Abstract
Objective:
Radiation-induced sensorineural hearing loss is a common complication after radiotherapy in patients with nasopharyngeal carcinoma (NPC) that significantly affects their quality of life. The goal of this study was to compare SmartArc-based volumetric modulated arc therapy (VMAT-S) with step-and-shoot intensity-modulated radiation therapy (IMRT) for patients with locoregionally advanced NPC with regard to the sparing effect on middle ear, vestibule and cochlea.
Methods:
20 patients with non-metastatic Stage III or IV NPC were selected to have planning with VMAT-S and IMRT [using Philips Pinnacle Planning System (Philips, Fitchburg, WI) for Varian accelerator] for dosimetric comparison. Mean middle ears, vestibule and cochlea doses for the two planning techniques were compared using a paired t-test. Target coverage and dose homogeneity were evaluated by calculating conformity index (CI) and homogeneity index (HI) values.
Results:
VMAT-S had significantly improved homogeneity and conformity compared with IMRT. Mean HI of planning target volume of gross tumour volume (PGTV) was better with VMAT-S (1.05 ± 0.02) than IMRT (1.09 ± 0.03) (p < 0.001). Mean CI of PGTV is also better with VMAT-S (0.59 ± 0.12) than IMRT (0.54 ± 0.12) (p < 0.001). Mean doses to the left cochleas were 43.8 ± 3.6 and 47.8 ± 4.0 (p < 0.001) for VMAT-S and IMRT plans, respectively. Mean doses to the right cochleas were 42.7 ± 4.7 and 47.6 ± 5.4 (p < 0.001) for VMAT-S and IMRT plans, respectively. VMAT-S also significantly reduced the mean doses to middle ears (p < 0.001 for both) and vestibule (p < 0.001 for both).
Conclusion:
Our results indicate that VMAT-S provides better sparing of hearing apparatus in locoregionally advanced NPC.
Advances in knowledge:
VMAT-S can improve the middle ear, vestibule and cochlea sparing in patients with locoregionally advanced NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignant tumours of head and neck in the South-east Asia. Globally, NPC accounts for 84,400 new cases and 51,600 deaths annually.1 More than 60% of new diagnostic cases are III or IV stage.2 Over the past decade, intensity-modulated radiation therapy (IMRT) has gained popularity in the treatment of NPC because of its excellent local control with decreased normal tissue effects.3
However, it is still difficult to spare the organs at risk (OARs) without compromising the tumour coverage in planning IMRT for locoregionally advanced NPC, where primary tumours are often large and concave around nearby critical normal tissues.4
Radiation-induced sensorineural hearing loss (SNHL) is a common complication after radiotherapy (RT) in patients with NPC, which significantly affects their quality of life.5–7 In a recent report, the frequency of radiation-induced damage to ear function was as high as 37% in patients treated with IMRT.6 As cisplatin-based chemoradiotherapy has become the standard treatment for advanced NPC, the synergistic ototoxic effect of radiation and cisplatin has been observed in patients.8,9 By lowering the dose to the hearing apparatus, the incidence of hearing loss is likely to decline.
In recent years, volumetric modulated arc therapy (VMAT) has become widely available. Compared with standard IMRT, VMAT can provide more precise conformal dose distribution through modulated gantry rotation speed, dose rate (DR) and multileaf collimator (MLC) pattern in linear accelerator.10,11 Some studies have demonstrated that VMAT provide superior OARs/target coverage and fewer monitor units (MUs) per fraction over conventional IMRT for NPC.12,13 However, few publications focused on the protection of hearing apparatus have been reported to compare between the two techniques. As the auditory apparatus is very close to the nasopharynx it usually receives a higher radiation dose; therefore, protection of the ears is difficult, especially for advanced NPC. Whether VMAT can provide better sparing of middle and inner ears than IMRT in locoregionally advanced NPC is still unknown.
The aim of this study was to investigate the impact of using SmartArc-based VMAT (VMAT-S) and step-and-shoot IMRT on hearing apparatus doses, as well as other involved OARs such as brain stem and spinal cord.
METHODS AND MATERIALS
Patients and treatment
Between 2013 and 2014, a total of 20 patients with locoregionally advanced NPC were enrolled in the study at our institute. These patients had been irradiated with a VMAT. Their IMRT plans were only generated for the purpose of comparing two RT plans for the same tumour. In accordance with the American Joint of Cancer Committee's staging system 2009, the tumour stages of patients were as follows: Stage III, 11 (55%) and Stage IV, 9 (45%). None of the patients had received prior RT. Patient characteristics are summarized in Table 1. This study was approved by the Institutional Review Board of Anhui Provincial Hospital Affiliated to Anhui Medical University, Hefei, China.
