Abstract
In terms of reproductive and social functions, vasoactive intestinal polypeptide (VIP) is best known as a major regulator of prolactin secretion in vertebrates and hence, as an essential contributor to parental care. However, VIP and its cognate VPAC receptors are distributed throughout the social behavior network in the brain, suggesting that VIP circuits may play important roles in a variety of behaviors. With the exception of VIP neuronal populations in the suprachiasmatic nucleus and tuberal hypothalamus (which regulate circadian rhythms and prolactin secretion, respectively), we have known very little about the functional properties of VIP circuits until recently. The present review highlights new roles for VIP signaling in avian social behaviors such as affiliation, gregariousness, pair bonding and aggression, and discusses recent advances in VIP’s role as a regulator of biological rhythms, including the potential timing of ovulation, photoperiodic response and seasonal migration.
Introduction
Vasoactive intestinal polypeptide (VIP) is a neuropeptide that is produced and released by numerous hypothalamic and extrahypothalamic cell groups and is perhaps best known as a major releasing factor of prolactin (PRL) from the pituitary in both birds [1] and mammals [2]. Because PRL regulates many reproductive and parental behaviors, such as egg incubation in bantam hens and turkeys [3, 4], parental regurgitation and feeding in ring doves [5], lactation in mammals [6] and chick-rearing in native Thai hens [7], VIP has been assumed to play an important role in reproductive behaviors. Indeed, VIP-immunization blocks VIP-induced increases in plasma PRL levels, reducing nesting activity in turkeys [3] and increasing nest desertion in incubating bantam hens [4]. This immunization can be either passive, through injections of anti-chicken VIP serum [4], or active, whereby synthetic chicken VIP is conjugated to keyhole limpet hemocyanin (KLH) for injections [8]. In the latter case, KLH serves as a carrier protein for VIP that enables a robust immune response in the form of antibody production. Furthermore, changes in VIP within the portal blood, as well as VIP expression and immunoreactivity in hypothalamic regions, such as the infundibular nucleus (INF) and median eminence, closely mirror changes in plasma PRL levels across the reproductive cycle and different stages of parental care [7, 9-11]. Interestingly, VIP’s stimulation of PRL release from the pituitary in turkeys appears to be regulated via dopamine (DA) and its receptors [12, 13], and changes in hypothalamic VIP are associated with changes in tyrosine hydroxylase-immunolabeling during incubation and nest deprivation in native thai hens [14], suggesting that DA may modulate the VIP/PRL signaling cascade in certain avian species.
VIP’s other well-known role is as a regulator of circadian rhythms in mammals [15, 16], mediated by GABAergic cells that contain VIP within the ventral core of the suprachiasmatic nucleus (SCN), the brain’s main pacemaker nucleus. These core cells are retinorecipient cells that sense and respond to light [17] and mediate the phase shifting of activity rhythms [18, 19]. VIP’s effects can be mediated by VPAC receptors (VPAC1 and VPAC2), which bind both VIP and pituitary adenylate cyclase activating peptide (PCAP) [20]. However, PCAP also acts through the PACAP receptor, PAC1, and binds to this receptor with high affinity, as compared to VIP, which binds PAC1 with low affinity [20, 21]. In animals lacking VIP or VPAC2 (one of the VIP receptors), circadian rhythms of rest/activity are disrupted and the circadian system shows deficits in response to photic stimuli [22-24]. In tissue slices lacking the VPAC2 receptor gene, both molecular timekeeping within individual SCN cells and spontaneous synchronization between SCN cells, is lost [25]. Thus, VIP signaling plays very important roles in the generation, maintenance and synchronization of circadian rhythms (for reviews see [15, 16]) and is likely at the top of a hierarchy of paracrine signals that control SCN molecular pacemaking [26].
In addition to the hypothalamic INF and SCN, VIP elements (i.e. cells, fibers and receptors) are present in virtually every brain area that is known to be important for social behavior [27-30], including core nodes of the brain’s “social behavior network” [31, 32], which include the preoptic area (POA), anterior hypothalamus (AH), ventromedial hypothalamus (VMH), medial extended amygdala (medial amygdala, MeA, and medial bed nucleus of the stria terminalis, BSTm), midbrain central gray, ventral tegmental area (VTA) and lateral septum (LS). However, until recently, VIP’s role in the social behaviors affiliated with this network remained largely unexplored. In the present review, we highlight research conducted within the last 3 years that describes new roles for VIP signaling in avian social behaviors such as aggression, affiliation, gregariousness, pair bonding and nesting, as well as recent advances in VIP’s role as a regulator of reproductive and seasonal rhythms in both mammals and birds.
The role of VIP in grouping behavior, affiliation and pair bonding
Cross-species comparisons of VIP circuitry provided the first insight that VIP signaling may modulate grouping preferences and affiliative behavior in birds. VPAC receptors are found at higher densities within the subpallial LS and BSTm of year-round gregarious finch species relative to territorial species [27] and VIP innervation of the BSTm and PVN is greater in sparrow species that flock during the winter compared to species that do not winter flock [33].
