Abstract
Objective
Reduced expression or increased degradation of BK (large conductance Ca2+-activated K+) channel β1-subunits has been associated with increased vascular tone and hypertension in some metabolic diseases. The contribution of BK channel function to control of blood pressure (BP), heart rate (HR) and vascular function/structure was determined in wild-type and BK channel β1-subunit knockout mice fed a high-fat or control diet.
Methods and Results
After 24 weeks of high-fat diet, wild-type and BK β1-knockout mice were obese, diabetic, but normotensive. High-fat-BK β1-knockout mice had decreased HR while high-fat-wild-type mice had increased HR compared with mice on the control diet. Ganglion blockade caused a greater fall in BP and HR in high-fat mice than in mice on the control diet. β1-adrenergic receptor blockade reduced BP and HR equally in all groups. α1-adrenergic receptor blockade decreased BP in high-fat-BK β1-knockout mice only. Echocardiographic evaluation revealed left ventricular hypertrophy in high-fat-BK β1-knockout mice. Although under anesthesia, mice on a high-fat had higher absolute stroke volume and cardiac output, these measures were similar to control mice when adjusted for body weight. Mesenteric arteries from high-fat-BK β1-knockout mice had higher norepinephrine reactivity, greater wall thickness and collagen accumulation than high-fat-wild-type mesenteric arteries. Compared with control-wild-type mesenteric arteries, high-fat-wild-type mesenteric arteries had blunted contractile responses to a BK channel blocker, although BK α-subunit (protein) and β1-subunit (mRNA) expression were unchanged.
Conclusions
BK channel deficiency promotes increased sympathetic control of blood pressure, and vascular dysfunction, remodeling and fibrosis, but does not cause hypertension in high-fat fed mice.
Keywords: BK β1-subunit knockout, blood pressure and heart rate, cardiac function, high-fat diet, vascular remodeling and fibrosis,
Introduction
Obesity is a major risk factor for the development of hypertension and 75% of the incidence of hypertension is obesity-related.[1] The pathophysiological mechanisms responsible for obesity-induced hypertension, however, are complex and incompletely understood.[1,2] Increased peripheral resistance contributes to obesity-associated hypertension.[3,4] Vascular tone, an important contributor to peripheral resistance, is modulated by K+ and Ca2+ channel function, and vascular ion channel dysfunction occurs in hypertension.[5-7] BK channels are expressed by vascular smooth muscle cells (VSMC) wherein they regulate vascular contractility. BK channels are assembled from pore forming α-subunits and accessory β-subunits that modulate α-subunit Ca2+ sensitivity and channel activity.[8,9] In VSMC, BK channels maintain membrane potential and myogenic tone through negative-feedback modulation. Activation of BK channels causes VSMC hyperpolarization, L-type Ca2+ channel closure and vasodilation.[8,9] There are four subtypes of BK channel β-subunits (β1-β4), and β1-subunits are specific for SMC. BK β1-knockout reduces α-subunit Ca2+ sensitivity causing VSMC membrane depolarization, increased vascular tone and enhanced norepinephrine sensitivity.[8,9] Vascular BK channel dysfunction alone is not sufficient to cause hypertension as C57BL/6 BK β1-knockout mice are normotensive when blood pressure is measured using radiotelemetry.[10-12] Vascular BK channel dysfunction may promote hypertension, however, when combined with other pathophysiological conditions.[5-7]
Vascular BK channel function is often impaired in diabetic patients.[13] Animal models of metabolic disorders such as insulin resistance[14], diabetes[15], and genetic[16]or high-fat diet-induced obesity[17-19] also show vascular BK channel dysfunction. Reduced expression[17-19] or increased degradation of BK β1-subunit protein caused by oxidative signaling[14,15] appears responsible for vascular BK channel dysfunction, as α-subunit protein expression is unchanged. BK channel deficiency contributes to increased vascular tone and blood pressure regulation in these animal models of metabolic disease.[16-19] One possible explanation for this link is that the anti-contractile activity of adiponectin is mediated by BK channel activation[20], and lower adiponectin levels are associated with human cardiovascular and metabolic diseases.[21] Thus maintenance of BK channel activity may protect against vascular dysfunction and hypertension, and reduced BK β1-subunit expression/function may be responsible for impaired vascular BK channel function in obesity.[17-19] We hypothesized that preexisting SMC BK channel deficiency would promote vascular dysfunction and hypertension in mice fed a high-fat diet. As there is no specific BK β1-subunit inhibitor, we used high-fat-fed BK β1-knockout mice to model human high-fat diet-induced obesity[22], and by utilizing mice with SMC specific knockout of BK β1-subunit, we minimized interference of BK channel function in other tissues. The high-fat diet fed rodent models have been used extensively in studies on the pathophysiology of obesity-associated metabolic disorders, and are presumed to mimic the etiology of primary human diet-induced obesity[17-19, 22]. BK β1-knockout mice have been used by others to mimic the SMC BK channel deficiency known to occur in hypertension[10,12], septic shock[11], and metabolic disorder.[20]
Mterials and Methods
Animals and diets
BK β1-knockout mice used in our studies are congenic as a result of seven generations of inbreeding to the C57BL/6J line (From Jacksons Laboratory) and maintained as homozygous lines in Dr. Brenner's laboratory.[8] Breeding and maintenance of BK β1-knockout mice have been described previously.[10,11] Pups of BK β1-knockout mice were weaned at 4 weeks. Mice were divided into two groups at the end of 4 weeks and fed either a control diet D12450B (10 kcal% fat) or high fat diet (high-fat) D12492 (60 kcal% fat, Research Diets, Inc.) adlibitum. Age and diet matched wild-type (C57BL/6J) mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA). Mice were maintained on their diets for up to 24 weeks. Mice were weighed weekly. All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and approved by the Michigan State University Institutional Animal Care and Use Committee.
Measurement of mean arterial blood pressure (MAP) and heart rate (HR)
Procedures used for implantation of BP telemeters in mice have been described previously.[10,11] After 12 weeks on the control or high-fat diet, radiotelemeters (PA-C10, Data Sciences International, St. Paul, Minnesota, USA) were implanted under isoflurane anesthesia (2-3%). After the mice recovered from surgery (> 3 days), BP and HR were sampled continuously for 10 seconds every 10 minutes. Daily MAP and HR were collected up to 24 weeks during dietary treatment.
Contributions of the sympathetic nervous system and salt sensitivity to control of BP and HR
At week 20, salt sensitivity of blood pressure was evaluated in all groups by providing 1% NaCl in the drinking water for 7 days. MAP and HR were measured 2 days before and 3 days after salt loading.
The effects of high-fat diet on chronic β1-adrenergic and α1-adrenergic receptor mediated cardiovascular regulation were determined by treating all mice with the receptor antagonist, first with atenolol (β1-adrenergic receptor antagonist, 1 mg/ml in drinking water) for 4 days, followed by a 7 day washout with drug-free drinking water. Thereafter, all mice were treated for 7 days with prazosin (α1- adrenergic receptor antagonist, 3 mg/ml in drinking water).[23]
Following 7 days prazosin washout with drug-free drinking water, neurogenic pressor and cardiac activity (net autonomic effect on BP) were evaluated by measuring the maximal changes in MAP and HR within 30 minutes after injection of hexamethonium (HEX, a nicotinic nACh receptor antagonist, 30 mg/kg, ip).