Table 1.
Clinical characteristic data of patients
| Characteristic | Case (%) |
|---|---|
| Age (years) | |
| ≤51 | 10 (50.0) |
| >51 | 10 (50.0) |
| Gender | |
| Male | 11 (55.0) |
| Female | 9 (45.0) |
| Clinical stage | |
| III | 11 (55.0) |
| IV | 9 (45.0) |
| T stage | |
| T1 | 0 (0) |
| T2 | 4 (20.0) |
| T3 | 10 (50.0) |
| T4 | 6 (30.0) |
| N stage | |
| N0 | 1 (5.0) |
| N1 | 2 (10.0) |
| N2 | 14 (70.0) |
| N3 | 3 (15.0) |
All patients were immobilized in the supine position with a head, neck and shoulder thermoplastic mask. CT simulation was performed with a slice thickness of 2.5 mm extending from the vertex to 2 cm below the clavicle. The target volumes were defined in accordance with the International Commission on Radiation Units and Measurements reports 50, 62 and 83.14–16 All target volumes were delineated slice by slice on the treatment planning CT scan. The primary nasopharygeal gross tumour volume and that for the involved cervical lymph nodes were determined from the imaging and clinical findings. Two clinical target volumes (CTVs) were delineated: CTV1 and CTV2. CTV1 was defined as the nasopharynx gross target volume plus a 5- to 10-mm margin (2- to 3-mm margin posteriorly) to encompass the high-risk sites of microscopic extension and the whole nasopharynx. CTV2 was defined by adding a 5- to 10-mm margin to the CTV1 (when the CTV2 was adjacent to critical organs, such as the brain stem and spinal cord, the margin was reduced to 3–5 mm) and included the lymph nodal regions, clivus, skull base, pterygoid fossae, parapharyngeal space, inferior sphenoid sinus, and posterior edge of the nasal cavity and maxillary sinuses. The planning target volume (PTV) was created based on each volume with an additional 3-mm margin, allowing for setup variability. The prescribed dose was 68–70 Gy to the nasopharynx gross target volume, 66–70 Gy to the positive neck lymph nodes, 60 Gy to CTV1 and 54 Gy to CTV2.
Regarding the OARs, the near maximal dose (D2%) to the brain stem and the spinal cord were set as 54 and 45 Gy, respectively. In addition, at least one side of the parotid glands should receive a mean dose of ≤26 Gy, or the volume receiving 30 Gy radiation should be <50%. The dose constraints to other normal tissues are all listed in Table 2.
Table 2.
Dose constraints for the critical structures
| Organs at risk | Dose constraints |
|---|---|
| Brain stem | D2% < 54 Gy |
| Spinal cord | D2% < 45 Gy |
| Parotid glands | Mean dose <26 Gy or V30Gy < 50% (at least one side) |
| Eyes | D2% <50 Gy |
| Optic nerves/chiasm | D2% <54 Gy |
| Lenses | D2% <10 Gy |
| Mandible | D1 cm3 < 75 Gy |
| Temporal lobe | D1 ≤ 65 Gy |
| Larynx | Mean dose <45 Gy |
D1, the dose of the 1% volume received; D1 cm3, the dose of the 1 cm3 volume received; D2%, near maximal dose.
All patients received concomitant chemotherapy using platinum-based regiments.
TECHNIQUES
SmartArc-based volumetric modulated arc therapy planning
The Philips Pinnacle Planning System v. 9.2 (Philips, Fitchburg, WI) was adopted for VMAT planning using SmartArc module. Details of the SmartArc planning algorithm were as described by Bzdusek et al.17 The VMAT-S plan consisting of two coplanar arcs of 360° were optimized simultaneously, to be delivered with opposite rotation (clockwise and counter clockwise). No limitations were used on the delivery time. Continuous gantry motion, dose-rate variation and MLC motion were approximated by optimizing individual beams at 4° gantry angle increments.
Intensity-modulated radiation therapy planning
Plans were designed according to the step-and-shoot methods with nine fixed gantry beams. Direct machine parameter optimization module was adopted for the planning, which used nine angles to evenly separate coplanar fields. The minimum segment area was set to 4 cm2, and minimum segment MU was 5 MUs. A collapsed cone convolution algorithm was used to calculate dosage, with a dose grid resolution of 4 mm.