Given these links of increased VIP elements to flocking, and the well documented role of the BSTm and LS in grouping behavior and social affiliation, particularly with regards to nonapeptide circuitry [34], we hypothesized that activation of VPAC receptors promotes social contact and preference for larger groups, as well as pair bonding in the monogamous and highly gregarious zebra finch (Taeniopygia guttata). Indeed, central antagonism of VPAC receptors using a selective VPAC receptor antagonist (neurotensin6-11-mouseVIP7-28) that is known to block VIP stimulation of cyclic AMP production in chick brain slices without blocking PAC1 receptors [35], significantly impacts many affiliative behaviors (Figure 1). When exposed to a group choice apparatus that allows zebra finches to affiliate with either 2 or 10 unfamiliar conspecifics (Figure 1a), VPAC antagonism significantly decreases the amount of time subjects spend in social contact with either group, compared to control infusions, but only in the first trial when animals are tested in a novel social environment (Figure 1b). The antagonist has no effect in the second trial (Figure 1b), in subsequent novel-familiar conspecific choice tests in the same apparatus or in behavioral tests of general anxiety, suggesting that endogenous VIP signaling specifically promotes social contact in response to contextual novelty [36]. This finding complements studies in mice where reduction of VIP signaling in utero leads to deficits in social approach and sociability in adolescent and adult offspring [37, 38].
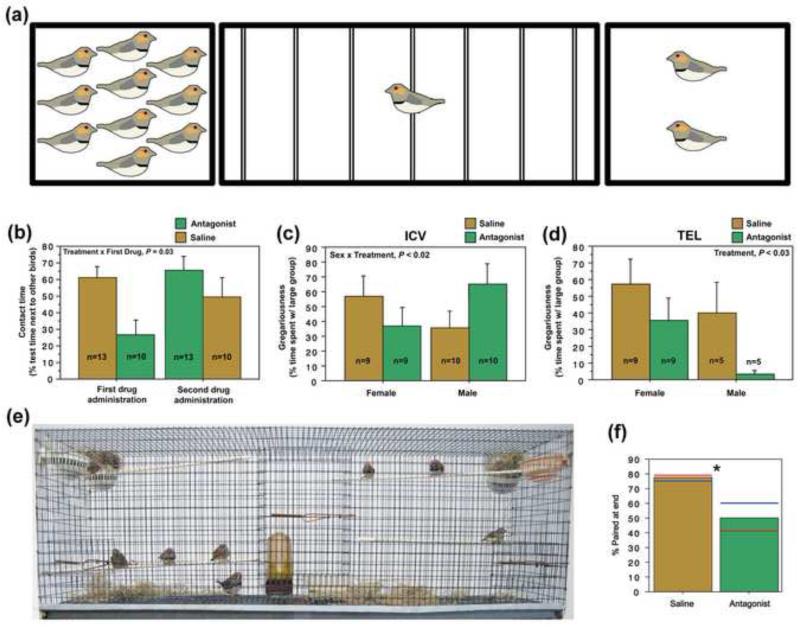
Fig. 1.
VPAC antagonism alters social contact in a novel environment, gregariousness and pair bonding in zebra finches. (a) Our choice test apparatus consists of a 1 M wide testing cage subdivided into zones by seven perches with stimulus cages containing either two or ten familiar same-sex conspecifics at either end. The percent of test time spent on the two end perches combined (within 6 cm of a stimulus cage) provides a measure of “social contact time”. The percent of social contact time spent with the large group provides a measure of “gregariousness” (group size preference). (b) Ventricular VPAC antagonist infusions decrease social contact time in the choice apparatus (shown in a) on the first test day when the testing paradigm is novel, but not on the second test day. (c, d) VPAC antagonism has site- and sex-specific effects on gregariousness in choice tests. (c) A significant interaction of Sex and Treatment is observed following ICV antagonist infusions whereby VPAC antagonism tends to decrease gregariousness in females and increase it in males. (d) A main effect of Treatment is observed following VPAC antagonist infusions into the telecephalon whereby VPAC antagonism reduces gregariousness in both males and females. (a-d), modified from [36]. (e) A 1 M wide cage showing the standard setup for colony observations, with a single nest cup in each of the 4 corners, and a food dish, water sipper and burlap nesting material placed on the cage floor. Modified from Kelly and Goodson [41]. (f) Chronic VPAC receptor antagonism significantly decreases the percentage of zebra finches that are paired in the final behavioral session, as compared to control animals. Blue bars denote male values and red bars denote female values. Modified from [45].
VPAC receptor antagonism also impacts gregariousness in zebra finches, with sex-specific and site-specific effects [36]. Intracerebroventricular (ICV) antagonist infusions tend to reduce gregariousness (i.e. preferences for the larger of two groups) in females but increase gregariousness in males (Figure 1c). Since we have not observed sex differences in VIP binding densities for the BSTm and subpallial LS [27], areas likely impacted by the ICV infusions, we speculate that the sex difference in gregariousness may arise from sex differences in VIP innervation and/or sex-specific VIP neuronal activation. Interestingly, the sex-specific modulation of gregariousness by the nonapeptides [39-41] is now known to be associated with sex-specific activation of oxytocin (OT) or vasopressin (VP) cells following social stimuli exposure [42]. We further speculate that the sex-specific modulation of gregariousness by VIP could arise from an interaction with OT and/or VP, acting together to regulate behavior. Importantly, ICV infusions of VIP in rats increases plasma OT and VP [43], likely by means of the hypothalamo-neurohypophyseal system [44], suggesting that VIP may serve as a releasing factor for these hormones.
