Abstract
Objective:
To optimize the flattening filter-free (FFF) beam selection in stereotactic body radiotherapy (SBRT) treatment for Stage I lung cancer in different fraction schemes.
Methods:
Treatment plans from 12 patients suffering from Stage I lung cancer were designed using the 6XFFF and 10XFFF beams in different fraction schemes of 4 × 12, 3 × 18 and 1 × 34 Gy. Plans were evaluated mainly in terms of organs at risk (OARs) sparing, normal tissue complication probability (NTCP) estimation and treatment efficiency.
Results:
Compared with the 10XFFF beam, 6XFFF beam showed statistically significant lower dose to all the OARs investigated. The percentage of NTCP reduction for both lung and chest wall was about 10% in the fraction schemes of 4 × 12 and 3 × 18 Gy, whereas only 7.4% and 2.6% was obtained in the 1 × 34 Gy scheme. For oesophagus, heart and spinal cord, the reduction was greater with the 6XFFF beam, but their absolute estimates were <10−6%. The mean beam-on time for 6XFFF and 10XFFF beams at 4 × 12, 3 × 18 and 1 × 34 Gy schemes were 2.2 ± 0.2 vs 1.5 ± 0.1, 3.3 ± 0.9 vs 2.0 ± 0.5 and 6.3 ± 0.9 vs 3.5 ± 0.4 min, respectively.
Conclusion:
The 6XFFF beam obtains better OARs sparing and lower incidence of NTCP in SBRT treatment of Stage I lung cancer, whereas the 10XFFF beam improves the treatment efficiency. To balance the OARs sparing and intrafractional variation owing to the prolonged treatment time, the authors recommend using the 6XFFF beam in the 4 × 12 and 3 × 18 Gy schemes but the 10XFFF beam in the 1 × 34 Gy scheme.
Advances in knowledge:
This study optimizes the FFF beam selection in different fraction schemes in SBRT treatment of Stage I lung cancer.
INTRODUCTION
Lung cancer is the first and second leading cause of cancer death both in males and females, respectively, accounting for 13% of the total cases and 18% of deaths in 2008.1 Surgery is the recommended choice for patients diagnosed as early stage non-small cell lung cancer (NSCLC),2 but many patients do not undergo surgery owing to comorbidities, patient preferences or other reasons.3 Stereotactic body radiotherapy (SBRT) is a novel non-invasive radiation therapy that delivers a highly conformal and hypofractionated ablative radiation dose to the tumours over several fractions and provides sharp fall-off dose gradients outside the target.4 Therefore, it is increasingly recognized as a preferred treatment choice in patients who cannot undergo surgery.5,6 Recent publications7,8 demonstrated that SBRT treatment of early stage NSCLC achieved similar disease-free survival, local control and distant control as surgery.
In the past few years, Varian's RapidArc® solution (Varian® Medical Systems, Palo Alto, CA) combined with flattening filter-free (FFF) beam has become an extraordinarily attractive dose delivery technique for lung SBRT.9–11 FFF beams are characterized by high dose rate and are most frequently utilized for treatments where higher fraction dose is delivered, including hypofractionated SBRT for NSCLC, liver and other sites.9–12 6XFFF and 10XFFF are the only FFF beam modes in Varian's TrueBeam™ accelerator (Varian® Medical Systems), and they are both widely used in SBRT treatment for lung cancer.9,10,12–14 It is well known that the 10XFFF beam is produced with a dose rate of up to 2400 monitor units (MU) min−1 than 1400 MU min−1 for 6XFFF beam and consequently improves the treatment efficiency.9,13,14 However, the treatment efficiency is not the only factor that needs taking into account for SBRT treatment, the organs at risk (OARs) sparing is equally important and also worthy of considering according to radiation therapy oncology group (RTOG) 0915 report.15 Up till now, the dosimetric impact to the normal tissue for the two beam energies remains unknown. Only Hrbacek et al16 suggested that a 6XFFF beam was more suitable for lung SBRT, owing to the sharper dose fall-off than in 10XFFF beam, but they did not perform additional experiments to confirm that. On the other hand, although Weiss et al17 analysed 6- vs 18-MV flattened photon energy plans for intensity modulated radiation therapy of lung cancer and found that 6-MV beam reduced the lung, heart and oesophagus dose, few studies18,19 have investigated the dosimetric analysis with FFF beams in lung SBRT. Unlike conventional beam, the FFF beams involve cone-like profile and less out-of-field dose. Whether the 6XFFF beam really provides better OARs sparing than the 10XFFF beam still needs further investigation.
Additionally, many publications reported the clinical feasibility of FFF beams for SBRT treatment of NSCLC;9–13 however, the most suitable fraction scheme for peripheral lung lesion is still questionable. Lower dose fraction schemes will narrow the treatment efficiency difference between the 6XFFF and 10XFFF beams according to our preliminary experiences. In that case, beam energy selection is fraction scheme dependent if the 6XFFF beam really provides better OARs sparing. Consequently, how to select appropriate beam energy is an issue that needs to be addressed during SBRT treatment for Stage I lung cancer.
Therefore, the main purpose of the study is two fold: firstly, we want to confirm the better OARs sparing with the 6XFFF beam than the 10XFFF beam; secondly, the planning study aims to optimize the beam energy selection in different fraction schemes by evaluating the differences in dosimetry, tumour control probability (TCP), normal tissue complication probability (NTCP) and treatment efficiency between the 6XFFF and 10XFFF beams based on the Varian's RapidArc solution. It is hoped that the problem of beam energy selection in different fraction schemes for the SBRT treatment of lung cancer will be resolved.
The work is specific to the Varian RapidArc solution because Elekta (Elekta AB, Stockholm, Sweden) FFF solution uses a different approach regarding the energy modulation. It increases the beam energy in order to account for loss of beam hardening,20 and this may change the results if repeated using those beams and a different planning algorithm/system.