Cardiac function in control and mice on a high-fat diet by echocardiography
Echocardiographic assessments of myocardial function were made using the Vevo® 2100 system (VisualSonics, Toronto, Ontario, Canada). A MS550D microscan transducer (40 MHz) was used to collect images. At 24 weeks of feeding, mice (n=6 in each group) were anesthetized using 2% - 2.5% isoflurane anesthesia with oxygen (1 l/min). 100-frame B-Mode and M-Mode cine image sequences were obtained and were used to calculate HR, cardiac output (CO), stroke volume (SV), left ventricular ejection fraction (LVEF); fractional shortening (FS); left ventricular internal dimension at diastole (LVID;d,); left ventricular internal dimension at systole (LVID;s,); left ventricular posterior wall in diastole (LVPW;d,); left ventricular posterior wall at systole (LVPW;s), and estimated left ventricular mass weight (LV mass).
Supplemental Materials
Methods for blood sampling and tissue collection, vascular reactivity and morphology in pressurized mesenteric arteries ex vivo, histological assessment of collagen deposition and inflammation in mesenteric arteries, intrarenal, and coronary arteries, measurements of plasma insulin, creatinine, and tumour necrosis factor-alpha (TNF-α) levels, quantitative RT-PCR for BK β1- and α-subunits, and western blot analysis for BK α-subunits are provided in Supplemental Materials.
Statistics
Data are reported as mean ± SEM. Paired and unpaired t-tests were used for two group comparisons. HR and MAP in different groups were compared using a mixed design, two-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test. Changes in MAP and HR in the same treatment groups were assessed by one-way ANOVA, followed by Dunnett's test. Data were analyzed using GraphPad Prism 5.0 software. A P-value of <0.05 was considered statistically different.
Results
Body weight and metabolic measures
After 24 weeks on high-fat diet, wild-type and BK β1-knockout mice had similar increases in body weight, adipose accumulation, plasma glucose, insulin, and creatinine levels compared with control-fed mice. high-fat-wild-type mice displayed higher hematocrit (Hct) values than control and high-fat-BK β1-knockout mice. Plasma TNF-α levels were similar in all mice. High-fat-BK β1-knockout mice had greater BW adjusted LV mass than wild-type mice (see Supplemental Materials, Table S1).
Cardiovascular responses to high-fat feeding
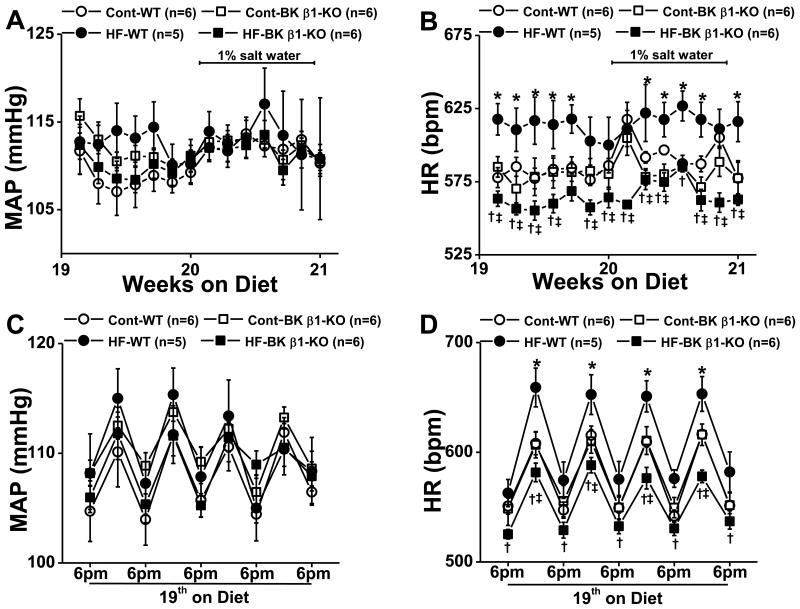
At week 19 of feeding, the 24-h MAP were similar in all mice (Fig 1A) with no differences during daytime (0600-1800 h) or nighttime (1800-0600 h) (Fig. 1C).
Figure 1.
Mean arterial blood pressure (MAP) and heart rate (HR) in control and high-fat (HF) fed wild-type (WT) and BK β1-knockout (KO) mice at 19-20 weeks on a high-fat diet. 24-h averaged MAP (A) and HR (B) from control and HF mice treated with or without saline (1% NaCl). Day and night (12-h averaged) MAP (C) and HR (D) from control and HF mice at 19 weeks. Cont, control diet. Data are presented as mean ± SE. *P<0.05, HF-WT vs control WT; †P<0.05, HF-BK β1-KO vs control BK β1-KO; ‡P<0.05, HF-BK β1-KO vs HF-WT .
At 14 - 15 weeks, HR in high-fat-wild-type mice was elevated (610 ± 10 bpm, P<0.05) compared to all other groups. HR in high-fat-BK β1-knockout mice (580 ± 10 bpm) was similar to control wild-type (587 ± 6 bpm) and BK β1-knockout mice (585 ±8 bpm) (P>0.05). Thereafter, HR in high-fat-BK β1-knockout mice progressively declined with obesity development while tachycardia in high-fat-wild-type mice persisted to week 19 (Supplemental Fig S1). At week 19, 24-h average HR was similar in control wild-type and BK β1-knockout mice (Fig 1B), while HR in high-fat-wild-type mice was higher than control wild-type and high-fat-BK β1-knockout mice (P<0.05) (Fig 1B). High-fat-BK β1-knockout mice had the slowest HR. Tachycardia in high-fat-wild-type and bradycardia in high-fat-BK β1-knockout mice was more prominent at night (Fig 1D).
We evaluated MAP and HR variability at two frequencies (hour-to-hour and 10 minute-to-10 minute values) by calculating the standard deviation of representative time periods during the daytime and night-time periods and found no difference between the four groups of mice (data not shown).
Effects of salt loading
There was no change in 24-h average MAP in control or high-fat fed mice during salt loading (Fig 1A). HR in all control mice increased within 24-h in response to elevated salt intake (Fig 1A). 24-h average HR in all control mice increased from baseline levels by 30 bpm (P<0.05) for 1-2 days, then returned to pre-salt water levels within 2-3 days. HR in high-fat-wild-type mice did not change during the 7 days of salt water administration (Fig 1B). HR in high-fat-BK β1-knockout mice increased by 30 bpm (P<0.05) over 4 days then returned to pre-salt loading levels by day 5 (Fig 1B).
Adrenergic regulation of MAP and HR
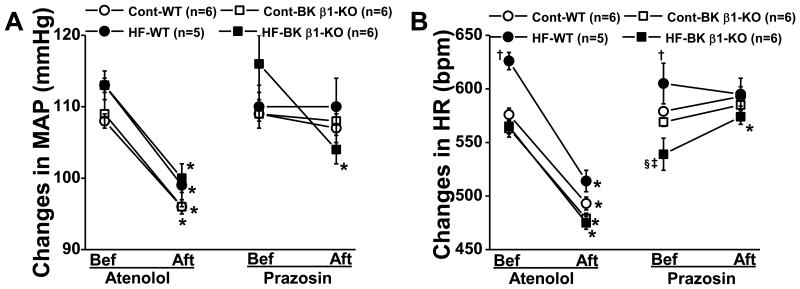
In response to atenolol treatment (Fig. 2A, 2B) within 24 h MAP and HR significantly decreased from baseline in all groups by nearly 10 mmHg and nearly 100 bpm, respectively, and this effect persisted for the 4 days of atenolol treatment.
Figure 2.
Atenolol and prazosin (administered via the drinking water)-induced peak changes in 24-h averaged MAP (A) and HR (B) in 21-23 week control and HF mice. Bef, before (pretreatment level), aft, after (at day 1 of the treatment). Bef, before treatment; Aft, after treatment. Data are presented as mean ± SE. *P<0.05, pretreatment vs after treatment; †P<0.05, HF-WT vs control WT; ‡P<0.05, HF-BK β1-KO vs control BK β1-KO; §P<0.05, HF-BK β1-KO vs HF-WT.