In contrast to ICV infusions, antagonism in the medial telencephalon reduces gregariousness in both sexes (Figure 1d). Together, these results suggest that gregariousness is modulated via VPAC activation in both the medial nidopallium and in one or more sites likely impacted by ICV infusions, such as the BSTm and LS.
Chronic VPAC receptor antagonism also significantly impairs zebra finches ability to pair bond. When tested in a colony environment (Figure 1e), zebra finches receiving chronic ICV infusions of the VPAC receptor antagonist took longer to form a pair bond [45], were paired for fewer observation sessions [45] and were less likely to be paired in the final observation session (Figure 1f). We hypothesize that VIP’s sites of action likely include brain areas that are known to be important for the establishment of pair bonding in prairie voles [46], including the LS, VTA, nucleus accumbens (NAcc), and ventral pallidum. These proposed sites of VIP action are based on VIP’s association with the mesolimbic dopamine system (for discussion, see [45]), including a strong expression of VIP mRNA in the VTA [29], a high density of 125I-VIP binding sites in the NAcc [27], and increased 125I-VIP binding sites in the LS of gregarious zebra finches [27]. Based on this association of VIP elements with the mesolimbic dopamine system, we hypothesize that VIP and dopamine may interact to mediate behaviors characterized by incentive motivation. VIP may also interact with the nonapeptides as they are also found within the SBN and are known to modulate affiliative behavior and/or pair bonding [34, 39, 40, 47].
VIP’s role in modulating aggression
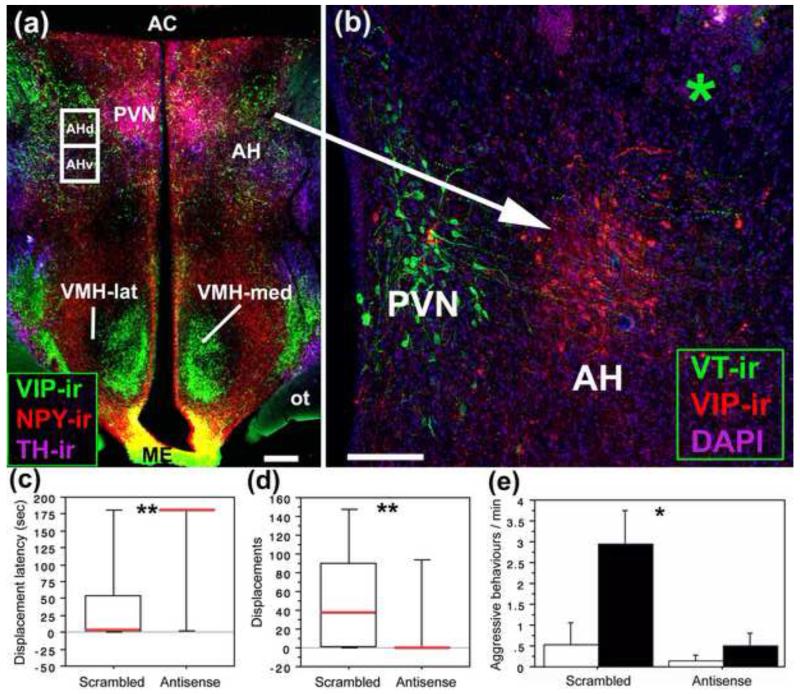
A role for VIP in aggressive behavior was first suggested by field and laboratory studies modulating central VIP in finches and sparrows in the late 1990s. Whereas VIP infusions into the LS of violet-eared waxbills, a highly territorial estrildid finch, facilitate aggression in the context of territorial defense [48], similar infusions decrease mate competition aggression in gregarious zebra finches [49] and inhibit agonistic dawn song in territorial field sparrows [50]. The LS receives projections from the AH [51], a brain area well known for the mediation of aggression and agonistic communication [52]. The dorsal AH (AHd) contains a discrete population of VIP cells that can be visualized by both in situ hybridization [53] and by immunolabeling after colchicine treatment (Figure 2a-b). Knockdown of VIP peptide production in AHd cells using antisense oligonucleotides essentially abolishes aggression in highly territorial violet-eared waxbills, as observed by an increase in latency to aggress (Figure 2c) and a reduction in displacements (Figure 2d) [53]. Within male zebra finches, VIP knockdown in the AHd decreases aggressive behaviors in the context of nest defense (Figure 2e). This AHd VIP cell population represents an aggression-specific cell type in the brain since VIP knockdown had no effect on social preferences, anxiety-like behaviors, courtship, pair bonding or maintenance behaviors [53]. Consistent with these findings are the observations that VIP immunolabeling in the AH and caudocentral septum correlates positively with aggression in sparrows [33] and VIP cell numbers in the AH correlate positively with aggressive behaviors in waxbills [53].
Fig. 2.