Dose calculation of the cochlea, vestibule and middle ear
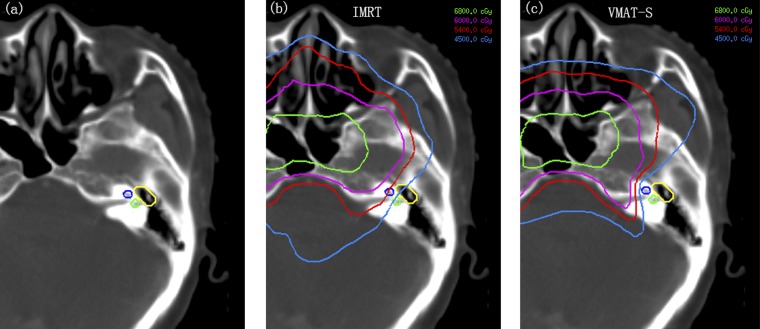
The auditory structures were contoured according to the guidelines established by Pacholke et al18 (Figure 1a). The mean doses to cochlea, vestibule and middle ear were limited to 45, 45 and 34 Gy, respectively.19–21 The dose distributions were calculated after delineation of the hearing apparatus as shown in Figure 1b,c.
Figure 1.
Hearing apparatus contouring and the dose distributions on hearing apparatus of intensity-modulated radiation therapy (IMRT) and SmartArc-based volumetric modulated arc therapy (VMAT-S). (a) Blue line, cochlea; green line, vestibule; yellow line, middle ear. (b) IMRT. (c) VMAT-S. The green, purple, red and light blue lines were isodose curves of 68, 60, 54 and 45 Gy. See online for colour images.
Planning comparison
During planning, the primary goal was to meet the required coverage of target volumes. The plans were generated first to achieve coverage of 95% of the prescribed dose to each target. The secondary goal was to minimize OARs doses as much as possible, without compromising coverage to the target volumes.
Two sets of plans were compared in this study, all designed on the pinnacle treatment planning system with 6-MV photon beams from a Varian Trilogy linear accelerator equipped with a Millennium MLC (Varian Oncology Systems, Palo Alto, CA) with 120 leaves (spatial resolution of 5 mm at isocentre for the central 20 cm and of 10 mm in the outer 2 × 10 cm, maximum leaf speed of 2.5 cm s−1 and leaf transmission of 1.8%). For the optimization, the PTVs were reduced to 5 mm under the skin surface to prevent optimization problems in the build-up region.
Plans for VMAT-S were optimized selecting a maximum DR of 600 MU/min, and a fixed DR of 400 MU/min was selected for IMRT. To ensure consistency of planning techniques, all treatment plans were devised by physicists with over 2-year clinical experience in IMRT and VMAT-S planning.
An analysis was performed based on the cumulative dose–volume histograms (DVHs) for each patient/plan/region of interest. The average cumulative DVH for PTV and OARs were built from the individual DVH obtained by averaging the corresponding volumes at each dose bin (0.01 Gy in this case).
Homogeneity index (HI) and conformity index (CI) values were calculated in all cases and used to compare the quality of the two plans. The dosimetric comparison criteria were as follows:
(1) HI: measurement of how even the dose distributes in PTV. Formula: HI = D5%/D95%. A higher HI indicates poorer homogeneity.
(2) CI: measurement of how conformed the dose distribution is along the target volume. Formula: CI = VPTV × (VTV/TVPV2), where VTV is the treatment volume of the prescribed isodose lines, VPTV is the volume of PTV and TVPV is the volume of VPTV within the VTV. CI value will be <1, and the closer the CI to 1, the better the conformality.22
(3) OARs: the normal tissue doses of both VMAT-S and IMRT plans were calculated. Planning OAR volumes were generated with a 3-mm setup margin for brain stem and 5-mm margin for spinal cord.
Statistical analysis
All statistical analyses were performed using SPSS® v. 13.0 (SPSS Inc., Chicago, IL). The paired t-test was used to compare the dosimetric differences in VMAT-S vs step-and-shoot IMRT. The threshold for statistical significance was set at p < 0.05.
