | −0.36* | −0.26† |
| NEPSY-II Contextual | 62 | −0.31* | −0.29* | −0.30* | −0.29* | −0.31* |
| NEPSY-II Affect Recognition | 60 | −0.33** | −0.29* | −0.32** | −0.31* | −0.28* |
| Reading the Mind in the Eyes | 63 | −0.54*** | −0.48** | −0.54*** | −0.51*** | −0.50*** |
p < 0.1;
p < 0.05;
p < 0.01;
p < 0.001.
r shows the relation between AA/day and each of the ToM measures. β is the standardised regression coefficient relating AA/day to the ToM measure after statistical adjustment for executive function mediating variables.
Estimated for 6 cases.
Controlling for drug use
Because very few mothers reported using drugs, we reran the effect of alcohol on the RME task excluding the cases that reported drug use. The effect of alcohol on RME remained significant when analysed in relation both to diagnostic groups, F(3,54)=9.86, p<.001, and to the continuous alcohol measure, r=−.54, p<.001.
PAE and ADHD
ADHD was significantly related to RME (r=−.25, p=.047), not significantly related to First-order False Belief (r=−.23, p=.071), and not related to any of the other ToM measures (all ps>.15). There was an adverse effect on RME of number of Inattentive (r=−.31, p=.014), but not Hyperactive-Impulsive (r=−.15, p=.255), symptoms of ADHD. Multivariate analyses showed that FASD diagnosis (F(3,58)=10.46, p<.001) and AA/day (β=−0.51, p=.001) were each significantly related to RME after control for ADHD, whereas ADHD was no longer significantly related to RME (β=−0.14, p=.20) after control for alcohol exposure. Similarly, regression analyses showed that prenatal alcohol (β=−0.54, p=.001) was independently related to the RME, whereas Inattentive (β=−0.13, p=.25) and Hyperactive symptoms were not (β=−0.07, p=.559).
DISCUSSION
Although children with FASD have been described as having alcohol-related social problems, the potential of an environmental insult, such as PAE, to alter ToM function has received little attention. Despite cross-cultural variations, children understand First- and Second-order False Belief by preschool and early school age, respectively. Consistent with these findings, we found no group differences on First- and Second-order False Belief tasks in our Cape Town cohort at 9–11 years, suggesting that the ability to infer epistemic mental states at both First- and Second-order levels is equally developed across all groups by this age. Previous research on First-order False Belief in FASD found significant impairment in younger alcohol-exposed children (Rasmussen et al. 2009). Our findings showing that the deficit in this elementary form of ToM is no longer evident in older school-age children suggest that the earlier deficit was attributable to developmental delay rather than arrested development and are consistent with findings from another study of school-aged children with FASD in which no differences were found on Second-order False-Belief tasks (Greenbaum et al. 2009).
Our most robust findings were the effects of alcohol on the RME task, which were seen when the data were analyzed using FASD diagnostic group as the predictor and when using the continuous measure of PAE as the predictor. These findings suggest that alcohol-exposed children have deficits in the attribution of mental states, a critical aspect of ToM. The alcohol effect on RME was not accounted for by deficits in IQ or EF or by ADHD, nor by sociodemographic background. This deficit is likely most clearly evident, in part, because RME is the ToM task that is least dependent on general cognitive function. Baron-Cohen et al. (1997) characterize it as a “pure” ToM task as it clearly does not require engagement of EF domains, such as attentional control. In contrast to Faux-Pas Comprehension, the RME task also does not require cognitive flexibility. It makes only minimal demands on WM because a written version of the affective words and mental state phrases are left in front of the child and do not need to be recalled. Also, knowledge of the meaning of the words is not essential as participants are encouraged to ask the meaning of any unfamiliar words, and there is no time limit. Thus, the RME data indicate that impaired responsiveness to emotional cues in children with FASD cannot be attributed to poor attention but reflect specific alcohol-related deficits in affective processing necessary for interpretation of complex mental states. These data are consistent with Kerns et al.’s (2015) recent findings that children with FASD have more difficulty with emotion recognition than controls.
We demonstrated that the effect of alcohol on RME persists after adjustment for IQ, which is consistent with previous findings in which no significant association was found between RME performance and IQ in adults (Baron-Cohen et al., 2001a). In the current study, the RME effects also persisted after adjustment for EF. These findings extend this work by showing the RME task to be a useful and valid test for detecting impairment in social cognition independent of IQ and EF in children with FASD. Previous studies have found activation in the amygdala, the superior temporal gyrus and the posterior superior temporal sulcus, using RME or other affective processing tasks (Adams et al., 2009; Barbour et al., 2010; Baron-Cohen et al., 1999b). Hence, in future neuroimaging research one would expect alcohol-related deficits in brain regions related to affective processing in children with FASD.