Knockdown of VIP peptide in the dorsal anterior hypothalamus (AHd) greatly reduces aggression in territorial and gregarious finches. (a) VIP elements in the hypothalamic region of the zebra finch. TH-ir, tyrosine hydroxylase immunoreactivity (-ir) (purple); NPY-ir, neuropeptide Y-ir (red); VIP-ir, vasoactive intestinal polypeptide-ir (green). Scale bar, 200 μm. (b) The AHd region is defined by a distinct VIP cell group, shown here for a violet-eared waxbill. VIP-ir, vasoactive intestinal polypeptide immunoreactivity (red); VT-ir, vasotocin-ir (green); DAPI nuclear stain (purple). Green asterisk shows site of cannula tip for VIP antisense or scrambled oligonucleotide infusions. Scale bar, 100 μm. AC, anterior commissure; AHd and AHv, anterior hypothalamus, dorsal and ventral; ME, median eminence; ot, optic tract; PVN, paraventricular nucleus; VMH-lat and VHM-med, ventral medial hypothalamus, lateral and medial. (c, d) Compared to control (scrambled) infusions, VIP antisense infusions significantly increase the latency to displace an intruder (c) and eliminate aggressive displacements in most male and female violet-eared waxbills during a resident-intruder test (d). **P = 0.01. (e) VIP Antisense infusions in male zebra finches significantly reduce aggressive behaviors in the context of nest defense within a colony environment. Males were placed into either high aggression (black bars) and low aggression (white bars) colony cages based on aggressive behavior during pre-screenings. *P = 0.02, main effect of Treatment. Modified from [53].
As with affiliative behaviors, VIP may interact with other neuropeptides to modulate aggressive behavior. Whereas infusions of VIP and VP in the lateral septum have opposing roles in regulating aggressive behaviors in violet-eared waxbills, zebra finches and field sparrows [48-50], both OT and AHd VIP appear to facilitate aggression in violet-eared waxbills [53, 54]. Thus, we are currently investigating if VIP may interact with OT to mediate aggression and response to stressors in both social and asocial finches.
In summary, through the use of ICV and site-specific infusions of VIP and VIP receptor antagonists, site-specific infusions of VIP antisense oligonucleotides and cross species comparisons of VIP binding, we have demonstrated a role for VIP signaling in avian affiliative and aggressive behaviors. We have also identified particular SBN nodes that are key players for these social behaviors. Based on the ideas put forth by Newman and Goodson [32, 55], we believe many nodes within the SBN network respond to a social stimulus, yet it is the unique pattern of activation throughout the network that is associated with a particular social context and behavioral response [55]. Currently, we are examining network-wide VIP cell activation in response to social and non-social stressors to gain insight as to how VIP might regulate responses to stress and how these responses relate to an animal’s social phenotype (i.e. asocial versus gregarious). We are also exploring whether VIP may interact with OT to modulate stress responses as OT is known to be anxiolytic and to mediate stress-coping [34].
Recent advances in VIP’s role as a regulator of biological rhythms
In 1976, the estrous cycle was shown to have a circadian organization and it was proposed that estrus and daily activity rhythms might be controlled by a single pacemaker nucleus such as the SCN or by multiple oscillators working within a hierarchical organization [56]. There is now support for both hypotheses. Furthermore, while the SCN does provide a daily signal to the gonadotropin-releasing hormone (GnRH) cells of the hypothalamus that are part of the hypothalamic-pituitary-gondal axis, these GnRH cells only stimulate the preovulatory surge in luteinizing hormone (LH) if there is concomitantly sufficient levels of estradiol [57]. From studies conducted in the late 1990s and early 2000s in rodents, it is now known that hypothalamic GnRH cells express VPAC2, and that VIP projections from the SCN to these GnRH cells regulate the surge in GnRH and LH that stimulate ovulation (reviewed in [57]). Consistent with this research, it has recently been shown that female VIP knockout mice, in addition having a disrupted circadian system, have disrupted reproduction, characterized by a delayed estrous cycle, less frequent ovulation and a reduced number of offspring, compared to wild-type sisters [58].
Circadian rhythms in the SCN also encode seasonal changes, displaying different neuronal activity patterns in long versus short days and a means for storing a memory of photoperiod [59, 60]. Recently it has been proposed that VIP is required for this seasonal encoding since VIP knockout mice display no “after-effects” in either their locomotor activity patterns or SCN electrical activity when transferred to constant darkness. In contrast, wild-type controls do show these “after effects” that reflect a memory of the previous photoperiod [61].
Previous work suggests that VIP may have a related role in birds for sensing photoperiodic changes for the timing of seasonal reproduction [62, 63]. However, whereas the eyes, SCN and pineal gland are important for sensing and transducing photoperiodic information in mammals, deep brain photoreceptors in the lateral septal organ (LSO) and hypothalamic regions appear to detect photoperiodic cues in birds [64]. Furthermore, VIP cells within the mediobasal hypothalamus (MBH) and LSO likely serve as the encephalic photoreceptors [62-66]. Most recently it has been shown that VIP-immunoreactivity is significantly increased in all regions of the MBH of black-headed buntings in a migratory state, as compared to a non-migratory state, suggesting that the increase in VIP cells and fibers may mediate increased light sensitivity for seasonal night migration [65].
VIP’s role in preparatory nesting
Given VIP’s roles in reproductive behavior via its modulation of prolactin secretion, the timing of the preovulatory GnRH/LH surge, and the proposed encoding of seasonal photoperiod, VIP may also be important for behaviors that are preparatory to the arrival of offspring, such as “nesting” behaviors. In a recent study examining the activation of VIP brain cells groups within zebra finches in response to nesting, VIP mRNA and VIP-Fos co-expression were correlated with nesting behaviors (i.e. time in nest cup, nest items picked up, nest items carried to the nest) in virtually all key nodes of the social behavior network, with particularly strong positive correlations for the POA and MeA [67]. Future experiments manipulating VIP signaling in vivo will be useful to establish a causal link between VIP and nesting behavior, as well as other parental behaviors such as incubation and nestling care.