RESULTS
Target coverage
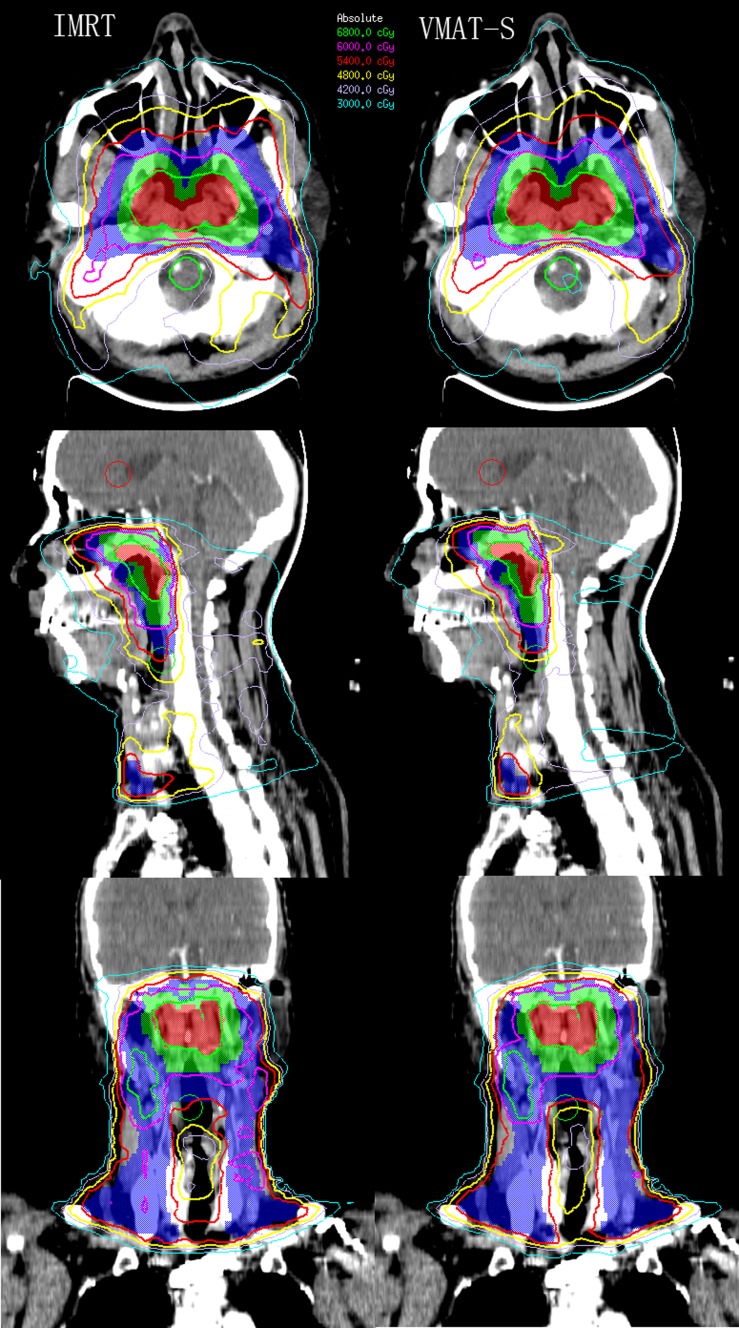
Typical dose distributions of VMAT-S and IMRT planned for one patient with NPC were shown in Figure 2. Target coverage and dose homogeneity were identical for both planning techniques. The results are summarized in Table 3. Regarding HI and CI, IMRT showed statistically inferior results compared with VMAT-S at all dose levels.
Figure 2.
The dose distributions for one patient with nasopharyngeal carcinoma planned for SmartArc-based volumetric modulated arc therapy (VMAT-S) (left) and intensity-modulated radiation therapy (IMRT) (right). Colour-wash areas: 68 Gy = red; 60 Gy = green; 54 Gy = blue. The green, purple, red, yellow, brown and indigo lines were isodose curves of 68, 60, 54, 48, 42, and 30 Gy, respectively. See online for colour images.
Table 3.