Investigation of higher-order ToM abilities revealed that alcohol-exposed and control participants performed equally well on the Strange Stories and Faux-Pas Detection task. These findings suggest that the ToM deficits in FASD are less severe than those seen in children with Autism Spectrum Disorders in whom deficits on Strange Stories and Faux-Pas continue to be detected at school age (Baron-Cohen et al., 1999a; Happé, 1994).
However, our data also indicate that other higher-order ToM domains are adversely affected by PAE at school age. Given that EF might be expected to play an important role in social information processing, it is impressive that virtually none of these effects were mediated by EF. Effects in two of the four affected ToM domains—Faux-Pas Comprehension and NEPSY-II AR—were mediated by alcohol effects on IQ. Dependence of these more complex tasks on higher-order cognitive function makes it difficult to determine whether the observed effects are attributable to the alcohol-related IQ effects or are merely more difficult to detect after adjustment for IQ. However, the findings that fetal alcohol-related ToM deficits on the NEPSY-II Contextual and RME tasks were not accounted for by IQ shows that PAE is independently related to specific higher-order ToM impairments in FASD.
The previous FASD study that used the NEPSY-II ToM assessment, which combines Verbal and Contextual tasks, found no alcohol-related impairment (Rasmussen et al., 2013). In our study we only administered the Contextual task from the NEPSY-II ToM assessment, since the verbal component provides a broad assessment of social cognition not specifically focused on ToM. In the Contextual task, the child cannot see the facial expression but must infer what the person might be feeling given the context. As noted earlier, the effect of PAE on performance on the NEPSY-II Contextual task was not mediated by IQ or EF or attributable to potential confounders. Thus, this task and RME, which both assess mental state attribution, were sensitive to PAE.
We evaluated performance on the ToM tasks using both FASD diagnosis and a measure of continuous alcohol exposure as a predictor. Although the FASD diagnostic groups clearly differed in RME performance, the continuous measure of alcohol exposure was more sensitive in detecting deficits on three other ToM tasks, even after adjustment for potential confounders, including maternal education and SES.
Limitations
This study has limitations common to other longitudinal studies of PAE in that it depends on the mother’s report of her alcohol consumption during pregnancy. A strength of this study, however, is that PAE was determined prospectively on the basis of maternal reports obtained during pregnancy. Moreover, our alcohol ascertainment protocol has been validated in relation to levels of fatty acid ethyl esters in meconium samples in this community (Bearer et al., 2003) and in relation to child cognitive outcomes (e.g., Jacobson et al., 2002; 2004). Given the sample size and that all of the children were from the Cape Coloured community, these findings warrant replication in other populations.
Conclusion
These findings suggest that deficits in higher-order ToM function, specifically mentalizing through eye expression, may play a significant role in the behavioral impairment often described clinically in children with FASD. People’s eyes are thought to provide us with invaluable social cues as they allow us to decode other’s intentions and are, thus, a crucial guide for our social interactions. Children with FASD appear to have difficulty reading people’s emotions, and this deficit may contribute to their deficits in social cognition. Interventions using developmentally appropriate tasks need to be designed to help alcohol-exposed children focus on and improve their ability to read affective cues and facial expressions and improve their social attention and affective information processing.
Acknowledgments
We thank Denis L. Viljoen, MD, for his collaboration on the recruitment and infant phase of the study; Maggie September, Anna Susan Marais, Julie Croxford, and Renee Sun, for their contributions to the subject recruitment and data collection. We thank the University of Cape Town Autism Research group for use of the UCT Theory of Mind battery. We appreciate the consultation and input from Joel Nigg, PhD, and Rafael Klorman, PhD, regarding the ADHD diagnoses. We also thank H. Eugene Hoyme, MD, Luther K. Robinson, MD, and Nathaniel Khaole, MD, who conducted the FAS dysmorphology examinations in conjunction with the NIAAA Collaborative Initiative on Fetal Alcohol Spectrum Disorders. We wish to express our appreciation to the mothers and children who have participated in the longitudinal study. Funding: NIH/NIAAA R01 AA09524, R01 AA016781, U01 AA014790, U24 AA014815; and Joseph Young, Sr., Fund from the State of Michigan. Portions of this research were presented at the 2012 meetings of the Research Society on Alcoholism.