Conclusions
It is well established that hypothalamic VIP is the primary regulator of prolactin secretion in vertebrates and a major regulator of mammalian circadian rhythms. We now describe new roles for VIP that likely include the modulation of affiliation within novel environments, group size preferences, pair bonding and nesting behavior, based on recent findings from central VPAC antagonism studies, immediate early gene studies, and comparisons of VIP circuitry in territorial and flocking species of finches and sparrows. In addition, VIP potently mediates aggression, based on recent findings of site-specific VIP knockdown in the dorsal AH of finches, effects of central VIP infusions, and correlations between VIP elements and aggressive behaviors. Recent advances within the field of circadian and biological rhythms propose roles for VIP in the timing of ovulation during the estrous cycle, the seasonal encoding of photoperiod, and perhaps the timing of seasonal migration. Based on the findings presented here and the distribution of VIP cells and receptors throughout the brain’s “social behavior network”, it is likely that VIP has significant roles in many aspects of social behavior, which are only just beginning to be elucidated (Table 1).
Table 1. Proposed associations between VIP signaling and various social behaviors across taxa1.
| Area | Taxonomic group or Species | Sex | Behavior/Physiological Response | Reference |
|---|---|---|---|---|
| Extended amyadala: | ||||
| MeA, anterior | zebra finches | M, F | nesting | [67•] |
| MeA, posterior | zebra finches | M, F | nesting | [67•] |
| BSTm | zebra finches | M | nesting | [67•] |
| BSTm | estrildid finches, emberizid sparrows2 | M, F | flocking | [27, 33••] |
| Septum: | ||||
| CcS | field sparrows, song sparrows | M | aggression | [33••] |
| LS | violet-eared waxbills | M | aggression | [48] |
| LS | zebra finches | M | aggression (−) | [49] |
| LS | field sparrows | M | agonistic song (−) | [48] |
| LSc, subpallial | estrildid finches2 | M, F | flocking | [27] |
| LSO, medial | chickens, doves, pigeons, quail2 | M | seasonal photoperiodic response | [62-64, 66] |
| Other Telencephalic: | ||||
| N, medial | zebra finches | M, F | grouping | [36••] |
| POA-hypothalamus: | ||||
| AH, dorsal | violet-eared waxbills, zebra finches | M, F | aggression | [53••] |
| AH, dorsal | zebra finches | M, F | nesting (−) | [67•] |
| IH/INF | native Thai hens | F | incubation | [14] |
| IH/INF | native Thai hens | F | rearing | [7] |
| INF/ME | zebra finches | M, F | nesting* | [67•] |
| INF/ME | bantam hens, turkeys | F | incubation | [3, 4] |
| INF/ME | ring doves | M, F | incubation | [10] |
| MBH | chickens, black-headed buntings | M | seasonal photoperiodic response | [62, 64, 65•] |
| MPA | zebra finches | M, F | nesting | [67•] |
| MPO | zebra finches | M, F | nesting | [67•] |
| SCN | hamsters, humans, mice, rats2 | M, F | circadian rhythms | [15, 16•, 26•] |
| SCN | mice, rats2 | F | reproduction, ovulation | [57••, 58••] |
| SCN | mice2 | M | seasonal encoding of photoperiod | [61••] |
| PVN | zebra finches | M, F | flocking | [33••] |
| VMH | zebra finches | M, F | nesting4 | [67•] |
| Midbrain: | ||||
| CG | zebra finches | F | nesting (−) | [67•] |
| ICo, medial | zebra finches | M | nesting (−) | [67•] |
| ICo, lateral | zebra finches | F | nesting | [67•] |
| VTA, caudal | zebra finches | F | nesting | [67•] |
| Other: | ||||
| ICV (VPAC antagonism) | zebra finches | M, F | Grouping3, social contact, pair bonding | [36••, 45•] |
| IP (VPAC antagonism) | NIH Swiss mice | M | sociability | [37] |
| VIP genetic knockdown | mice2 | M, F | sociability | [38] |
Abbreviations: Extended amygdala: BSTm, medial bed nucleus of the stria terminalis; MeA; medial amygdala. Septum: CcS, caudocentral septum; LS, lateral septum; LSc, caudal division of lateral septum (here subpallial includes ventrolateral and ventral LSc); LSO, lateral septal organ. Other telencephalic: N, nidopallium. POA-hypothalamus: AH, anterior hypothalamus; IH, inferior nucleus; INF, infundibular nucleus; MBH, mediobasal hypothalamus; ME, median eminence; MPA, medial preoptic area; MPO, medial preoptic nucleus; POA, preoptic area; PVN, paraventricular nucleus; SCN, suprachiasmatic nucleus; VMH, ventromedial hypothalamus. Midbrain: CG, central gray; ICo, intercollicular nucleus; VTA, ventral tegmental area. Other: ICV, intraventricular (infusion); IP, interperitoneal (injection).
Proposed relationship between VIP and behavior is positive unless otherwise indicated.
For these entries, please refer to the listed references for specific strains, crosses or species used.
A positive relationship is observed for females and a negative relationship is observed for males.
A positive relationship is observed for males and a negative relationship is observed for females.
Trends were observed between the amount of VIP/Fos cells and nesting in the INF/ME.
Highlights.
We propose new roles for VIP signaling in avian affiliation, pair bonding and aggression.
We describe advances in VIP’s role as a regulator of reproductive and seasonal rhythms.