MAP and HR were unchanged after 24h of prazosin treatment in control-fed and high-fat-wild-type mice (Fig 2A, 2B), however, in high-fat-BK β1-knockout mice, prazosin decreased MAP by 12 ± 3 mmHg (Fig 2A) (P<0.05) and increased HR by 25 ± 7 bpm (Fig 2B) (P<0.05). However, HR and MAP returned to baseline by day 3 of prazosin treatment.
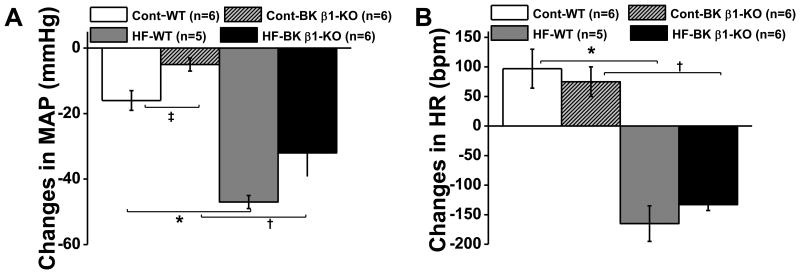
Overall neurogenic support of MAP and HR
Peak changes in MAP and HR caused by hexamethonium (HEX) in control and mice on high-fat diet are shown in Fig 3A and 3B. The HEX-induced fall in MAP in high-fat mice was not significantly different between groups (high-fat-wild-type: -47 ± 10 mmHg vs high-fat-BK β1-knockout: -32 ± 7 mmHg, P>0.05, Fig 3A). The peak drop in MAP in control wild-type mice was significantly larger than in control BK β1-knockout mice (wild-type: -16 ± 3 mmHg vs BK β1-knockout: -5 ± 2 mmHg, P<0.05, Fig. 3A). The fall in MAP was significantly larger (P<0.05) in BK β1-knockout mice on high-fat vs control diet. HEX decreased HR in all high-fat mice (high-fat-wild-type: -166 ± 30 bpm vs high-fat-BK β1-knockout: -133 ± 10 bpm, P>0.05) (Fig 3B), and increased HR in all control mice (wild-type: +97 ± 33 bpm vs BK β1-Knockout: +75 ± 25 bpm, P>0.05) (Fig. 3B).
Figure 3.
Hexamethonium (HEX, i.p) induced peak changes in MAP (A) and HR (B) in 24 week control and HF fed mice. MAP and HR were sampled continuously for 10 seconds every 10 minutes before and up to 120 minutes after injection. Peak changes were recorded at 20 minutes post-injection. Data are presented as mean ± SE. *P<0.05, HF-WT vs control WT; †P<0.05, HF-BK β1-KO vs control BK β1-KO; ‡P<0.05, control WT vs control BK β1-KO.
Cardiac structure and function
HR and cardiac ejection fraction under anesthesia were similar in all mice (Table 1). High-fat fed mice had higher CO and SV values than control mice, but these measures were similar across groups when adjusted for body weight. High-fat-BK β1-knockout mice had reduced LVID;S, thicker LVPW;d and LVPW;s, and increased LV mass, compared with control and high-fat-wild-type mice.
Table 1. Echocardiographic parameters of control and high-fat-wild-type and BK β1-knockout mouse heart measured and analyzed using a parasternal long axis view.
| Control | High-fat | ||||
|---|---|---|---|---|---|
|
| |||||
| (n=6) | WT | BK β1-KO | WT | BK β1-KO | |
| B-Mode | HR (bpm) | 527 ± 7 | 517 ± 5 | 528 ± 16 | 499 ± 19 |
| SV (μl) | 25 ± 8 | 23 ± 4 | 35 ± 5* | 34 ± 6† | |
| SV/BW (μl/10g) | 9 ± 3 | 8 ± 1 | 8 ± 1 | 7 ± 1 | |
| CO (ml/min) | 13.1 ± 4 | 11.9 ± 2 | 18.8 ± 2* | 17.1 ± 3† | |
| CO/BW (ml/min/10g) | 4.7 ± 1.6 | 4.3 ± 0.6 | 4.2 ± 0.6 | 3.6 ± 0.7 | |
| EF (%) | 53.4 ± 7.8 | 43.7 ± 3.5 | 52.8 ± 7.1 | 55.7 ± 8.2 | |
| FS (%) | 14.8 ± 4.2 | 9.2 ± 2.9 | 12.1 ± 3.5 | 17.0 ± 4.5 | |
| M-Mode | LVID;d (mm) | 3.7 ± 0.2 | 3.7 ± 0.2 | 3.8 ± 0.1 | 3.2 ± 0.2 |
| LVID:s (mm) | 2.9 ± 0.2 | 2.5 ± 0.3 | 2.7 ± 0.2 | 2.0 ± 0.2†‡ | |
| LVPW;d (mm) | 0.7± 0.1 | 1.0 ± 0.2 | 0.9 ± 0.1 | 1.7 ± 0.3†‡ | |
| LVPW;s (mm) | 1.1 ± 0.1 | 1.3 ± 0.2 | 1.1 ± 0.1 | 1.9 ±0.3†‡ | |
| LVmas (mg) | 95 ± 6 | 102 ± 8 | 109 ± 14 | 147 ± 19 | |
| LVmas/BW (mg/10g) | 35 ± 5 | 37 ± 3 | 19 ± 2 | 26 ± 3‡ | |
Data are presented as mean ± SE. BW, body weight; SV, stroke volume; CO, cardiac output; EF, ejection fraction; FS, left ventricle fractional shortening; LVID;d, left ventricular internal dimension at diastole; LVID;S, left ventricular internal dimension at systole; LVPW;d, left ventricular posterior wall at diastole; LVPW;s, left ventricular posterior wall at systole; LVmas, estimated left ventricle weight.
P<0.05, high-fat-WT vs control WT;
P<0.05, high-fat-BK β1-KO vs control BK β1-KO;
P<0.05, high-fat-BK β1-KO vs high-fat-WT.
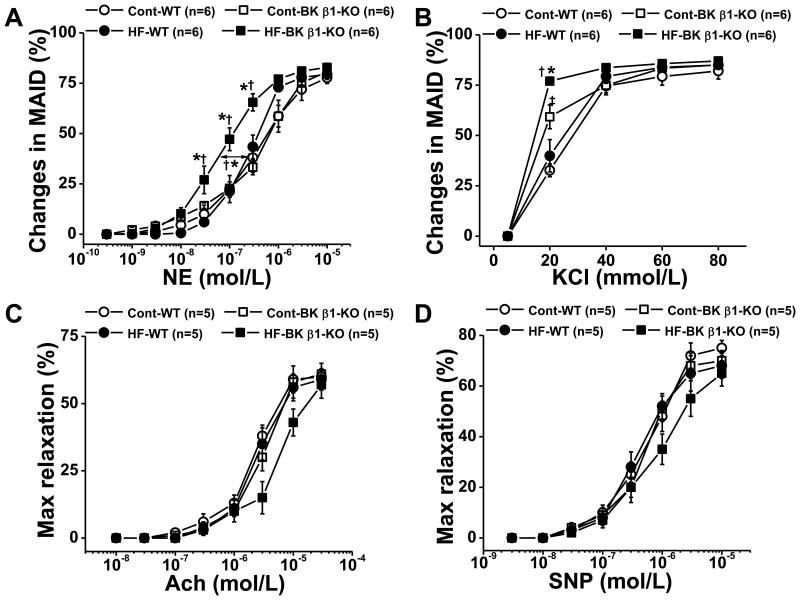
Vascular contractility in mesenteric arteries
Norepinephrine concentration-response curves were left-shifted in high-fat-BK β1-knockout mesenteric arteries (Fig. 4A) compared to all other groups. Norepinephrine concentration-response curves were similar in control and high-fat-wild-type mesenteric arteries. Maximal constrictions were similar in all mesenteric arteries, and peak constriction occurred at 1-3 μmol/l of norepinephrine in all groups.