Footnotes
CONFLICT OF INTEREST
None of the authors have any conflicts of interest related to this study.
References
- Adams RB, Rule NO, Franklin RG, Wang E, Stevenson MT, Yoshikawa S, Nomura M, Sato W, Kveraga K, Ambady N. Cross-cultural reading the mind in the eyes: an fMRI investigation. J Cognitive Neurosci. 2009;22:97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Barbour T, Murphy E, Pruitt P, Eickhoff SB, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Reduced intra-amygdala activity to positively valenced faces in adolescent schizophrenia offspring. Schizophr Res. 2010;123:126–136. doi: 10.1016/j.schres.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of Theory of Mind: evidence from very high functioning adults with autism or Asperger syndrome. J Child Psychol Psyc. 1997;38:813–822. doi: 10.1111/j.1469-7610.1997.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, O’Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. J Autism Dev Disord. 1999a;29:407–418. doi: 10.1023/a:1023035012436. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Moriarty J, Schmitz B, Costa D, Ell P. Recognition of mental state terms. Clinical findings in children with Autism and a functional neuroimaging study of normal adults. Br J Psychiatry. 1994;165:640–649. doi: 10.1192/bjp.165.5.640. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SCR. Social intelligence in the normal and autisitc brain: an fMRI study. European J Neurosci. 1999b;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001a;42:241–251. [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Scahill V, Lawson J, Spong A. Are intuitive physics and intuitive psychology independent? Journal of Developmental and Learning Disorders. 2001b;5:47–78. [Google Scholar]
- Bearer CF, Jacobson JL, Jacobson SW, Barr D, Croxford J, Molteno CD, Viljoen DL, Marais AS, Chiodo LM, Cwik AS. Validation of a new biomarker of fetal exposure to alcohol. J Pediatr. 2003;143:463–469. doi: 10.1067/S0022-3476(03)00442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, Jacobson SW, Jacobson JL. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum Brain Mapp. 2013;34:1931–1945. doi: 10.1002/hbm.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge NC, Jacobson JL, Jacobson SW. Protective effects of the alcohol dehydrogenase-ADH1B*3 allele on attention and behavior problems in adolescents exposed to alcohol during pregnancy. Neurotoxicology and Teratology. 2014;41:43–50. doi: 10.1016/j.ntt.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahie CM, Symons DK. Executive functioning and theory of mind in children clinically referred for attention and behavior problems. J Appl Dev Psychol. 2003;24:51–73. [Google Scholar]
- Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J. Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: a comparison with attention deficit hyperactivity disorder. Alcohol Clin Exp Res. 2009;33:1656–1670. doi: 10.1111/j.1530-0277.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- Halberstadt A, Denham SA, Dunsmore J. Affective social competence. Soc Dev. 2001;10:79–119. [Google Scholar]
- Happé FGE. An advanced test of theory of mind: Understanding by story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24:129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale J Sociol. 2011;8:21–51. [Google Scholar]
- Hoogenhout M, Malcolm-Smith S. Theory of mind in autism spectrum disorder: Does DSM classification predict development? Res Autism Spectr Disord. 2014;8:597–607. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A Practical Clinical Approach to Diagnosis of Fetal Alcohol Spectrum Disorders: Clarification of the 1996 Institute of Medicine Criteria. Paediatrics. 2005;115:39–48. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson JL, Dodge ND, Burden MJ, Klorman R, Jacobson SW. Number processing in adolescents with prenatal alcohol exposure and ADHD: Differences in the neurobehavioral phenotype. Alcohol Clin Exp Res. 2011;35:431–442. doi: 10.1111/j.1530-0277.2010.01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Carr LG, Croxford J, Sokol RJ, Li T, Jacobson JL. Protective effects of the alcohol dehydrogenase-ADH1B allele in children exposed to alcohol during pregnancy. J Pediatr. 2006;148:30–37. doi: 10.1016/j.jpeds.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Chiodo LM, Jacobson JL, Sokol RJ. Validity of maternal report of alcohol, cocaine, and smoking during pregnancy in relation to infant neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden M, Fuller D, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32:365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. The schedule for affective disorders and schizophrenia for school aged children (6–18 years) Lifetime version. 1996 doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kerns KA, Siklos S, Baker L, Müller U. Emotion recognition in children with fetal alcohol spectrum disorders. Child Neuropsychol. 2015:1–21. doi: 10.1080/09297049.2014.993310. [DOI] [PubMed] [Google Scholar]