METHODS AND MATERIALS
Patient characteristics
From March 2013 to October 2014, 12 patients suffering from Stage I lung cancer were enrolled in this study. Tumour staging was according to the American Joint Committee on Cancer, seventh edition. 10 of them were males and the remaining 2 were females. Their age ranged from 40 to 68 years with the mean age of 51.7 ± 9.2 years, and their median planning target volume (PTV) was 32.2 ± 12.7 cm3. Detailed information of the 12 patients investigated was listed in Table 1.
Table 1.
Detailed information of the patients enrolled
| Patient index | Age (years) | Gender | Planning target volume (cm3) | Tumour location |
|---|---|---|---|---|
| 1 | 56 | M | 28.7 | LLL |
| 2 | 67 | M | 16.3 | RML |
| 3 | 54 | M | 27.6 | RML |
| 4 | 43 | M | 31.3 | LLL |
| 5 | 45 | F | 23.0 | RML |
| 6 | 57 | M | 22.4 | RML |
| 7 | 43 | M | 40.8 | RLL |
| 8 | 53 | M | 63.5 | RUL |
| 9 | 46 | M | 39.9 | RML |
| 10 | 40 | F | 39.5 | RUL |
| 11 | 68 | M | 20.1 | LUL |
| 12 | 48 | M | 33.7 | RML |
F, female; LLL, left lower lobe; LUL, left upper lobe; M, male; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
CT scanning and contouring of organs at risk
Immobilization was performed by using thermoplastic masks (Guangzhou Klarity Medical & Equipment Co., Ltd, Guangzhou, China), with arms resting at both sides of the body in the supine position. Patients were subjected to a four-dimensional CT (4DCT) scans (The Philips Brilliance CT Big Bore Oncology Configuration; Philips Healthcare, Cleveland, OH) under uncoached free breathing. The CT images were then transferred to Varian Eclipse™ treatment planning system v. 10 (Varian® Medical Systems) for target, OARs delineation and treatment planning. The gross tumour volume (GTV) accounting for the 10 breathing phases were contoured in the CT pulmonary windows by a radiation oncologist expertized in lung SBRT. 10 phases of the GTV were then combined and formed the internal target volume. To account for setup inaccuracies and potential intrafractional tumour shifts, a 0.5-cm setup margin was added to the ITV to form the PTV. All plans were carried out on the averaged 4DCT. OARs, including aorta, oesophagus, bronchial tree, heart, spinal cord, lung and chest wall (CW) were contoured according to the RTOG 0915 report.15
Treatment planning
Prescription was set to three different fraction schemes of 4 × 12, 3 × 18 and 1 × 34 Gy according to RTOG 0915 and other clinically feasible publications.15,21,22 Treatment planning was designed with partial arcs to exclude the entrance of the beam through the contralateral lung. All plans were generated from a clockwise and a counterclockwise arc with the same isocentre. Collimator angles for all plans were set to 30° in one arc and the complementary angle for another to minimize the contribution of the tongue-and-groove effect to the dose.23 The same beam arrangement and optimization constraints were employed for the 6XFFF and 10XFFF beams. Plans of different beam energy were optimized, selecting a maximum dose rate of 1400 and 2400 MU min−1 for the 6XFFF and 10XFFF beams, respectively. Optimization was performed with the progressive resolution optimizer algorithm implemented in Eclipse 10.0. The optimizing objectives were adjusted to make sure the maximum dose was between 120% and 130% of the prescription dose and centred in the GTV. Dose calculations were carried out using the anisotropic analytic algorithm (AAA) taking into account heterogeneity correction with a calculation grid size of 1 mm. The final dose calculation was normalized to guarantee that 95% of PTV received the prescribed dose. All of the dose constraints to PTV, conformity of prescribed dose, intermediate dose spillage and critical organ dose–volume limits should meet the criterion of the RTOG 0915 protocol and other publications.4,15
Evaluation of treatment planning
The evaluating parameters include the maximum, minimum and mean dose to the PTV. CI100%, defined as the ratio of prescription isodose volume to the PTV volume,15 was employed to evaluate the conformity of the prescribed dose. D2cm (defined as the maximum dose 2 cm away from PTV in any direction) and R50% (defined as the ratio of 50% prescription isodose volume to the PTV volume) were used to evaluate the intermediate dose spillage according to the RTOG 0915 protocol.15 For the OARs, the analysis included the maximum dose to the aorta, oesophagus, bronchial tree, heart and spinal cord. Meanwhile, a set of appropriate Vx values and Dmean were used to assess the lung. Vx was the volume of the organ receiving a dose of x or more. For example, V40 was the volume of organ receiving a dose of 40 Gy or more. Dmean represents the mean dose to the OARs. V30 and V40 were reported to be the predictive factors of radiation-induced CW pain.24,25 As the minimum fraction scheme in our study was 1 × 34 Gy, we only used V30 to gauge the risk of complication of CW pain. The beam-on time (the time when beam is actually delivered) was recorded on the TrueBeam accelerator (Varian® Medical Systems) by adding the “automation” function.
Radiobiological effect calculation
Both of TCP and NTCP were calculated with in-house developed programs using MATLAB® 7.0 (MathWorks®, Natick, MA). We employed the equivalent uniform dose-based biological model to estimate TCP.26 Values of α, TCD50 (tumour control dose 50%) and γ50 in the model was according to the work by Park et al27 and Okunieff et al.28 For NTCP estimation, we utilized the Lyman–Kutcher–Burman (LKB) model to predict the complication probability of the lung, oesophagus, heart and spinal cord. Values of n, m and TD50 (the probability of 50% complication) in the LKB model followed the results of the studies by Emami et al29 and Burman et al.30 Because Woody et al31 found better prediction using the modified equivalent uniform dose model with moderate weighting over maximum dose to a 1 cm3 volume and V30 models, we incorporated it for prediction of CW pain. Unfortunately, complication probability of bronchial tree and aorta was unfinished owing to the lack of parameters of n, m and TD50 in the references. In detail, the statistics from cumulative dose volume histogram (cDVH) was exported at a resolution of 5 cGy and imported to the MATLAB® software. Before computing the TCP and the NTCP, the program needed to convert the cDVH to the differential DVH is required by the mathematical model. Then, the program converted the dose in each volume element to a biological equivalent dose (BED) with the formula from the work by Liu et al.32 The value of α/β in the BED conversion was assigned to 10 Gy for the TCP calculation and 3 Gy for NTCP estimation of oesophagus, heart, spinal cord and CW.33 Particularly, α/β value of the lung was equal to 1.3 Gy according to study result by Scheenstra et al.34
Statistical analysis
All data in this study were presented as the mean ± standard deviation. To determine the difference between plans, the Wilcoxon signed-rank tests were performed using SPSS® v. 17.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL). Differences were considered statistically significant when p < 0.05.