Figure 4.
Concentration-response curves for norepinephrine (A), KCl (B), Ach (acetylcholine) (C) and SNP (sodium nitroprusside) (D) in pressurized mesenteric arteries from 24 weeks diet treated control and HF mice. Ach and SNP-induced Relaxation were determined in pre-constricted mesenteric arteries and normalized to the maximal relaxation caused by papaverine. Data are presented as mean ± SE. *P<0.05, HF-BK β1-KO vs control BK β1-KO; †P<0.05, HF-BK β1-KO vs HF-WT ; ‡P<0.05, control BK β1-KO vs control WT .
KCl concentration-response curves were also left-shifted in high-fat-BK β1-knockout mesenteric arteries compared with all other groups (Fig 4B). KCl contractile responses were similar in control and high-fat-wild-type mesenteric arteries. Acetylcholine (Ach) and sodium nitroprusside (SNP)-induced relaxations of mesenteric arteries were similar across all groups (Fig 4C, 4D).
Arterial remodeling and fibrosis
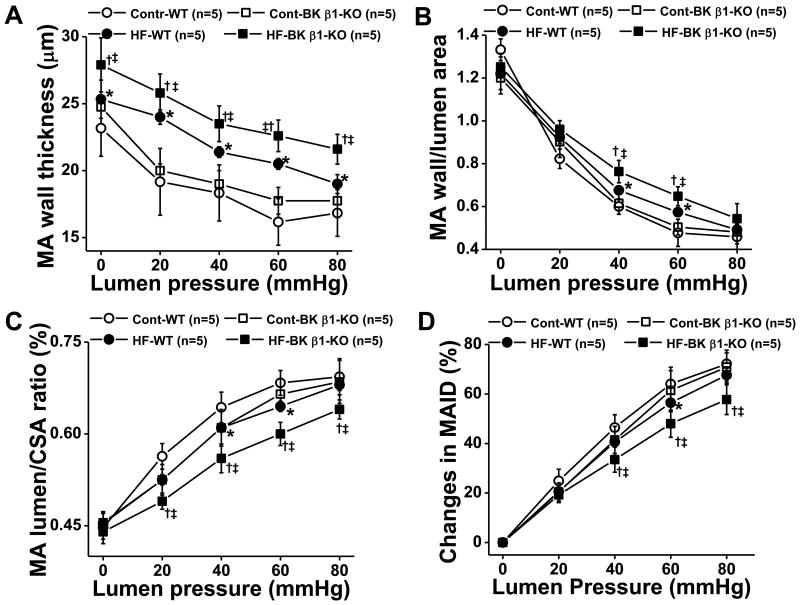
Vascular structure in pressurized control and high-fat mesenteric arteries were compared in Ca2+ free buffer ex vivo (Fig. 5). When lumen pressure was gradually increased to 80 mmHg, high-fat-wild-type mesenteric arteries and high-fat-BK β1-knockout mesenteric arteries exhibited a thicker wall (Fig. 5A), smaller lumen/wall ratio (Fig. 5B, 5C) and less distensibility (Fig. 5D) than control mesenteric arteries. These changes were greater in high-fat-BK β1-knockout mesenteric arteries than high-fat-wild-type mesenteric arteries (Fig. 5A-5D).
Figure 5.
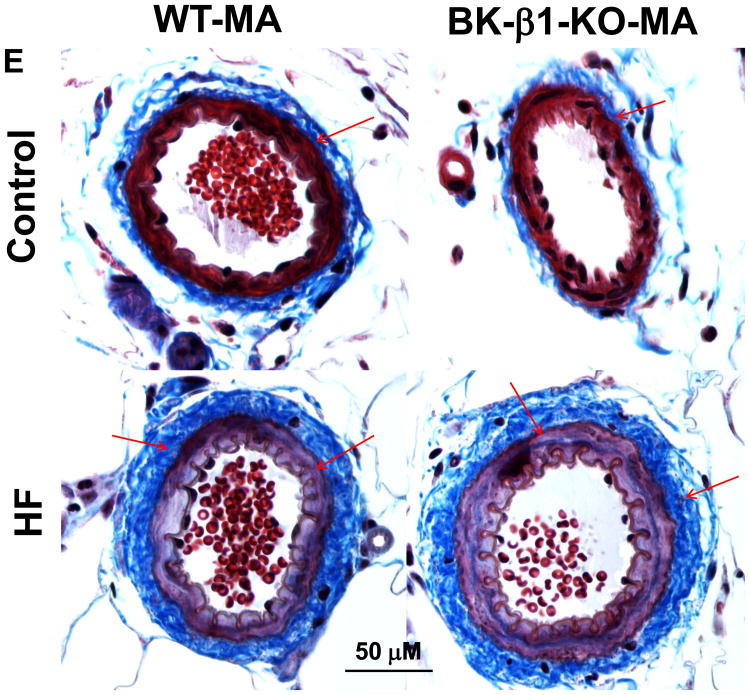
Comparison of mechanical properties in 24 week diet fed control and HF mesenteric arteries (MA). All measures were taken in Ca2+ free buffer with gradually increased lumen pressure. A), wall thickness, B), wall/lumen ratio; C) lumen/CSA (cross section area) ratio, D) changes in MA ID (MA inner diameter). E, light photomicrographs of control and HF MA. All tissues were stained with Masson's Trichrome stain to highlight perivascular fibrosis (blue-stained connective tissue; arrows). MA in HF fed mice had remarkable vascular remodeling and perivascular fibrosis than vessels from control fed mice, the changes were more predominant in BK β1-KO mice. Images are representative of 6 mice in each group. Data are presented as mean ± SE. *P<0.05, HF-WT vs control WT ; †P<0.05, HF-BK β1-KO vs control BK β1-KO; ‡P<0.05, HF-BK β1-KO vs HF-WT.
Myogenic tone was calculated and shown in Fig S2 (Supplemental data). Control BK β1-knockout mesenteric arteries displayed greater myogenic tone than control wild-type mesenteric arteries. Myogenic tone was significantly reduced in all high-fat mesenteric arteries, compared to control mesenteric arteries. The myogenic tone was similar in high-fat-wild-type mesenteric arteries and high-fat-BK β1-knockout mesenteric arteries.
Masson's trichrome staining showed collagen accumulation in adventitial and SM layers in high-fat mesenteric arteries (Fig. 5E); vascular hypertrophy and collagen accumulation were more prominent in high-fat-BK β1-knockout mesenteric arteries (Fig. 5E). Increased collagen accumulation occurred in coronary and intrarenal arteries from high-fat-BK β1-knockout mice (Supplemental Fig. S3).
We did not find monocytes/macrophages or T-cells around the mesenteric arteries or coronary arteries from either control or mice on high-fat diet (supplemental data Fig. S4, S5). Very few macrophages and T-cells were found around the intrarenal arteries (supplemental data Fig S4, S5), although significant macrophage infiltration was detected in renal tubules and glomeruli from mice on high-fat diet (data not shown).