Plan comparison for planning target volume of gross tumour volume (PGTV), planning target volume (PTV)1, PTV2, monitor units (MUs) and delivery time
| Parameter | Intensity-modulated radiation therapy, mean ± SD (Gy) | SmartArc-based volumetric modulated arc therapy, mean ± SD (Gy) | p-value |
|---|---|---|---|
| PGTV | |||
| D98% | 65.2 ± 2.9 | 64.5 ± 3.4 | 0.140 |
| D2% | 73.5 ± 1.2 | 72.2 ± 1.2 | 0.001 |
| Dmean | 70.7 ± 0.9 | 70.2 ± 0.6 | 0.045 |
| CI | 0.54 ± 0.12 | 0.59 ± 0.12 | <0.001 |
| HI | 1.09 ± 0.03 | 1.05 ± 0.02 | <0.001 |
| PTV1 | |||
| D98% | 58.7 ± 1.9 | 58.8 ± 2.3 | 0.898 |
| D2% | 69.2 ± 2.1 | 68.5 ± 1.9 | 0.036 |
| Dmean | 65.2 ± 1.1 | 64.7 ± 0.8 | 0.023 |
| CI | 0.42 ± 0.07 | 0.49 ± 0.08 | <0.001 |
| HI | 1.17 ± 0.02 | 1.14 ± 0.03 | <0.001 |
| PTV2 | |||
| D98% | 51.9 ± 3.2 | 52.3 ± 2.9 | 0.510 |
| D2% | 65.2 ± 3.8 | 64.4 ± 3.8 | 0.059 |
| Dmean | 58.9 ± 1.3 | 58.4 ± 1.2 | 0.009 |
| CI | 0.47 ± 0.05 | 0.51 ± 0.07 | <0.001 |
| HI | 1.18 ± 0.02 | 1.16 ± 0.03 | 0.001 |
| MUs | 741.9 ± 48.2 | 625.7 ± 66.6 | <0.001 |
| Time (min) | 14.2 ± 1.1 | 6.3 ± 0.4 | <0.001 |
CI, conformity index; D2%, near maximum dose; D98%, near minimum dose; Dmean, mean dose; HI, homogeneity index; SD, standard deviation.
Middle and inner ears
A statistically significant advantage in the dose received by the middle ears, vestibules and cochleas were found for VMAT-S (Table 4).
Table 4.
The dose result of both intensity-modulated radiation therapy (IMRT) and SmartArc-based volumetric modulated arc therapy (VMAT-S) for ear (Gy)
| Structure | Dose–volume index | IMRT (mean ± SD) | VMAT-S (mean ± SD) | p-value |
|---|---|---|---|---|
| Middle ear-L | Dmean | 39.7 ± 5.7 | 32.9 ± 4.7 | <0.001 |
| D2% | 50.3 ± 5.7 | 47.8 ± 5.0 | 0.001 | |
| Middle ear-R | Dmean | 41.3 ± 5.6 | 33.4 ± 5.0 | <0.001 |
| D2% | 50.7 ± 6.0 | 48.7 ± 5.9 | 0.001 | |
| Cochlea-L | Dmean | 47.8 ± 4.0 | 43.8 ± 3.6 | <0.001 |
| D2% | 53.0 ± 3.9 | 49.9 ± 3.0 | <0.001 | |
| Cochlea-R | Dmean | 47.6 ± 5.4 | 42.7 ± 4.7 | <0.001 |
| D2% | 52.1 ± 4.6 | 48.6 ± 4.7 | <0.001 | |
| Vestibule-L | Dmean | 38.2 ± 5.1 | 35.2 ± 5.2 | <0.001 |
| D2% | 43.9 ± 5.1 | 41.2 ± 5.2 | <0.001 | |
| Vestibule-R | Dmean | 38.1 ± 5.3 | 34.9 ± 5.3 | <0.001 |
| D2% | 44.2 ± 5.1 | 40.3 ± 5.6 | <0.001 |
D2%, near maximum dose; Dmean, mean dose; L, left; R, right; SD, standard deviation.