RESULTS
Table 2 presents the difference of the PTV minimum, maximum, mean dose, CI100%, R50% and D2cm between the 6XFFF and 10XFFF beams in different fraction schemes. It was observed that 10XFFF beam predicted about 1% higher maximum and mean dose to the PTV in the three fraction schemes, and the comparisons were statistically significant (p < 0.05). But the two beam modalities obtained comparable CI100% (p > 0.05). As to the intermediate dose spillage, the 10XFFF beam provided 4–5% higher R50% in the three fraction schemes (p < 0.05), but the D2cm in the two beam energies was similar (p > 0.05).
Table 2.
Dosimetric comparison between the 6XFFF and 10XFFF beams in the three fraction schemes
| Parameters | 4 × 12 Gy |
3 × 18 Gy |
1 × 34 Gy |
|||
|---|---|---|---|---|---|---|
| 6XFFF | 10XFFF | 6XFFF | 10XFFF | 6XFFF | 10XFFF | |
| PTV | ||||||
| Dmin (%) | 93.2 ± 0.5 | 93.1 ± 1.2 | 93.4 ± 0.9 | 93.3 ± 0.8 | 93.2 ± 0.7 | 93.1 ± 0.9 |
| Dmax (%) | 123.4 ± 0.9 | 124.4 ± 0.8a | 123.3 ± 1.1 | 124.1 ± 0.9b | 123.4 ± 1.2 | 124.6 ± 1.1c |
| Dmean (%) | 108.6 ± 1.5 | 108.9 ± 1.4a | 108.6 ± 1.5 | 108.9 ± 1.3b | 108.6 ± 1.4 | 109.0 ± 1.4c |
| CI100% | 0.968 ± 0.014 | 0.973 ± 0.013 | 0.963 ± 0.011 | 0.975 ± 0.016b | 0.961 ± 0.013 | 0.975 ± 0.025 |
| Intermediate dose spillage | ||||||
| R50% | 4.1 ± 0.3 | 4.3 ± 0.3a | 4.1 ± 0.3 | 4.3 ± 0.3b | 4.1 ± 0.3 | 4.3 ± 0.3c |
| D2cm (%) | 49.1 ± 3.3 | 49.6 ± 3.6 | 49.0 ± 3.8 | 49.2 ± 3.4 | 49.0 ± 3.0 | 49.6 ± 3.2c |
| Aorta | ||||||
| Dmax (Gy) | 11.6 ± 6.7 | 12.8 ± 6.6a | 13.3 ± 6.9 | 14.2 ± 7.3b | 8.4 ± 4.6 | 8.9 ± 4.8c |
| Bronchial tree | ||||||
| Dmax (Gy) | 15.5 ± 9.6 | 16.6 ± 9.6a | 15.0 ± 11.1 | 16.7 ± 12.2b | 10.8 ± 6.1 | 11.6 ± 6.7c |
| CW | ||||||
| V30 (cm3) | 11.1 ± 11.2 | 12.4 ± 11.9a | 17.8 ± 15.6 | 19.3 ± 16.6b | 2.3 ± 1.9 | 2.7 ± 2.3c |
| Spinal cord | ||||||
| Dmax (Gy) | 9.1 ± 4.7 | 9.9 ± 4.5a | 10.8 ± 5.4 | 11.5 ± 5.2b | 6.5 ± 3.2 | 7.1 ± 3.2c |
| Oesophagus | ||||||
| Dmax (Gy) | 8.8 ± 2.7 | 9.8 ± 2.6a | 10.0 ± 2.8 | 10.9 ± 3.2b | 6.3 ± 2.0 | 6.9 ± 2.1c |
| Heart | ||||||
| Dmax (Gy) | 12.2 ± 4.2 | 13.4 ± 4.7a | 13.9 ± 5.1 | 15.1 ± 5.4b | 8.6 ± 3.2 | 9.3 ± 3.2c |
| Lung | ||||||
| V5 (%) | 13.9 ± 4.0 | 14.5 ± 4.2a | 14.6 ± 4.0 | 15.5 ± 4.4b | 12.0 ± 4.3 | 12.3 ± 4.4c |
| V10 (%) | 9.8 ± 4.1 | 10.0 ± 4.1a | 10.5 ± 4.2 | 10.8 ± 4.4b | 6.9 ± 3.3 | 7.1 ± 3.4c |
| V20 (%) | 4.5 ± 2.5 | 4.7 ± 2.6a | 5.1 ± 2.9 | 5.2 ± 3.0b | 2.7 ± 1.6 | 2.8 ± 1.6c |
| Dmean (Gy) | 3.2 ± 1.2 | 3.4 ± 1.2a | 3.6 ± 1.3 | 3.8 ± 1.4b | 2.3 ± 0.8 | 2.4 ± 0.9c |
CI100%, conformity of the prescribed dose; CW, chest wall; D2cm, maximum dose 2 cm from PTV in any direction; Dmax, maximum dose; Dmean, mean dose; Dmin, minimum dose; FFF, flattening filter free; PTV, planning target volume; R50% = ratio of 50% prescription isodose volume to the PTV volume; Vx, the volume of the organ receiving a dose of x or more.
Wilcoxon signed-rank tests with p < 0.05 between 6XFFF and 10XFFF plans in the 4 × 12 Gy fraction scheme.
Wilcoxon signed-rank tests with p < 0.05 between 6XFFF and 10XFFF plans in the 3 × 18 Gy fraction scheme.