Arterial BK channel function
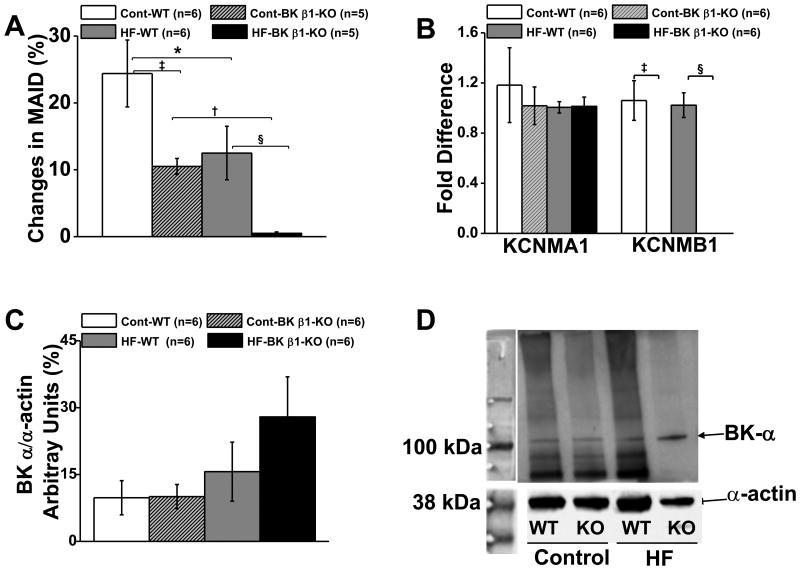
Arterial BK channel function was tested with paxilline in pressurized mesenteric arteries ex vivo (Fig. 6A). Paxilline constricted wild-type mesenteric arteries by 25 ± 4%, and BK β1-knockout mesenteric arteries by 12 ± 2%, (Fig. 6A). Paxilline constricted high-fat-wild-type mesenteric arteries by 11 ± 3%, but did not constrict high-fat-BK β1-knockout mesenteric arteries (0.5 ±0.2%).
Figure 6.
A. Comparison of paxilline induced contractions in 24 week diet fed control and HF MA. B. Expression of BK α- and β1-subunt mRNA levels in control and HF MA. KCNMA1, gene code of BK α-subunit; KCNMB1, gene code of BK β1-subunit. C. Expression of BK α-subunit protein levels in control and HF MA. D. BK α-subunit blots from control and HF MA. Data are presented as mean ± SE. *P<0.05 HF-WT MA vs control WT MA; †P<0.05 HF BK β1-KO MA vs control BK β1-KO MA; ‡P<0.05 control WT MA vs control BK β1-KO MA; §P<0.05, HF-WT MA vs HF-BK β1-KO MA.
High-fat diet did not affect α-subunit mRNA expression in high-fat mesenteric arteries (Fig 6B). β1-subunit mRNA levels were similar in control and high-fat-wild-type mesenteric arteries, and it was undetectable in control and high-fat-BK β1-knockout mesenteric arteries (Fig. 6B). High-fat diet did not reduce the α-subunit protein expression in high-fat-wild-type mesenteric arteries (Fig 6C, 6D). Expression of β1-subunit protein could not be assessed as all commercially available antibodies lack specificity for BK β1-subunits.[24]
Discussion
Vascular BK channel deficiency does not cause hypertension in high-fat-fed mice
Although BK channels play a major role in maintenance of VSMC membrane potential and vascular tone, the contribution of BK channel dysfunction to development of hypertension is controversial. For example, a recent study strongly supports the idea that impaired BK channel activity is associated with increased VSMC excitability and vascular tone in arteries from hypertensive patients in vitro.[5] However, integrative evidence to support a role for BK channel dysfunction as a sufficient cause of hypertension is lacking. Several studies in animal models of hypertension provide evidence that both supports and minimizes a role for BK channel dysfunction in hypertension.[6,7, 10-12,17-19] Studies in C57BL/6J BK β1-knockout mice do not support an association of vascular BK channel dysfunction with hypertension, as these mice are not hypertensive under normal conditions.[10-12] More recent work in high-fat fed rats and mice indicated that impaired BK channel function contributes to elevated BP through increased tone in resistance arteries, and that reduced BK β1-subunit expression plays a major role in obesity associated vascular dysfunction.[17-19] Therefore, we tested the hypothesis that normotensive BK β1-knockout mice would become hypertensive when given a high-fat diet.
We show that high-fat-wild-type mice were normotensive, as was also shown previously.[25] Nystoriak[19] et al reported that MAP was about 20 mmHg higher in high-fat-fed vs control-fed C57BL/6J mice (95 ± 5 mmHg in control diet mice vs 115 ± 2 mmHg in high-fat mice). Interestingly, MAP in their C57BL/6J mice on control diet was notably lower than in our previous study and those of other.[25,26] For example, MAP was nearly 110 mmHg in our C57BL/6J mice on control diets. Therefore, differences in conclusions among these studies could be based on different baseline BP in control mice, but we cannot explain why the measures of baseline BP were different in mice with a similar genetic background and local environmental factors (diet, housing etc.) might account for baseline BP measures. Nevertheless, our studies indicate that high-fat-diet induced obesity does not reliably promote hypertension in mice.
Previous studies of obesity-induced hypertension indicated that increased sympathetic effects on peripheral resistance are the hemodynamic basis for established hypertension.[27,28] On the contrary, unchanged BP in high-fat-fed mice has been explained by impaired arterial reactivity to norepinephrine due to reduced α1-adrenergic receptor signaling.[25] In our studies, ex vivo α1-adrenergic receptor mediated arterial vasoconstriction was similar in control wild-type and high-fat-wild-type mice tested ex vivo. This result differs from a previous report showing impaired α1-adrenergic receptor mediated vasoconstriction in high-fat mice [25]. It is also surprising that α1-adrenergic receptor mediated vasoconstriction was similar in control wild-type and BK β1-knockout mice, which is contradictory to our own previous studies.[10] We found increased reactivity to KCl in both control and high-fat-BK β1-knockout mesenteric arteries, but not increased reactivity to norepinephrine. The different outcomes in our two studies could be explained by the fact that α1-adrenergic receptor mediated vasoconstriction was tested in the current study in arteries taken from prazosin-treated animals. It has been shown that treatment with α1-adrenergic receptor antagonists restores α1-adrenergic receptor expression and function in high-fat mice[25,26]; however, it is unclear how long α1-adrenergic receptor blockade persists after prazosin withdrawal. Our studies were done 7 days after stopping prazosin administration. Thus, α-adrenergic receptor blocked by prazosin could explain why our findings here differed from previous work.[10,12] To determine whether BK β1-knockout specifically affects vascular adrenergic tone in obese mice, the vascular responses to norepinephrine and other vasoconstrictors need to be tested in vessels from nondrug treated control and high-fat mice.
Alternatively, the different outcome here also could be explained by the fact that vasoconstriction was tested in arteries taken from the animals of different ages (∼10-12 weeks in our previous work vs. 30 weeks in the current study). It has been reported that sensitivity to vasoconstrictors in isolated small arteries declines with aging[29]; therefore, our current results may not be strictly comparable to those from our previous studies. In any case, it is unlikely that differences in arterial reactivity to vasoconstrictors in control and high-fat fed mice explain why the high-fat-BK β1-knockout mice are not hypertensive. Compared to high-fat-wild-type mice, high-fat-BK β1-knockout mice showed increased depressor responses to α1-adrenergic receptor blockade in vivo, increased mesenteric arteries reactivity to norepinephrine ex vivo, and extensive arterial remodeling and fibrosis. We speculate that conscious high-fat-BK-β1 knockout mice should have higher peripheral resistance compared to control and high-fat-wild-type mice, and this needs to be tested directly in future studies. Although BP was similar in all high-fat fed mice, the LV hypertrophy we observed in high-fat-BK β1-knockout mice may have been induced by increased cardiac afterload caused by higher peripheral resistance.