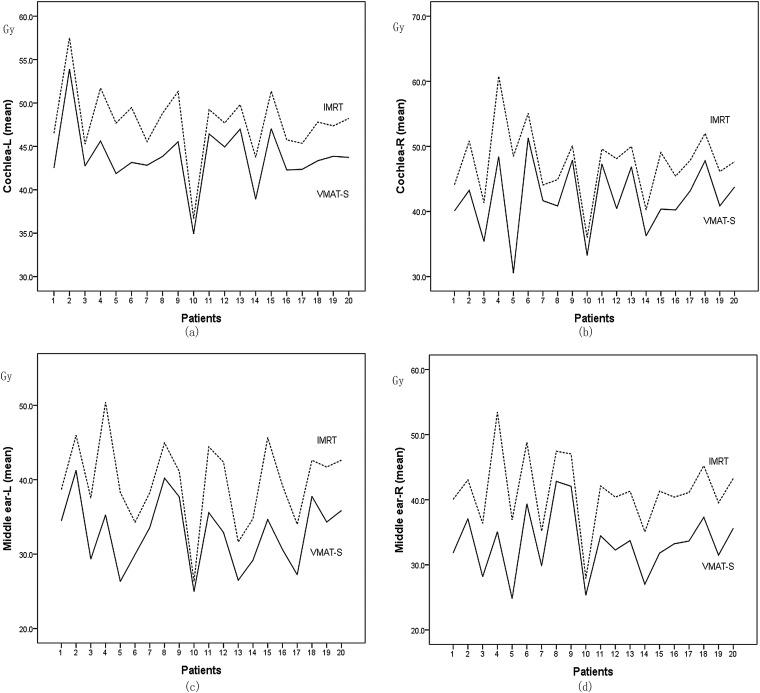
The mean left middle ear doses were 39.7 ± 5.7 and 32.9 ± 4.7 Gy for IMRT and VMAT-S plans, respectively (Figure 3). The mean left cochlea doses were 47.8 ± 4.0 and 43.8 ± 3.6 Gy for IMRT and VMAT-S plans, respectively (Figure 3). There was a statistically significant difference between the VMAT-S and IMRT in reducing the doses of vestibules.
Figure 3.
Comparison of the cochlea and middle ear mean doses between intensity-modulated radiation therapy (IMRT) and SmartArc-based volumetric modulated arc therapy (VMAT-S) plans. (a) Left cochlea mean doses. (b) Right cochlea mean doses. (c) Left middle ear mean doses. (d) Right middle ear mean doses.
Brain stem
The values of D2% and Dmax1% were significantly lower in VMAT-S than in IMRT. A dose reduction of D2% by 1.4 Gy was obtained from VMAT-S to IMRT (Table 5).
Table 5.
Comparison of doses to other critical structures (Gy)
| Structure | Dose–volume index | Intensity-modulated radiation therapy (mean ± SD) | SmartArc-based volumetric modulated arc therapy (mean ± SD) | p-value |
|---|---|---|---|---|
| Brain stem | D2% | 53.6 ± 0.7 | 52.2 ± 1.7 | <0.001 |
| Dmax1% | 51.2 ± 1.6 | 49.8 ± 2.4 | <0.001 | |
| Spinal cord | D2% | 43.8 ± 1.6 | 42.2 ± 1.9 | <0.001 |
| Dmax1% | 41.6 ± 1.2 | 40.3 ± 1.4 | <0.001 | |
| Temporal lobe | Dmax1% | 60.0 ± 4.5 | 58.5 ± 4.9 | <0.001 |
| Parotid-R | Dmean | 30.9 ± 5.6 | 26.1 ± 3.4 | <0.001 |
| V20 Gy (%) | 65.3 ± 1.1 | 46.3 ± 1.2 | <0.001 | |
| V30 Gy (%) | 43.4 ± 1.9 | 31.8 ± 1.4 | <0.001 | |
| Parotid-L | Dmean | 31.1 ± 5.9 | 26.3 ± 3.8 | <0.001 |
| V20 Gy (%) | 65.7 ± 1.1 | 47.7 ± 1.1 | <0.001 | |
| V30 Gy (%) | 44.8 ± 1.8 | 32.8 ± 1.1 | <0.001 | |
| Optical nerve-R | D2% | 37.7 ± 10.4 | 36.7 ± 11.8 | 0.39 |
| Dmean | 20.6 ± 7.5 | 19.9 ± 7.7 | 0.41 | |
| Optical nerve-L | D2% | 36.7 ± 10.4 | 34.7 ± 12.1 | 0.14 |
| Dmean | 19.6 ± 8.6 | 18.9 ± 6.9 | 0.49 | |
| Lens-R | D2% | 5.9 ± 0.8 | 6.0 ± 0.7 | 0.84 |
| Lens-L | D2% | 5.4 ± 0.7 | 5.3 ± 0.5 | 0.76 |
| Chiasm | D2% | 40.6 ± 8.5 | 38.5 ± 8.9 | 0.19 |
D2%, near maximum dose; Dmax1%, maximum dose encompassing 1% of the organ at risk volume; Dmean, mean dose; L, left; R, right; SD, standard deviation; V20 Gy, volume receiving at least 20 Gy; V30 Gy, volume receiving at least 30 Gy.