Wilcoxon signed-rank tests with p < 0.05 between 6XFFF and 10XFFF plans in the 1 × 34 Gy fraction scheme.
Considering the OARs sparing, the 6XFFF beam energy contributed lower dose to the aorta, oesophagus, bronchial tree, heart and spinal cord in the three fraction schemes. Dose to OARs with the 6XFFF and 10XFFF beams in the three fraction schemes are displayed in Table 2. We need to state that the critical organ dose presented in the table is only simple doses without considering the radiobiological effect. However, we found that the dose difference became larger when taking the BED into account with the formula from the work by Liu et al.32
As to the radiobiological effect estimation, it is observed from Table 3 that TCP in the 3 × 18 and 1 × 34 Gy schemes were about 6% higher than the 4 × 12 Gy, but comparable TCP were obtained between the 6XFFF and 10XFFF beams in all the three fraction schemes. We also found that about 10% percentage of NTCP reduction for both of lung and CW was achieved with 6XFFF beam compared to the 10XFFF beam in the fractions schemes of 4 × 12 and 3 × 18 Gy. However, only 7.4% and 2.6% NTCP reduction were acquired with the 1 × 34 Gy scheme. For oesophagus, heart and spinal cord, the percentage of NTCP reduction with the 6XFFF beam was greater but the absolute NTCP estimates was extremely low (<10−6%).
Table 3.
Tumour control probability (TCP) and normal tissue complication probability (NTCP) prediction between the 6XFFF and 10XFFF beams in the three fraction schemes
| Parameters | 4 × 12 Gy |
3 × 18 Gy |
1 × 34 Gy |
|||
|---|---|---|---|---|---|---|
| 6XFFF | 10XFFF | 6XFFF | 10XFFF | 6XFFF | 10XFFF | |
| TCP (%) | 88.7 ± 1.9 | 88.7 ± 1.9 | 94.7 ± 2.1 | 94.7 ± 2.0 | 94.8 ± 2.0 | 94.8 ± 2.1 |
| NTCP | ||||||
| Lung (%) | 5.3 ± 3.0 | 5.9 ± 3.1a | 10.4 ± 5.9 | 11.3 ± 6.1b | 12.5 ± 7.3 | 13.4 ± 7.8c |
| NRlung (%) | 10.3 ± 4.5 |
9.9 ± 3.6 |
7.4 ± 2.2 |
|||
| CW | 33.6 ± 33.9 | 35.5 ± 34.9a | 66.1 ± 57.6 | 69.8 ± 58.5b | 113.8 ± 99.1 | 116.3 ± 99.8c |
| NRCW (%) | 11.9 ± 13.7 |
10.0 ± 8.8 |
2.6 ± 2.5 |
|||
| Oesophagus | NS | NS | NS | NS | NS | NS |
| Heart | NS | NS | NS | NS | NS | NS |
| Spinal cord | NS | NS | NS | NS | NS | NS |
CW, chest wall; FFF, flattening filter free; NR, 100% × (NTCP10X−NTCP6X)/NTCP10X; NRCW, percentage of NTCP reduction for CW; NRlung, percentage of NTCP reduction for lung; NS, not significant (NTCP values were <10−6%).
Wilcoxon signed-rank tests with p < 0.05 between 6XFFF and 10XFFF plans in the 4 × 12 Gy fraction scheme.
Wilcoxon signed-rank tests with p < 0.05 between 6XFFF and 10XFFF plans in the 3 × 18 Gy fraction scheme.
Wilcoxon signed-rank tests with p < 0.05 between 6XFFF and 10XFFF plans in the 1 × 34 Gy fraction scheme.
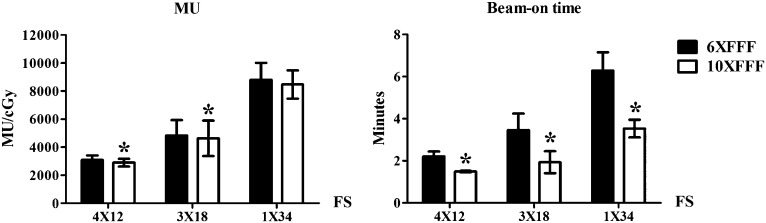
5–6% of MU reduction was observed with the 10XFFF beam in the 4 × 12 and 3 × 18 Gy schemes (p = 0.003 and 0.030), whereas the MU in the 1 × 34 Gy was similar (p = 0.119). Most importantly, the 10XFFF beam reduced the beam-on time by 31.9 ± 7.2%, 38.7 ± 2.8% and 43.6 ± 4.0% compared with the 6XFFF beam in the 4 × 12, 3 × 18 and 1 × 34 Gy schemes, respectively. It was 2.2 ± 0.2 vs 1.5 ± 0.1, 3.3 ± 0.9 vs 2.0 ± 0.5 and 6.3 ± 0.9 vs 3.5 ± 0.4 min for the 6XFFF and 10XFFF beams in the three fraction schemes (Figure 1).
Figure 1.
Monitor unit (MU) and beam-on time comparison between the 6XFFF and 10XFFF beams in the three fraction schemes (FS). *Statistically significant.
DISCUSSION
The most optimal FFF beam energy for lung SBRT is controversial, particularly when the most suitable fraction scheme for peripheral lung lesion is still questionable, with the balance of sparing the OARs and the treatment time. In this study, we found that the 6XFFF and 10XFFF beams each offered advantages: the 6XFFF beam provided up to 15% lower dose to all the OARs whereas the 10XFFF beam improved the treatment delivery efficiency by 0.7, 1.3 and up to 2.8 min in the 4 × 12, 3 × 18 and 1 × 34 Gy, respectively. The results confirm the point of view proposed by Hrbacek that 6XFFF beam provides better OARs sparing and confirmed the need for optimizing the FFF beam selection under different fraction schemes in lung SBRT.