As conscious high-fat-BK β1-knockout mice were bradycardic, we speculated that these mice might have lower CO via reduced sympathetic/increased vagal control of HR, assuming SV is similar to that of high-fat-wild-type mice. However high-fat-BK β1-knockout mice did not have reduced CO (or HR) in our echocardiographic studies under anesthesia. Both groups of high-fat mice showed similar HR, but increased CO and SV absolute values in absolute terms though they were similar to control mice when adjusted for body weight. High-fat fed mice may have relatively higher blood flow to some tissues, particularly if the measures are normalized for tissues with less adiposity, for example, cardiac and skeletal muscles. Unfortunately, we did not measure blood flow directly in control and high-fat fed mice, so we cannot interpret the CO measures in light of organ blood flow.[30] In our current studies, body weight and body fat distribution were similar in all high-fat fed mice, and therefore, we conclude that overall cardiac function in high-fat-wild-type and high-fat-BK β1-knockout mice is similar.
Vascular BK channel deficiency reduces neurogenic pressor activity
As has been reported by others [27,28], high-fat feeding in our experiments led to increased sympathetic/reduced parasympathetic drive of BP and HR (based on responses to ganglion blockade) in wild-type and BK β1-knockout mice. Interestingly, by the same measure, vascular BK channel deficiency tended to reduce sympathetic/increase parasympathetic drive of BP and HR in both control and high-fat fed mice. This may partially explain why high-fat feeding failed to cause hypertension in high-fat-BK β1-knockout mice. It is important to stipulate here, however, that we did not directly evaluate parasympathetic activity in our study, so further investigation of parasympathetic activity in the model is needed.
We were unable to determine precisely how high-fat feeding increases neurogenic support of BP by assessing BP changes to short-term treatment with α1- and β1-adrenergic receptor blockers. The fall in BP in response to β1-adrenergic receptor blockade with atenolol was identical in control-fed and high-fat fed mice. There was no steady-state fall in BP in response to α1-adrenergic receptor blockade in control fed wild-type or high-fat fed wild-type mice. Interestingly, however, in BK β1-knockout mice, although the BP response to β1-adrenergic receptor blockade was identical in control and high-fat fed mice, high-fat-BK β1-knockout mice showed a significant fall in BP during α1-adrenergic receptor blockade that was not seen in control fed BK β1-knockout mice. This may reflect their increased arterial reactivity to norepinephrine and structural remodeling as discussed earlier.
In high-fat fed wild-type mice, both increased sympathetic activity and decreased vagal activity appear to contribute to their tachycardia based on a larger fall in HR to atenolol and a reversal in the HR response to ganglion blockade (increase to decrease). This is consistent with studies in humans showing that obesity and elevated SBP are associated with increases in sympathetic and reduces vagal effects on heart.[31,32] In high-fat-BK β1-knockout mice, cardiac sympathetic activity was not increased compared to control fed BK β1-knockout mice based on responses to atenolol, whereas the decrease in vagal activity during high-fat diet appeared to be less in BK β1-knockout mice, thereby accounting for their relative high vagal activity and bradycardia. We speculate that the mechanism for this difference in vagal activity may be related to higher peripheral vascular resistance in conscious BK β1-knockout mice and thus increased chronic engagement of the baroreflex.
The role of post-capillary venular function in obesity?
We found that the hematocrit level was significantly increased in high-fat-wild-type mice. Our studies are consistent with a previous report of increased hematopoiesis in high-fat diet-induced obesity in mice due to enhanced bone marrow haematopoiesis.[33] However, high-fat-BK β1-knockout mice, on the contrary, did not show increased hematocrit values compared with control fed BK β1-knockout mice. This could be the result of altered haematopoiesis in BK β1-kockout mice, but to the best of our knowledge, the BK channel β1-subunit is not involved in the regulation of haematopoietic function. Another possibility is that the higher hematocrit level in high-fat-wild-type mice reflects hemoconcentration secondary to reduced circulating fluid volume. Capacitance vein function is highly dependent on sympathetic activity, so sympathetically mediated venoconstriction could have increased capillary pressure and fluid filtration, thus leading to a reduced circulating fluid volume.[34] if so, this effect would presumably be similar in high-fat-BK β1-knockout mice, especially as we previously reported a lack of functional BK channels in mesenteric veins in C57BL/6J mice.[10] We speculate that the capillary pressure may be increased to a greater degree in high-fat-wild-type mice, however, due to reduced prearteriolar contractile responses to norepinephrine in these animals, whereas high-fat-BK β1-knockout mice may have lower capillary pressure (and thus fluid filtration) because of increased arterial reactivity to norepinephrine. However, this is only the speculation at this time, as we did not directly measure blood volume.
Vascular BK channel deficiency exacerbates the obesity-associated systemic arterial remodeling and fibrosis
Previous studies indicated that vascular BK channel dysfunction causes tissue hypoperfusion and related complications in humans[13], and in animal models of metabolic diseases, due to increased VSMC contractility and insensitivity to endothelium-dependent NO.[14,15,35,36] There is no literature showing an association of BK channel deficiency with vascular remodeling and fibrosis in obesity. Thus we are the first to report that BK channel deficiency exacerbates systemic vascular remodeling and fibrosis in obesity. Exacerbated remodeling and fibrosis are clearly associated with enhanced BK channel activity directly, not with pressure overloading (a major trigger for inward hypertrophic remodeling) secondary to BK channel dysfunction-induced vasoconstriction.
Obesity induces systemic vascular remodeling and fibrosis through inflammation and/or oxidative stress associated with hyperglycemia.[37] Although we have not identified a mechanism to explain exacerbated hypertrophic remodeling in high-fat BK β1-knockout mice, this may be caused by elevated VSMC intracellular Ca2+ combined with high-fat diet induced hyperglycemia. Although measures of metabolic dysfunction were similar in high-fat-wild-type and high-fat-BK β1-knockout mice, that hypothesis is supported by reports showing that hyperglycemia inhibits BK channel activity, and BK channel deficiency stimulates SMC proliferation by disrupting the stability of cell apoptosis/proliferation in cell culture. Therefore, BK channel deficiency-induced SMC proliferation could occur in the absence of pressure loading.[38,39] These may explain why high-fat-BK β1-knockout mice developed sever vascular remodeling and fibrosis even without increases in BP.
Although our high-fat-BK β1-knockout mice developed severe systemic arterial fibrosis, this response was not associated with immune cell infiltration into the arterial wall. Very few macrophages and T-cells were detected by light microscopy in the interstitium surrounding renal arteries in control and high-fat fed mice, although significant macrophage infiltration occurred in the renal tubules and glomeruli in all high-fat fed mice (data not shown). High-fat-wild-type and BK β1-knockout mice had moderately elevated plasma creatinine levels and lipid droplet accumulation in the renal tubules. These results are consistent with inflammation and obesity-related renal dysfunction. High-fat-BK β1-knockout mice showed myocardial hypertrophy and severe coronary arterial fibrosis, but functional and histological changes were absent in the myocardium. Obesity-associated inflammatory responses and tissue disease are not uniform across all organs.[40] Obesity-induced cardiac inflammation and compromised energy metabolism have been detected in mice after 32 weeks on a high-fat diet.[40] These changes occur much later than those that occur in kidneys. Furthermore, there is no evidence indicating that the BK β1-subunit is expressed and functional in immune cells; our animal model has SMC specific loss of BK β1-subunit expression. Taken together, our studies do not support inflammation as a cause of BK channel deficiency-induced vascular remodeling and fibrosis. Alternatively, inflammation-induced vascular remodeling and fibrosis may not be detectable simply by light microscopy and immunohistochemistry. Previous studies demonstrated an association between the vascular extracellular matrix and vascular BK channel activity. Fibronectin, an α5β1 integrin ligand, enhanced BK channel activity through Ca2+ and phosphorylation-dependent mechanisms in VSMC.[41] It is not known if vascular BK β1-subunit deficiency affects extracellular matrix (collagen) degradation or synthesis, but cloned BK channels directly regulated apoptosis and proliferation of HEK293 cell under hyperglycemic conditions.[38] BK channels also mediate laminar shear stress-induced VSMC proliferation in cell culture.[39] Further studies will be needed to determine the mechanisms responsible for BK channel deficiency associated vascular remodeling and fibrosis in obesity.