VIP has widespread actions throughout the “social behavior network” in the brain.
Acknowledgements
We would like to thank Leah C. Wilson for comments on this manuscript. National Institutes of Health grant R01 MH092331 supports work in the laboratory of the author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
We have nothing to declare.
References
- 1.El Halawani M. Vasoactive intestinal peptide as the avian prolactin-releasing factor. In: Harvey S, Etches RJ, editors. Perspectives In Avian Endocrinology. Wiley-Blackwell; 1997. pp. 403–416. [Google Scholar]
- 2.Kato Y, Iwasaki Y, Iwasaki J, Abe H, Yanaihara N, Imura H. Prolactin release by vasoactive intestinal polypeptide in rats. Endocrinology. 1978;103:554–558. doi: 10.1210/endo-103-2-554. [DOI] [PubMed] [Google Scholar]
- 3.El Halawani ME, Pitts GR, Sun S, Silsby JL, Sivanandan V. Active immunization against vasoactive intestinal peptide prevents photo-induced prolactin secretion in turkeys. Gen Comp Endocrinol. 1996;104:76–83. doi: 10.1006/gcen.1996.0143. [DOI] [PubMed] [Google Scholar]
- 4.Sharp PJ, Sterling RJ, Talbot RT, Huskisson NS. The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: observations using passive immunization, radioimmunoassay and immunohistochemistry. J Endocrinol. 1989;122:5–13. doi: 10.1677/joe.0.1220005. [DOI] [PubMed] [Google Scholar]
- 5.Buntin JD, Becker GM, Ruzycki E. Facilitation of parental behavior in ring doves by systemic or intracranial injections of prolactin. Horm Behav. 1991;25:424–444. doi: 10.1016/0018-506x(91)90012-7. [DOI] [PubMed] [Google Scholar]
- 6.Grattan DR, Bridges RS. Prolactin actions in the brain. In: Pfaff DW, editor. Hormones, Brain and Behavior. Vol. 4. Elsevier; Oxford, UK: 2009. pp. 2471–2503. [Google Scholar]
- 7.Chaiyachet OA, Chokchaloemwong D, Prakobsaeng N, Sartsoongnoen N, Kosonsiriluk S, Rozenboim I, El Halawani ME, Porter TE, Chaiseha Y. Neuroendocrine regulation of rearing behavior in the native Thai hen. Acta Histochem. 2013;115:209–218. doi: 10.1016/j.acthis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Youngren OM, Silsby JL, Rozenboim I, Phillips RE, el Halawani ME. Active immunization with vasoactive intestinal peptide prevents the secretion of prolactin induced by electrical stimulation of the turkey hypothalamus. Gen Comp Endocrinol. 1994;95:330–336. doi: 10.1006/gcen.1994.1130. [DOI] [PubMed] [Google Scholar]
- 9.Chaiseha Y, Tong Z, Youngren OM, El Halawani ME. Transcriptional changes in hypothalamic vasoactive intestinal peptide during a photo-induced reproductive cycle in the turkey. J Mol Endocrinol. 1998;21:267–275. doi: 10.1677/jme.0.0210267. [DOI] [PubMed] [Google Scholar]
- 10.Cloues R, Ramos C, Silver R. Vasoactive intestinal polypeptide-like immunoreactivity during reproduction in doves: influence of experience and number of offspring. Horm Behav. 1990;24:215–231. doi: 10.1016/0018-506x(90)90006-j. [DOI] [PubMed] [Google Scholar]
- 11.Youngren O, Chaiseha Y, Phillips R, El Halawani M. Vasoactive intestinal peptide concentrations in turkey hypophysial portal blood differ across the reproductive cycle. Gen Comp Endocrinol. 1996;103:323–330. doi: 10.1006/gcen.1996.0128. [DOI] [PubMed] [Google Scholar]
- 12.Al Kahtane A, Chaiseha Y, El Halawani M. Dopaminergic regulation of avian prolactin gene transcription. J Mol Endocrinol. 2003;31:185–196. doi: 10.1677/jme.0.0310185. [DOI] [PubMed] [Google Scholar]
- 13.Youngren OM, Chaiseha Y, El Halawani ME. Regulation of prolactin secretion by dopamine and vasoactive intestinal peptide at the level of the pituitary in the turkey. Neuroendocrinol. 1998;68:319–325. doi: 10.1159/000054380. [DOI] [PubMed] [Google Scholar]
- 14.Prakobsaeng N, Sartsoongnoen N, Kosonsiriluk S, Chaiyachet OA, Chokchaloemwong D, Rozenboim I, El Halawani M, Porter TE, Chaiseha Y. Changes in vasoactive intestinal peptide and tyrosine hydroxylase immunoreactivity in the brain of nest-deprived native Thai hen. Gen Comp Endocrinol. 2011;171:189–196. doi: 10.1016/j.ygcen.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16•.Hastings MH, Brancaccio M, Maywood ES. Circadian pacemaking in cells and circuits of the suprachiasmatic nucleus. J Neuroendocrinol. 2014;26:2–10. doi: 10.1111/jne.12125. A current comprehensive review of SCN circadian pacemaking that details the role of VIP and VPAC receptors within this pacemaking circuitry.