Our finding that the 6XFFF beam better spared all of the related OARs supports the opinion that 6XFFF beam was more suitable for lung SBRT.16 It was reported that dose deposited outside the PTV was determined by beam penumbra and the scattered dose. 6XFFF beam has sharper penumbra compared with 10XFFF beam for small fields19 and thus tended to minimize irradiation to nearby OARs, although the regions near beam entry ports receive higher doses.35 Although we found that the 6XFFF beam provided better OARs sparing in peripheral lung cancer patients, our data was also useful for SBRT in central lung tumours. Treating central lung tumours with SBRT has been reported to induce oesophageal toxicities. Modh et al36 reported that 12.8% patients with central lung tumour experienced ≥ grade 2 oesophageal toxicity after SBRT treatment. Wu et al37 found that the incidence of grade ≥2 acute toxicity was 12%. In these cases, the use of 6XFFF beam will lower the incidence rate of oesophageal toxicities. The newly introduced SBRT was reported with higher incidence of complications than conventional techniques,4 such as pulmonary toxicity,24,38–41 CW pain24,25,31,42 and oesophageal toxicity,37 especially when the target was adjacent to the OARs. Previous studies found that V5, V10, V20 and mean lung dose (MLD) were predictive of radiation pneumonitis (RP).38–41 Other studies reported that the V30 was mostly associated with CW pain.25,31,42 Wu et al37 reported the maximum dose (Dmax) was the dosimetric predictors of oesophageal toxicity after SBRT for lung tumour. The dosimetric predictors mentioned above are improved with the 6XFFF beam and that may help to lower the incidence of RP, CW pain and oesophageal toxicity.
Our finding that the 10XFFF beam obtained 30–45% reduction of beam-on time was in accordance with other researches.9,12,43 Because the difference in beam-on time may differ with different fraction schemes, we analysed it by use of three widely used fraction schemes. All of the fraction schemes were widely introduced for clinical use in recent publications and RTOG guideline. Our study found that the average beam-on time was reduced by 31.9 ± 7.2%, 38.7 ± 2.8% and 43.6 ± 4.0% with the 10XFFF beam for the three introduced fraction schemes, respectively. The beam-on time reduction was greater with the fraction dose increasing. Treatment efficiency was a critical factor that needed to be taken into account during lung SBRT. Reduction of delivery time was of utmost importance for SBRT.44 The importance of treatment time was investigated by a series of clinical studies. It was reported that the incidence of intrafractional variation in the target was found to be proportional to the treatment time.45,46 The shorter treatment time generally introduces substantially superior patient stability and treatment accuracy, simultaneously reduces the likelihood of intrafractional baseline shifts in tumour position.47,48 Other studies also found that the target shift could lead to inferior GTV coverage and lower the minimum dose to the GTV.49
Because 6XFFF and 10XFFF beams have competing benefits, it's challenging to optimize the beam selection in SBRT for lung cancer. In this situation, to find the effect of treatment time on the tumour variation and patient stability is critical. To date, the only report regarding the target motion as a function of treatment time was conducted by Ma et al.46 Their result found the average time needed to maintain the target motion within 1 mm of translation or 1° of rotational deviation was 5.9 min for thoracic tumours. Meanwhile, Verbakel et al suggested a single CBCT setup would be sufficient if the entire radiation delivery could be completed in 6 min,44 implying patients' setup was stable within 6 min of delivery time. Based on these results, we take 6 mins as a threshold to keep the tumour and patient in a stable situation. Therefore, the difference in treatment time between the 6XFFF and 10XFFF beams is clinically negligible in the 4 × 12 and 3 × 18 Gy dose fraction schemes in which the delivery time ranges from 1.5 to 4 min. However, it can't be neglected in the 1 × 34 Gy scheme with 6XFFF beam due to the long delivery time (>6 min). Therefore, we recommended the use of 10XFFF beam in the 1 × 34 Gy scheme to improve the treatment efficiency and the use of 6XFFF beam in the 4 × 12 and 3 × 18 Gy to maintain better OARs sparing in SBRT treatment for lung cancer. With the radiobiological model, we found that the TCP in the 3 × 18 and 1 × 34 Gy schemes was significantly higher than in the 4 × 12 Gy scheme. As the 4 × 12 Gy scheme is recommended for SBRT treatment of Stage I lung cancer by RTOG guideline, further investigation are needed to follow its clinical results. On the other hand, we found that the ratio of expected NTCP with 6XFFF beam showed a potential 10% reduction for lung and CW with the 4 × 12 and 3 × 18 schemes, and 7.5% and 3% were achieved with the 1 × 34 Gy scheme. The results indicated the potential of 6XFFF beam to lower the risk of radiation-induced complication than the 10XFFF beam, and its clinical effects needed further observation. For oesophagus, heart and spinal cord, the percentage of NTCP reduction was much bigger. However, the absolute NTCP values were extremely low (<10−6%), and their clinical significance is limited.
Owing to the competition between the benefits provided by the 6XFFF and 10XFFF beams, the plan optimization warrants further study. Maybe the hybrid energy technique is the solution. Hybrid energy technique which has the potential to combine the benefits in different modalities had been investigated in the lung and prostate cancers.35,50–53
Regarding the inhomogeneity management in treatment sites with heterogeneous density, the accuracy of dose calculation algorithm is an issue of concern. In the present study, we implemented AAA to finish dose calculation. However, AAA was reported to be less accurate than Acuros® XB algorithm (AXB; Varian Medical Systems).54,55 AXB explicitly solves the linear Boltzmann transport equation that describes the macroscopic behaviour of radiation particles as they travel through and interact with matters,56 thus enhancing the modelling of lateral scatter than AAA. Several publications have investigated the dose difference between AXB and AAA in lung cases. Fogliata et al reported that up to 2% of dose deviation from AAA with high energy beams.57 Tsuruta et al58 found that AXB yielded more accurate dose calculations that were closer to those with Monte Carlo than did AAA in lung SBRT cases. Our previous research also indicated that dose difference between AXB and AAA in lung and tumour regions was different.59 AXB principally predicted lower dose to PTV_lung (PTV with density in lung range) than AAA, whereas the dose difference in PTV_soft (PTV with density in water-equivalent range) was comparable. On the other hand, we also found that AXB predicted only a slightly lower dose to the oesophagus, spinal cord, heart, CW and lung. But, the dose difference was so smaller that it was clinically negligible. Based on the above results, we believed that the AXB algorithm principally influenced the dose to PTV_lung, while the dose to the PTV_soft and OARs were slight. Therefore, the conclusion drew from our study was unchanged even with the AXB algorithm.