High-fat diet induced obesity impairs vascular BK channel function
Studies using models of insulin resistance[14,15], diabetes and genetic obesity[15,16], and high-fat-diet induced obesity[17-19], show that BK β1-subunits are down-regulated due to either decreased synthesis[19] or increased degradation.[14,15] These changes are caused by oxidative stresses associated with hyperglycemia[14,19] or mutations of A-kinase anchoring protein signaling pathways[19], which uncouple BK α- and β1-subunits. In our studies, all high-fat mice exhibited similar levels of hyperglycemia and insulin. Mesenteric arteries from high-fat-wild-type and BK β1-knockout mice had reduced BK channel function as indicated by reduced constrictor responses to the BK channel agonist paxilline. However, β1-subunit mRNA expression in high-fat-wild-type mice was not affected by high-fat diet, and similar data have been obtained in other animal models of metabolic disease[14] and high-fat-induced obesity.[17-19] We were not able to assess β1-subunit protein expression due to the unavailability of reliable antibodies, the six commercially available anti BK β1-subunit antibodies from five manufacturers either detected protein bands of the appropriate molecular weight in tissues from both wild-type and BK β1-knockout mice, or failed to detect protein bands at the appropriate molecular weight in tissues from wild-type mice [24].
It is also noteworthy that high-fat-BK β1-knockout mice had further reduction in BK channel function compared to high-fat-wild-type and control-BK β1-knockout mice. This additional reduction in BK channel activity in high-fat-BK β1-knockout mice occurred without changes in α-subunit expression, because the α-subunit expression was similar in all mice. Therefore, our data indicate that changes in BK α-subunit protein expression are not responsible for impaired BK channel function in high-fat fed mice. As this is the first study that determined the role of obesity associated vascular BK channel dysfunction using vascular BK channel deficient mice, our finding is very novel. These results also suggested that downregulation of BK β1-subunit may not be the only mechanism in obesity associated vascular BK channel dysfunction. BK channel α-subunits can function without β1-subunits (see Fig 6A), but generally, this makes the BK channel harder to open; there is about a 30 to 40-mV positive shift in the voltage required to open BK channels. In addition, reduced vascular BK channel function in BK β1-knockout mice may be mediated by other mechanisms that directly inhibit BK channel α-subunit activity. This speculation is supported by the studies that high glucose inhibits BK channel α-subunit activity in BK α-subunit transfected HEK 293 cells.[38] Therefore, the impaired BK channel function in high-fat-wild-type mice could be caused by both downregulated BK β1-subunit expression and direct inhibition of BK channel α-subunit activity. Nevertheless, our studies show that BK β1-subunit dysfunction contributes to obesity associated systemic vascular dysfunction, remodeling and fibrosis. The increased vascular reactivity combined with exacerbated systemic vascular remodeling and fibrosis likely accelerates obesity associated organ dysfunction. Our studies indicate that maintenance of vascular BK channel protects against obesity-associated organ dysfunction/damage (kidney and cardiac failure), the major causes of morbidity and mortality in metabolic disorders.
Limitation and future studies
We have not identified a specific mechanism that explains why BK channel dysfunction does not increase BP, even in obese mice, although BK channels regulate peripheral resistance in normal and pathophysiological situations. Constitutive reductions in BK channel function (as occurs in BK β1-knockout mice) may be offset by adaptive responses of cellular or integrative BP control mechanisms. For example, our data suggest that sympathetic stimulation of blood pressure may be reduced in BK β1-knockout mice. It will be crucial to determine baroreflex responses, CO and peripheral resistance in conscious BK β1-knockout mice, as we found that the lower HR in high-fat-BK β1-Knockout mice was not preserved in anesthetized mice. Future studies will also be conducted to determine the mechanisms responsible for the effects of BK channel deficiency on vascular remodeling and fibrosis in obesity.
Novelty, Significance and Perspective
Hypertension occurs in ∼75% of obese patients, greatly increasing the risk for cardiac, renal and cerebrovascular disease. Increased vascular tone and contractility as well as vascular remodeling and fibrosis are common features in patients with metabolic disorders who also have impaired vascular BK channel function. This supports the hypothesis that BK channel dysfunction in obesity is associated with vascular dysfunction, remodeling, and fibrosis.[14,15,35,36] Our studies were the first to show that specific vascular BK channel deficiency does not promote obesity-associated hypertension, but promotes obesity-associated vascular dysfunction, remodeling, fibrosis, and the cardiac consequences. This is significant because it suggests that vascular BK channel activity protects against obesity-associated vascular remodeling and fibrosis via blood pressure-independent mechanisms. It is the first studies that determined the role of obesity associated vascular BK channel dysfunction using vascular BK channel deficient mice. Our data suggest that vascular BK channel activators are a new target for therapeutic intervention to protect against vasculopathy-induced tissue hypoperfusion and complications in patients with obesity and metabolic disorders.
Supplementary Material
Acknowledgments
We thank Dr. Robert Brenner (University of Texas Health Science Center at San Antonio, TX) for the gift of BK β1-Knockout mice. We thank the staff of the in Histopathology laboratory at Michigan State University for the tissue processing and histological staining.
Grants: Supported by National Heart, Lung and Blood Institute grant number: P01 HL070687 (HX, JJG and GDF) and American Heart Association Midwest Affiliate 11GRNT6690010 (HX).
Footnotes
The content of the study was presented in abstract form at Experimental Biology meeting, San Diego, April 2014.