- 17.Romijn HJ, Sluiter AA, Pool CW, Wortel J, Buijs RM. Differences in colocalization between Fos and PHI, GRP, VIP and VP in neurons of the rat suprachiasmatic nucleus after a light stimulus during the phase delay versus the phase advance period of the night. J Comp Neurol. 1996;372:1–8. doi: 10.1002/(SICI)1096-9861(19960812)372:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain Res. 2000;877:361–366. doi: 10.1016/s0006-8993(00)02724-4. [DOI] [PubMed] [Google Scholar]
- 19.Piggins HD, Antle MC, Rusak B. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci. 1995;15:5612–5622. doi: 10.1523/JNEUROSCI.15-08-05612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 21.Zawilska JB, Niewiadomski P, Nowak JZ. PAC1 receptors in chick cerebral cortex: characterization by binding of pituitary adenylate cyclase-activating polypeptide, [125I]-PACAP27. Neurosci Lett. 2003;338:155–158. doi: 10.1016/s0304-3940(02)01397-6. [DOI] [PubMed] [Google Scholar]
- 22.Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am Journal Physiol Regul Integr Comp Physiol. 2003;285:939–949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- 24.Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 25.Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol: CB. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 26•.Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci U S A. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. The authors demonstrates that there is a hierarchy of neuropeptide signals that contribute to SCN molecular pacemaking, with a preeminent role for VIP signaling.
- 27.Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- 29.Kuenzel WJ, McCune SK, Talbot RT, Sharp PJ, Hill JM. Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus) J Comp Neurol. 1997;381:101–118. doi: 10.1002/(sici)1096-9861(19970428)381:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 30.Sims KB, Hoffman DL, Said SI, Zimmerman EA. Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res. 1980;186:165–183. doi: 10.1016/0006-8993(80)90263-2. [DOI] [PubMed] [Google Scholar]
- 31.Goodson JL, Kingsbury MA. What’s in a name? Considerations of homologies and nomenclature for vertebrate social behavior networks. Horm Behav. 2013;64:103–112. doi: 10.1016/j.yhbeh.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 33••.Goodson JL, Wilson LC, Schrock SE. To flock or fight: Neurochemical signatures of divergent life histories in sparrows. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10685–10692. doi: 10.1073/pnas.1203394109. This study demonstrates that VIP circuitry is characterized by widespread and remarkable seasonal remodeling in emberizid sparrows that differ in their territorial and flocking behavior. Some major findings are that 1) winter flocking species show greater VIP innervation of the BSTm and PVN than do winter non-flocking species; and 2) VIP immunolabeling in the AH and caudocentral septum correlates positively with territorial aggression in the spring.
- 34.Kelly AM, Goodson JL. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol. 2014;35:512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Nowak JZ, Sedkowska P, Zawilska JB, Gozes I, Brenneman DE. Antagonism of VIP-stimulated cyclic AMP formation in chick brain. J Mol Neurosci. 2003;20:163–172. doi: 10.1385/JMN:20:2:163. [DOI] [PubMed] [Google Scholar]
- 36••.Kingsbury MA, Miller KM, Goodson JL. VPAC receptor signaling modulates grouping behavior and social responses to contextual novelty in a gregarious finch: A role for a putative prefrontal cortex homologue. Horm Behav. 2013;64:511–518. doi: 10.1016/j.yhbeh.2013.07.004. This study is the first to show that VPAC receptor signaling regulates grouping behavior and social contact in birds. Specifically, signaling through VIP VPAC receptors promotes social contact in a novel environment and regulates gregariousness in a sex-specific and site-specific manner in zebra finches.
- 37.Hill JM, Cuasay K, Abebe DT. Vasoactive intestinal peptide antagonist treatment during mouse embryogenesis impairs social behavior and cognitive function of adult male offspring. Exp Neurol. 2007;206:101–113. doi: 10.1016/j.expneurol.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Stack CM, Lim MA, Cuasay K, Stone MA, Seibert KM, Spivak-Pohis I, Crawley JN, Waschek JA, Hill JM. Deficits in social behavior and reversal learning are more prevalent in male offspring of VIP deficient female mice. Exp Neurol. 2008;211:67–84. doi: 10.1016/j.expneurol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly AM, Kingsbury MA, Hoffbuhr K, Schrock SE, Waxman B, Kabelik D, Thompson RR, Goodson JL. Vasotocin neurons and septal V1a-like receptors potently modulate songbird flocking and responses to novelty. Horm Behav. 2011;60:12–21. doi: 10.1016/j.yhbeh.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly AM, Goodson JL. Hypothalamic oxytocin and vasopressin neurons exert sex-specific effects on pair bonding, gregariousness, and aggression in finches. Proc Natl Acad Sci U S A. 2014;111:6069–6074. doi: 10.1073/pnas.1322554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly AM, Goodson JL. Personality is tightly coupled to vasopressin-oxytocin neuron activity in a gregarious finch. Front Behav Neurosci. 2014;8:55. doi: 10.3389/fnbeh.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bardrum B, Ottesen B, Fahrenkrug J, Fuchs AR. Release of oxytocin and vasopressin by intracerebroventricular vasoactive intestinal polypeptide. Endocrinology. 1988;123:2249–2254. doi: 10.1210/endo-123-5-2249. [DOI] [PubMed] [Google Scholar]
- 44.Bardrum B, Ottesen B, Fuchs AR. Preferential release of oxytocin in response to vasoactive intestinal polypeptide in rats. Life Sci. 1987;40:169–173. doi: 10.1016/0024-3205(87)90356-0. [DOI] [PubMed] [Google Scholar]
- 45•.Kingsbury MA, Goodson JL. Pair bond formation is impaired by VPAC receptor antagonism in the socially monogamous zebra finch. Behav Brain Res. 2014;272:264–268. doi: 10.1016/j.bbr.2014.06.042. This study shows that signaling through VIP VPAC receptors promotes pair bonding in birds.