Although we have optimized the FFF beam selection in SBRT for Stage I lung cancer based on the balance of the OARs sparing and treatment efficiency, it does have some limitations: (1) only partial arc was included in this study, the difference in beam-on time between the 6XFFF and 10XFFF beam would be potentially narrowed with full arcs owing to the restricted speed of gantry rotation; (2) the induced radioactivity generated from the 10XFFF beam has not been taken into account in our study. A large amount of MU (about 3000 or more per fraction in our study) required in the lung SBRT treatment increases the probability of production of induced radioactivity. However, it is worthy of concern that the linear accelerator engineers, physicists or the technicians are the ones who are at potential risk from induced radioactivity, and the radiation oncologists and therapists are not at risk from induced radioactivity in the head of the machine.
In summary, our study found that the 6XFFF beam obtained better OARs sparing and lower NTCP than did the 10XFFF beam, whereas the 10XFFF beam improved treatment efficiency in SBRT treatment for Stage I lung cancer. Considering the tumour displacement and patient stability as a function of treatment time, we recommend the use of 6XFFF beam in the 4 × 12 and 3 × 18 Gy schemes and the 10XFFF beam in the 1 × 34 Gy scheme.
CONCLUSION
Our study demonstrates that the 6XFFF beam obtains better OARs sparing, but the 10XFFF beam improves treatment efficiency in SBRT treatment for Stage I lung cancer. To balance the OARs sparing and tumour displacement induced by prolonged treatment time, we recommend the use of 6XFFF beam in the 4 × 12 and 3 × 18 Gy schemes, and the 10XFFF beam in the 1 × 34 Gy scheme.
Contributor Information
J-Y Lu, Email: 328796180@qq.com.
Z Lin, Email: 35105524@qq.com.
P-X Lin, Email: 150252317@qq.com.
B-T Huang, Email: 254954693@qq.com.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K; American College of Chest Physicians. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132: 234S–42S. doi: 10.1378/chest.07-1378 [DOI] [PubMed] [Google Scholar]
- 3.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010; 28: 5153–9. doi: 10.1200/jco.2010.30.0731 [DOI] [PubMed] [Google Scholar]
- 4.Munshi A, Krishnatry R, Banerjee S, Agarwal JP. Stereotactic conformal radiotherapy in non-small cell lung cancer—an overview. Clin Oncol (R Coll Radiol) 2012; 24: 556–68. doi: 10.1016/j.clon.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 5.Bradley JD, El Naqa I, Drzymala RE, Trovo M, Jones G, Denning MD. Stereotactic body radiation therapy for early stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys 2010; 77: 1146–50. doi: 10.1016/j.ijrobp.2009.06.017 [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. doi: 10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang B, Zhu F, Ma X, Tian Y, Cao D, Luo S, et al. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol 2014; 112: 250–5. doi: 10.1016/j.radonc.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014; 90: 603–11. doi: 10.1016/j.ijrobp.2014.05.055 [DOI] [PubMed] [Google Scholar]
- 9.Lang S, Shrestha B, Graydon S, Cavelaars F, Linsenmeier C, Hrbacek J, et al. Clinical application of flattening filter free beams for extracranial stereotactic radiotherapy. Radiother Oncol 2013; 106: 255–9. doi: 10.1016/j.radonc.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 10.Navarria P, Ascolese AM, Mancosu P, Alongi F, Clerici E, Tozzi A, et al. Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiother Oncol 2013; 107: 414–18. doi: 10.1016/j.radonc.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 11.Scorsetti M, Alongi F, Castiglioni S, Clivio A, Fogliata A, Lobefalo F, et al. Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments. Radiat Oncol 2011; 6: 113. doi: 10.1186/1748-717x-6-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong CL, Verbakel WF, Dahele M, Cuijpers JP, Slotman BJ, Senan S. Fast arc delivery for stereotactic body radiotherapy of vertebral and lung tumors. Int J Radiat Oncol Biol Phys 2012; 83: e137–43. doi: 10.1016/j.ijrobp.2011.12.014 [DOI] [PubMed] [Google Scholar]
- 13.Peguret N, Dahele M, Cuijpers JP, Slotman BJ, Verbakel WF. Frameless high dose rate stereotactic lung radiotherapy: intrafraction tumor position and delivery time. Radiother Oncol 2013; 107: 419–22. doi: 10.1016/j.radonc.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 14.Prendergast BM, Dobelbower MC, Bonner JA, Popple RA, Baden CJ, Minnich DJ, et al. Stereotactic body radiation therapy (SBRT) for lung malignancies: preliminary toxicity results using a flattening filter-free linear accelerator operating at 2400 monitor units per minute. Radiat Oncol 2013; 8: 273. doi: 10.1186/1748-717x-8-273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radiation Therapy Oncology Group. A randomized Phase II study comparing 2 stereotactic body radiation therapy (SBRT) schedules for medically inoperable patients with Stage I peripheral non-small cell lung cancer. [Updated 6 March 2014; cited 20 April 2015]. Available from: http://wwwrtogorg/ClinicalTrials/ProtocolTable/StudyDetailsaspx?study=0915
- 16.Hrbacek J, Lang S, Graydon S, Klöck S, Riesterer O. Contribution of flattening filter free beams to extracranial stereotactic VMAT treatment of NSCLC patients (Abstr). Radiother Oncol 2011; 99: S160. [DOI] [PubMed] [Google Scholar]