Disclosures: No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Kotchen TA. Obesity-associated hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23:1170–8. doi: 10.1038/ajh.2010.172. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen T, Lau DC. The obesity epidemic and its impact on hypertension. Can J Cardiol. 2012;28:326–333. doi: 10.1016/j.cjca.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Morris MJ. Cardiovascular and metabolic effects of obesity. Clin Exp Pharmacol Physiol. 2008;35:416–419. doi: 10.1111/j.1440-1681.2008.04912.x. [DOI] [PubMed] [Google Scholar]
- 4.Hodnett BL, Hester RL. Regulation of muscle blood flow in obesity. Microcirculation. 2007;14:273–288. doi: 10.1080/10739680701282143. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Liu ZF, Zeng XR. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 2013;61:519–525. doi: 10.1161/HYPERTENSIONAHA.111.00211. [DOI] [PubMed] [Google Scholar]
- 6.Joseph BK, Thakali KM, Moore CL, Rhee SW. Ion channel remodeling in vascular smooth muscle during hypertension: Implications for novel therapeutic approaches. Pharmacol Res. 2013;70:126–138. doi: 10.1016/j.phrs.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pintérová M, Behuliak M, Kuneš J, Zicha J. Involvement of BK(Ca) and K(V) potassium channels in cAMP-induced vasodilation: their insufficient function in genetic hypertension. Physiol Res. 2014 Jan 8; doi: 10.33549/physiolres.932718. [DOI] [PubMed] [Google Scholar]
- 8.Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 9.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Garver H, Galligan JJ, Fink GD. Ca2+-activated K+ channel beta1-subunit knockout mice are not hypertensive. Am J Physiol Heart Circ Physiol. 2011;300:H476–H485. doi: 10.1152/ajpheart.00975.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Garver H, Fernandes R, Galligan JJ, Fink GD. Altered L-type Ca2+ channel activity contributes to exacerbated hypoperfusion and mortality in smooth muscle cell BK channel deficient septic mice. Am J Physiol Regul Integr Comp Physiol. 2014;307:R138–R148. doi: 10.1152/ajpregu.00117.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachse G, Faulhaber J, Seniuk A, Ehmke H, Pongs O. Smooth muscle BK channel activity influences blood pressure independent of vascular tone in mice. J Physiol. 2014;592:2563–2574. doi: 10.1113/jphysiol.2014.272880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.González-Corrochano R, La Fuente J, Cuevas P, Fernández A, Chen M, Sáenz de Tejada I, Angulo J. Ca2+ -activated K+ channel (KCa) stimulation improves relaxant capacity of PDE5 inhibitors in human penile arteries and recovers the reduced efficacy of PDE5 inhibition in diabetic erectile dysfunction. Br J Pharmacol. 2013;169:449–461. doi: 10.1111/bph.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang DM, He T, Katusic ZS, Lee HC, Lu T. Muscle-specific f-box only proteins facilitate bk channel β(1) subunit downregulation in vascular smooth muscle cells of diabetes mellitus. Circ Res. 2010;107:1454–1459. doi: 10.1161/CIRCRESAHA.110.228361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu T, Chai Q, Yu L, d'Uscio LV, Katusic ZS, He T, Lee HC. Reactive oxygen species signaling facilitates FOXO-3a/FBXO-dependent vascular BK channel β1 subunit degradation in diabetic mice. Diabetes. 2012;61:1860–1868. doi: 10.2337/db11-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rueda A, Fernández-Velasco M, Benitah JP, Gómez AM. Abnormal Ca2+ spark/STOC coupling in cerebral artery smooth muscle cells of obese type 2 diabetic mice. PLoS One. 2013;8:e53321. doi: 10.1371/journal.pone.0053321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howitt L, Grayson TH, Morris MJ, Sandow SL, Murphy TV. Dietary obesity increases NO and inhibits BKCa-mediated, endothelium-dependent dilation in rat cremaster muscle artery: association with caveolins and caveolae. Am J Physiol Heart Circ Physiol. 2012;302:H2464–H2476. doi: 10.1152/ajpheart.00965.2011. [DOI] [PubMed] [Google Scholar]
- 18.Howitt L, Sandow SL, Grayson TH, Ellis ZE, Morris MJ, Murphy TV. Differential effects of diet-induced obesity on BKCa {beta}1-subunit expression and function in rat skeletal muscle arterioles and small cerebral arteries. Am J Physiol Heart Circ Physiol. 2011;301:H29–H40. doi: 10.1152/ajpheart.00134.2011. [DOI] [PubMed] [Google Scholar]
- 19.Nystoriak mesenteric arteries. Nieves-Cintrón M, Nygren PJ, Hinke SA, Nichols CB, Chen CY, Puglisi JL, Izu LT, Bers DM, Dell'acqua ML, Scott JD, Santana LF, Navedo MF. AKAP150 Contributes to Enhanced Vascular Tone by Facilitating Large-Conductance Ca2+-Activated K+ Channel Remodeling in Hyperglycemia and Diabetes Mellitus. Circ Res. 2014;114:607–615. doi: 10.1161/CIRCRESAHA.114.302168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, Heagerty AM. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;304:H786–H795. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 22.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–2159. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 23.Veitenheimer BJ, Engeland WC, Guzman PA, Fink GD, Osborn JW. Effect of global and regional sympathetic blockade on arterial pressure during water deprivation in conscious rats. Am J Physiol Heart Circ Physiol. 2012;303:H1022–1034. doi: 10.1152/ajpheart.00413.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattarai Y, Fernandes R, Kadrofske MM, Lockwood LR, Galligan JJ, Xu H. Western blot analysis of BK channel β1-subunit expression should be interpreted cautiously when using commercially available antibodies. Physiol Rep. 2014;2:e12189. doi: 10.14814/phy2.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belin de Chantemèle EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58:271–279. doi: 10.1161/HYPERTENSIONAHA.110.168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belin de Chantemèle EJ, Muta K, Mintz J, Tremblay ML, Marrero MB, Fulton DJ, Stepp DW. Protein tyrosine phosphatase 1B, a major regulator of leptin-mediated control of cardiovascular function. Circulation. 2009;120:753–763. doi: 10.1161/CIRCULATIONAHA.109.853077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012;590:1787–1801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 29.Hausman N, Martin J, Taggart MJ, Austin C. Age-related changes in the contractile and passive arterial properties of murine mesenteric small arteries are altered by caveolin-1 knockout. J Cell Mol Med. 2012;16:1720–1730. doi: 10.1111/j.1582-4934.2011.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll JF, Huang M, Hester RL, Cockrell K, Mizelle HL. Hemodynamic alterations in hypertensive obese rabbits. Hypertension. 1995;26:465–470. doi: 10.1161/01.hyp.26.3.465. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Xie G, Wang J, Yang S. Cardiovascular risk factors significantly correlate with autonomic nervous system activity in children. Can J Cardiol. 2012;28:477–82. doi: 10.1016/j.cjca.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Straznicky norepinephrine. Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep. 2008;10:440–447. doi: 10.1007/s11906-008-0083-1. 42. [DOI] [PubMed] [Google Scholar]
- 33.Trottier MD, Naaz A, Li Y, Fraker PJ. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci U S A. 2012;109:7622–7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maspers M, Björnberg J, Grände PO, Mellander S. Sympathetic alpha-adrenergic control of large-bore arterial vessels, arterioles and veins, and of capillary pressure and fluid exchange in whole-organ cat skeletal muscle. Acta Physiol Scand. 1990;138:509–521. doi: 10.1111/j.1748-1716.1990.tb08879.x. [DOI] [PubMed] [Google Scholar]
- 35.Lu T, Ye D, He T, Wang XL, Wang HL, Lee HC. Impaired Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels in the coronary artery smooth muscle cells of Zucker Diabetic Fatty rats. Biophys J. 2008;95:5165–5177. doi: 10.1529/biophysj.108.138339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGahon MK, Dash DP, Arora A, Wall N, Dawicki J, Simpson DA, Scholfield CN, McGeown JG, Curtis TM. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100:703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deutsch C, Portik-Dobos V, Smith AD, Ergul A, Dorrance AM. Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc Res. 2009;78:100–106. doi: 10.1016/j.mvr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang H, Ma YG, Wang YY, Song Z, Li Q, Yang N, Zhao HZ, Feng HZ, Chang YM, Ma J, Yu ZB, Xie MJ. High glucose alters apoptosis and proliferation in HEK293 cells by inhibition of cloned BK Ca channel. J Cell Physiol. 2011;226:1660–1675. doi: 10.1002/jcp.22497. [DOI] [PubMed] [Google Scholar]
- 39.Jia X, Yang J, Song W, Li P, Wang X, Guan C, Yang L, Huang Y, Gong X, Liu M, Zheng L, Fan Y. Involvement of large conductance Ca(2+)-activated K (+) channel in laminar shear stress-induced inhibition of vascular smooth muscle cell proliferation. Pflugers Arch. 2013;465:221–232. doi: 10.1007/s00424-012-1182-z. [DOI] [PubMed] [Google Scholar]
- 40.Noyan-Ashraf MH, Shikatani EA, Schuiki I, Mukovozov I, Wu J, Li RK, Volchuk A, Robinson LA, Billia F, Drucker DJ, Husain M. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85. doi: 10.1161/CIRCULATIONAHA.112.091215. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Wu X, Gui P, Wu J, Sheng JZ, Ling S, Braun AP, Davis GE, Davis MJ. Alpha5beta1 integrin engagement increases large conductance, Ca2+-activated K+ channel current and Ca2+ sensitivity through c-src-mediated channel phosphorylation. J Biol Chem. 2010;285:131–141. doi: 10.1074/jbc.M109.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.