- 46.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 47.Klatt JD, Goodson JL. Oxytocin-like receptors mediate pair bonding in a socially monogamous songbird. Proc Biol Sci. 2013;280:20122396. doi: 10.1098/rspb.2012.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodson JL. Vasotocin and vasoactive intestinal polypeptide modulate aggression in a territorial songbird, the violet-eared waxbill (Estrildidae: Uraeginthus granatina) Gen Comp Endocrinol. 1998;111:233–244. doi: 10.1006/gcen.1998.7112. [DOI] [PubMed] [Google Scholar]
- 49.Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 50.Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- 51.Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475:426–461. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- 52.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 53••.Goodson JL, Kelly AM, Kingsbury MA, Thompson RR. An aggression-specific cell type in the anterior hypothalamus of finches. Proc Nat Acad Sci U S A. 2012;109:13847–13852. doi: 10.1073/pnas.1207995109. This study shows that aggression is regulated in a very selective and potent manner by VIP cells in the dorsal anterior hypothalamus of both territorial and gregarious finches.
- 54.Goodson JL, Schrock SE, Kingsbury MA. Oxytocin mechanisms of stress response and aggression in a territorial finch. Physiol Behav. 2015;141:154–163. doi: 10.1016/j.physbeh.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci U S A. 1976;73:2923–2927. doi: 10.1073/pnas.73.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Williams WP, 3rd, Kriegsfeld LJ. Circadian control of neuroendocrine circuits regulating female reproductive function. Front Endocrinol. 2012;3:1–14. doi: 10.3389/fendo.2012.00060. This comprehensive review discusses the role of VIP circuity in the circadian control of female reproduction in mammals, particularly in regard to the preovulatory LH surge and prolactin secretion.
- 58••.Loh DH, Kuljis DA, Azuma L, Wu Y, Truong D, Wang HB, Colwell CS. Disrupted reproduction, estrous cycle, and circadian rhythms in female mice deficient in vasoactive intestinal peptide. J Biol Rhythm. 2014;29:355–369. doi: 10.1177/0748730414549767. This study directly addresses the impact of VIP deficiency on female biological rhythms. Female VIP-/-mice have disrupted circadian rhythms, disrupted estrous cycles, reduced ovulation, reduced nursing and fewer offspring, compared to wild-type sisters.
- 59.Sumova A, Travnickova Z, Peters R, Schwartz WJ, Illnerova H. The rat suprachiasmatic nucleus is a clock for all seasons. Proc Natl Acad Sci U S A. 1995;92:7754–7758. doi: 10.1073/pnas.92.17.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, Vansteensel MJ, Block GD, Meijer JH. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 61••.Lucassen EA, van Diepen HC, Houben T, Michel S, Colwell CS, Meijer JH. Role of vasoactive intestinal peptide in seasonal encoding by the suprachiasmatic nucleus clock. Eur J Neurosci. 2012;35:1466–1474. doi: 10.1111/j.1460-9568.2012.08054.x. Analyses in VIP-/-mice suggest that in the absence of VIP, the suprachiasmatic nucleus loses its ability to encode seasonal information (i.e. cannot retain a “memory” of photoperiod).
- 62.Li H, Kuenzel WJ. A possible neural cascade involving the photoneuroendocrine system (PNES) responsible for regulating gonadal development in an avian species, Gallus gallus. Brain Res Bull. 2008;76:586–596. doi: 10.1016/j.brainresbull.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Saldanha CJ, Leak RK, Silver R. Detection and transduction of daylength in birds. Psychoneuroendocrinology. 1994;19:641–656. doi: 10.1016/0306-4530(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 64.Kuenzel WJ, Seong KW, Zhou JZ. Exploring deep-brain photoreceptors and their role in activating the neuroendocrine regulation of gonadal development. Poult Sci. 2015;94:786–798. doi: 10.3382/ps.2014-04370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Rastogi A, Kumari Y, Rani S, Kumar V. Neural correlates of migration: activation of hypothalamic clock(s) in and out of migratory state in the blackheaded bunting (Emberiza melanocephala) PLoS One. 2013;8(10):e70065. doi: 10.1371/journal.pone.0070065. VIP-immunoreactivity is significantly increased in the mediobasal hypothalamus (proposed site of an avian photoperiodic clock) of blackheaded buntings in a migratory state, as compared to a non-migratory state. Authors propose that the increase in VIP elements may mediate increased light sensitivity for seasonal night migration.
- 66.Silver R, Witkovsky P, Horvath P, Alones V, Barnstable CJ, Lehman MN. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- 67•.Kingsbury MA, Jan N, Klatt JD, Goodson JL. Nesting Behavior is Associated with VIP Expression and VIP-Fos Colocalization in a Network-Wide Manner. Horm Behav. 2015;69:68–81. doi: 10.1016/j.yhbeh.2014.12.010. Number and activation of VIP cells correlates with nesting behaviors throughout the “social behavior network” in the brain and indicates a network-wide relationship between VIP production and preparatory nesting.