- 17.Weiss E, Siebers JV, Keall PJ. An analysis of 6-MV versus 18-MV photon energy plans for intensity-modulated radiation therapy (IMRT) of lung cancer. Radiother Oncol 2007; 82: 55–62. doi: 10.1016/j.radonc.2006.10.021 [DOI] [PubMed] [Google Scholar]
- 18.Kragl G, af Wetterstedt S, Knäusl B, Lind M, McCavana P, Knöös T, et al. Dosimetric characteristics of 6 and 10MV unflattened photon beams. Radiother Oncol 2009; 93: 141–6. doi: 10.1016/j.radonc.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 19.Hrbacek J, Lang S, Klöck S. Commissioning of photon beams of a flattening filter-free linear accelerator and the accuracy of beam modeling using an anisotropic analytical algorithm. Int J Radiat Oncol Biol Phys 2011; 80: 1228–37. doi: 10.1016/j.ijrobp.2010.09.050 [DOI] [PubMed] [Google Scholar]
- 20.Paynter D, Weston SJ, Cosgrove VP, Evans JA, Thwaites DI. Beam characteristics of energy-matched flattening filter free beams. Med Phys 2014; 41: 052103. doi: 10.1118/1.4871615 [DOI] [PubMed] [Google Scholar]
- 21.Coroller TP, Mak RH, Lewis JH, Baldini EH, Chen AB, Colson YL, et al. Low incidence of chest wall pain with a risk-adapted lung stereotactic body radiation therapy approach using three or five fractions based on chest wall dosimetry. PLoS One 2014; 9: e94859. doi: 10.1371/journal.pone.0094859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 70: 685–92. doi: 10.1016/j.ijrobp.2007.10.053 [DOI] [PubMed] [Google Scholar]
- 23.Clivio A, Fogliata A, Franzetti-Pellanda A, Nicolini G, Vanetti E, Wyttenbach R, et al. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009; 92: 118–24. doi: 10.1016/j.radonc.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 24.Asai K, Shioyama Y, Nakamura K, Sasaki T, Ohga S, Nonoshita T, et al. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy: risk factors and dose-volume relationship. Int J Radiat Oncol Biol Phys 2012; 84: 768–73. doi: 10.1016/j.ijrobp.2012.01.027 [DOI] [PubMed] [Google Scholar]
- 25.Creach KM, El Naqa I, Bradley JD, Olsen JR, Parikh PJ, Drzymala RE, et al. Dosimetric predictors of chest wall pain after lung stereotactic body radiotherapy. Radiother Oncol 2012; 104: 23–7. doi: 10.1016/j.radonc.2012.01.014 [DOI] [PubMed] [Google Scholar]
- 26.Gay HA, Niemierko A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys Med 2007; 23: 115–25. doi: 10.1016/j.ejmp.2007.07.001 [DOI] [PubMed] [Google Scholar]
- 27.Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70: 847–52. doi: 10.1016/j.ijrobp.2007.10.059 [DOI] [PubMed] [Google Scholar]
- 28.Okunieff P, Morgan D, Niemierko A, Suit HD. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys 1995; 32: 1227–37. doi: 10.1016/0360-3016(94)00475-Z [DOI] [PubMed] [Google Scholar]
- 29.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21: 109–22. doi: 10.1016/0360-3016(91)90171-Y [DOI] [PubMed] [Google Scholar]
- 30.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991; 21: 123–35. doi: 10.1016/0360-3016(91)90172-Z [DOI] [PubMed] [Google Scholar]
- 31.Woody NM, Videtic GM, Stephans KL, Djemil T, Kim Y, Xia P. Predicting chest wall pain from lung stereotactic body radiotherapy for different fractionation schemes. Int J Radiat Oncol Biol Phys 2012; 83: 427–34. doi: 10.1016/j.ijrobp.2011.06.1971 [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Bassano DA, Prasad SC, Hahn SS, Chung CT. The linear-quadratic model and fractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys 2003; 57: 827–32. doi: 10.1016/S0360-3016(03)00634-5 [DOI] [PubMed] [Google Scholar]
- 33.Fowler JF, Tomé WA, Fenwick JD, Mehta MP. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys 2004; 60: 1241–56. doi: 10.1016/j.ijrobp.2004.07.691 [DOI] [PubMed] [Google Scholar]
- 34.Scheenstra AE, Rossi MM, Belderbos JS, Damen EM, Lebesque JV, Sonke JJ. Alpha/beta ratio for normal lung tissue as estimated from lung cancer patients treated with stereotactic body and conventionally fractionated radiation therapy. Int J Radiat Oncol Biol Phys 2014; 88: 224–8. doi: 10.1016/j.ijrobp.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 35.Park JM, Choi CH, Ha SW, Ye SJ. The dosimetric effect of mixed-energy IMRT plans for prostate cancer. J Appl Clin Med Phys 2011; 12: 3563. doi: 10.1120/jacmp.v12i4.3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Modh A, Rimner A, Williams E, Foster A, Shah M, Shi W, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014; 90: 1168–76. doi: 10.1016/j.ijrobp.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu AJ, Williams E, Modh A, Foster A, Yorke E, Rimner A, et al. Dosimetric predictors of esophageal toxicity after stereotactic body radiotherapy for central lung tumors. Radiother Oncol 2014; 112: 267–71. doi: 10.1016/j.radonc.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW, et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int J Radiat Oncol Biol Phys 2013; 85: 190–5. doi: 10.1016/j.ijrobp.2012.03.041 [DOI] [PubMed] [Google Scholar]
- 39.Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012; 82: 457–62. doi: 10.1016/j.ijrobp.2010.08.056 [DOI] [PubMed] [Google Scholar]
- 40.Bongers EM, Botticella A, Palma DA, Haasbeek CJ, Warner A, Verbakel WF, et al. Predictive parameters of symptomatic radiation pneumonitis following stereotactic or hypofractionated radiotherapy delivered using volumetric modulated arcs. Radiother Oncol 2013; 109: 95–9. doi: 10.1016/j.radonc.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 41.Kanemoto A, Matsumoto Y, Sugita T. Timing and characteristics of radiation pneumonitis after stereotactic body radiotherapy for peripherally located stage I lung cancer. Int J Clin Oncol Nov 2014. Epub ahead of print. doi: 10.1007/s10147-014-0766-3 [DOI] [PubMed] [Google Scholar]