- Llorente AM, Williams J, Satz P, D’Elia LF. Children’s color trails test: professional manual. Odessa, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Madge EM, vandenBerg AR, Robinson M, Landman J. Human Sciences Research Council. Pretoria: 1981. Junior South African individual scales. [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. Parent ratings of behavior in children with heavy prenatal alcohol exposure and IQ-matched controls. Alcohol Clin Exp Res. 2000;24:226–231. [PubMed] [Google Scholar]
- May P, Gossage JP. Maternal risk factors for fetal alcohol spectrum disorders. Alcohol Res Health. 2011;34:15–26. [PMC free article] [PubMed] [Google Scholar]
- May P, Blankenship J, Marais A, Gossage JP, Kalberg WO, Barnard R, De Vries M, Robinson LK, Adnams CM, Buckley D, Manning M, Jones KL, Parry C, Hoyme HE, Seedat S. Approaching the prevalance of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol Clin Exp Res. 2013;37:818–830. doi: 10.1111/acer.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Bjorkquist OA, Price JM, Mattson S, Riley EP. Social information processing skills in children with histories of heavy prenatal alcohol exposure. J Abnorm Child Psychol. 2009;37:817–830. doi: 10.1007/s10802-009-9313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee CL, Fryer SL, Bjorkquist OA, Mattson S, Riley EP. Deficits in social problem solving in adolescents with prenatal exposure to alcohol. Am J Drug Alcohol Abuse. 2008;34:423–431. doi: 10.1080/00952990802122630. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Evans SW, Gnagy EM, Greenslade KE. Teacher ratings of DSM–III–R symptoms for the disruptive behavior disorders: Prevalence, factor analyses, and conditional probabilities in a special education sample. School Psychol Rev. 1992;21:285–299. [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29:1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Tamana S, Baugh L, Andrew G, Tough S, Zwaigenbaum L. Neuropsychological impairments on the NEPSY-II among children with FASD. Child Neuropsychol. 2013;19:337–349. doi: 10.1080/09297049.2012.658768. [DOI] [PubMed] [Google Scholar]
- Rasmussen C, Wyper K, Talwar V. The relation between theory of mind and executive functions in children with fetal alcohol spectrum disorders. Can J Clin Pharmacol. 2009;16:370–380. [PubMed] [Google Scholar]
- Roebuck TM, Mattson SN, Riley EP. Behavioral and psychosocial profiles of alcohol-exposed children. Alcohol Clin Exp Res. 1999;23:1070–1076. [PubMed] [Google Scholar]
- Rubia K, Oosterlaan J, Sergeant JA, Brandeis D, van Leeuwen T. Inhibitory dysfunction in hyperactive boys. Behav Brain Res. 1998;94:25–32. doi: 10.1016/s0166-4328(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychol. 2006;12:439–452. doi: 10.1080/09297040600611338. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- Steele S, Joseph RM, Tager-Flusberg H. Brief report: developmental change in theory of mind abilities in children with autism. J Autism Dev Disord. 2003;33:461–467. doi: 10.1023/a:1025075115100. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Carmichael Olson H, Sampson PD, Bookstein FL, Burgess DM. Drinking during pregnancy decreases word attack and arithmetic scores on standardized tests: adolescent data from a population-based prospective study. Alcohol Clin Exp Res. 1994;18:248–254. doi: 10.1111/j.1530-0277.1994.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Kogan J, Bookstein FL. Centers for Disease Control and Prevention; 1996. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE); Final Report. [Google Scholar]
- Suttie M, Foroud T, Wetherill L, Jacoboson JL, Molteno CD, Meintjes EM, Hoyme HE, Khaole N, Robinson LK, Riley EP, Jacobson SW, Hammond P. Facial dysmorphism across the fetal alcohol spectrum. Pediatrics. 2013;131:779–788. doi: 10.1542/peds.2012-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol Clin Exp Res. 1998;22:528–533. [PubMed] [Google Scholar]
- Uekermann J, Kraemer M, Abdel-Hamid M, Schimmelmann BG, Hebebrand J, Daum I, Wiltfang J, Kis B. Social cognition in attention-deficit hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2010;34:734–743. doi: 10.1016/j.neubiorev.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Vellante M, Baron-Cohen S, Melis M, Marrone M, Petretto DR, Masala C, Preti A. The “Reading the Mind in the Eyes” test: Systematic review of psychometric properties and a validation study in Italy. Cogn Neuropsychiatry. 2013;18:326–354. doi: 10.1080/13546805.2012.721728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman HM. Theory of mind: Better methods, clearer findings, more development. Eur J Dev Psychol. 2012;9:313–330. [Google Scholar]