- 42.Stephans KL, Djemil T, Tendulkar RD, Robinson CG, Reddy CA, Videtic GM. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT). Int J Radiat Oncol Biol Phys 2012; 82: 974–80. doi: 10.1016/j.ijrobp.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 43.Hrbacek J, Lang S, Graydon SN, Klöck S, Riesterer O. Dosimetric comparison of flattened and unflattened beams for stereotactic ablative radiotherapy of stage I non-small cell lung cancer. Med Phys 2014; 41: 031709. doi: 10.1118/1.4866231 [DOI] [PubMed] [Google Scholar]
- 44.Verbakel WF, Senan S, Cuijpers JP, Slotman BJ, Lagerwaard FJ. Rapid delivery of stereotactic radiotherapy for peripheral lung tumors using volumetric intensity-modulated arcs. Radiother Oncol 2009; 93: 122–4. doi: 10.1016/j.radonc.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 45.Shah C, Grills IS, Kestin LL, McGrath S, Ye H, Martin SK, et al. Intrafraction variation of mean tumor position during image-guided hypofractionated stereotactic body radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys 2012; 82: 1636–41. doi: 10.1016/j.ijrobp.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 46.Ma L, Sahgal A, Hossain S, Chuang C, Descovich M, Huang K, et al. Nonrandom intrafraction target motions and general strategy for correction of spine stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2009; 75: 1261–5. doi: 10.1016/j.ijrobp.2009.04.027 [DOI] [PubMed] [Google Scholar]
- 47.Hoogeman MS, Nuyttens JJ, Levendag PC, Heijmen BJ. Time dependence of intrafraction patient motion assessed by repeat stereoscopic imaging. Int J Radiat Oncol Biol Phys 2008; 70: 609–18. doi: 10.1016/j.ijrobp.2007.08.066 [DOI] [PubMed] [Google Scholar]
- 48.Purdie TG, Bissonnette JP, Franks K, Bezjak A, Payne D, Sie F, et al. Cone-beam computed tomography for on-line image guidance of lung stereotactic radiotherapy: localization, verification, and intrafraction tumor position. Int J Radiat Oncol Biol Phys 2007; 68: 243–52. doi: 10.1016/j.ijrobp.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 49.Li R, Han B, Meng B, Maxim PG, Xing L, Koong AC, et al. Clinical implementation of intrafraction cone beam computed tomography imaging during lung tumor stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys 2013; 87: 917–23. doi: 10.1016/j.ijrobp.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan OS, Lee MC, Hung AW, Chang AT, Yeung RM, Lee AW. The superiority of hybrid-volumetric arc therapy (VMAT) technique over double arcs VMAT and 3D-conformal technique in the treatment of locally advanced non-small cell lung cancer—a planning study. Radiother Oncol 2011; 101: 298–302. doi: 10.1016/j.radonc.2011.08.015 [DOI] [PubMed] [Google Scholar]
- 51.Blom GJ, Verbakel WF, Dahele M, Hoffmans D, Slotman BJ, Senan S. Improving radiotherapy planning for large volume lung cancer: a dosimetric comparison between hybrid-IMRT and RapidArc. Acta Oncol 2015; 54: 427–32. doi: 10.3109/0284186x.2014.963888 [DOI] [PubMed] [Google Scholar]
- 52.Mayo CS, Urie MM, Fitzgerald TJ, Ding L, Lo YC, Bogdanov M. Hybrid IMRT for treatment of cancers of the lung and esophagus. Int J Radiat Oncol Biol Phys 2008; 71: 1408–18. doi: 10.1016/j.ijrobp.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 53.Verbakel WF, van Reij E, Ladenius-Lischer I, Cuijpers JP, Slotman BJ, Senan S. Clinical application of a novel hybrid intensity-modulated radiotherapy technique for stage III lung cancer and dosimetric comparison with four other techniques. Int J Radiat Oncol Biol Phys 2012; 83: e297–303. doi: 10.1016/j.ijrobp.2011.12.059 [DOI] [PubMed] [Google Scholar]
- 54.Han T, Mikell JK, Salehpour M, Mourtada F. Dosimetric comparison of Acuros XB deterministic radiation transport method with Monte Carlo and model-based convolution methods in heterogeneous media. Med Phys 2011; 38: 2651–64. doi: 10.1118/1.3582690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fogliata A, Nicolini G, Clivio A, Vanetti E, Cozzi L. Dosimetric evaluation of Acuros XB advanced dose calculation algorithm in heterogeneous media. Radiat Oncol 2011; 6: 82. doi: 10.1186/1748-717x-6-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kan MW, Leung LH, Yu PK. Dosimetric impact of using the Acuros XB algorithm for intensity modulated radiation therapy and RapidArc planning in nasopharyngeal carcinomas. Int J Radiat Oncol Biol Phys 2013; 85: e73–80. doi: 10.1016/j.ijrobp.2012.08.031 [DOI] [PubMed] [Google Scholar]
- 57.Fogliata A, Nicolini G, Clivio A, Vanetti E, Cozzi L. Critical appraisal of Acuros XB and anisotropic analytic algorithm dose calculation in advanced non-small-cell lung cancer treatments. Int J Radiat Oncol Biol Phys 2012; 83: 1587–95. doi: 10.1016/j.ijrobp.2011.10.078 [DOI] [PubMed] [Google Scholar]
- 58.Tsuruta Y, Nakata M, Nakamura M, Matsuo Y, Higashimura K, Monzen H, et al. Dosimetric comparison of Acuros XB, AAA, and XVMC in stereotactic body radiotherapy for lung cancer. Med Phys 2014; 41: 081715. doi: 10.1118/1.4890592 [DOI] [PubMed] [Google Scholar]
- 59.Huang B, Wu L, Lin P, Chen C. Dose calculation of Acuros XB and anisotropic analytical algorithm in lung stereotactic body radiotherapy treatment with flattening filter free beams and the potential role of calculation grid size. Radiat Oncol 2015; 10: 53. doi: 10.1186/s13014-015-0357-0 [DOI] [PMC free article] [PubMed] [Google Scholar]