Spinal cord
The values of D2% and Dmax1% to the spinal cord were significantly lower in VMAT-S than in IMRT. A dose reduction of D2% by 1.6 Gy was obtained from VMAT-S to IMRT (Table 5).
Other structures
The average doses to the OARs in the 20 patients with NPC were listed in Table 4. In comparison with IMRT, VMAT-S performed better in sparing OAR, particularly the temporal lobe and parotid glands.
There was a remarkable decrease in the Dmean, volume receiving at least 20 Gy (V20) and volume receiving at least 30 Gy (V30) of VMAT-S to the parotid glands (p < 0.05). The mean optical nerve doses were lower in VMAT-S than in IMRT, although this difference was not statistically significant. No significant dose difference was noted in the lenses or chiasm.
Monitor units and delivery time
The mean values of MUs were 741.9 ± 48.2 in IMRT and 625.7 ± 66.6 in VMAT-S (Table 3). VMAT-S resulted in a 15% reduction in MUs per fraction consumed, compared with IMRT. The mean delivery time was 14.2 ± 1.1 min in IMRT, and a 56% reduction in delivery time was achieved by VMAT-S (6.3 ± 0.4 min).
DISCUSSION
In the study, we compared a nine-field IMRT and VMAT-S in terms of cochlea, vestibule and middle ear sparing and target coverage in patients with locoregionally advanced NPC. We found that VMAT-S showed significantly superior performance results in terms of PTV coverage and the protection of ear function compared with IMRT. The doses of middle ears, cochleas and vestibules were significantly lower in the VMAT-S plan. According to the study by Vanetti et al23 on the radiation doses in VMAT and IMRT for head and neck cancer, VMAT plans outperformed IMRT plans in terms of homogeneity and conformity in PTV, as well as providing a better sparing effect on the OARs, and our findings are in accord with theirs. To the best of our knowledge, this is the first report to investigate the impact of using VMAT-S techniques on the middle and inner ear doses in patients with locoregionally advanced NPC.
VMAT is a novel technology that employs a linear accelerator to conduct modulated arc therapy. It has been indicated that given the same target dose coverage, VMAT is able to treat NPC more efficiently with less damage to OARs.11,24 Ning et al13 compared VMAT with IMRT for NPC treatment. They found that VMAT offered better protection to the OARs, particularly the temporal lobe, brain stem and parotid glands than IMRT. Our results of OARs correspond to their findings. VMAT-S achieved significantly better spinal cord, brain stem and temporal lobe sparing than IMRT.
Interesting finding is the dose to the auditory apparatus. Based on our results, the dose to the cochlea, vestibule and middle ear is lower with VMAT-S than IMRT. We found that the dose fall-off was quite remarkable within the small volume of the hearing apparatus, especially using VMAT-S. These organs have been reported to be important factors in the development of hearing loss.7,25 Radiation damage to the auditory system is one of the major complications of RT in patients with NPC. The auditory apparatus lies in close proximity to the nasopharynx and usually receives a significant dose of radiation. It is difficult to spare the acoustic apparatus using conventional IMRT technique. This meant that some patients suffered varying degrees of hearing loss, which may progress to complete deafness. Moreover, hearing loss has become a serious threat since the intensification of NPC therapy by the concurrent use of cisplatin-based chemotherapy.26
RT-induced ototoxicity can lead to two kinds of hearing impairment-conductive hearing loss originating in the middle ear, and SNHL caused by damage to the inner ear. Bhandare et al27 undertook a retrospective study on 325 patients who received RT for head and neck tumours with curative intent. They found RT-induced morbidity in 41.8% of patients (involving the middle ear in 28.6% and the inner ear in 26.8%). Another study25 has investigated the association between radiation dosage to the ear and subsequent hearing loss. They found a statistically significant relationship between radiation dosage to the inner ear and long-term SNHL. To reduce the risk for SNHL, the mean dose to the cochlea should be limited to ≤45 Gy.19,20 Bhandare et al25 also suggested that the dose to the cochlea should be kept as low as possible. The otitis media with effusion (OME) is also a very common complication of NPC.28 Wang et al21 reported that the radiation-induced OME may decrease when the dose to the middle ear cavity was <34 Gy. In our study, the mean doses to the middle ear, vestibule and cochlea were obviously lower in VMAT-S than in IMRT. For middle ear cochlea and vestibule, VMAT-S achieved 6, 4 and 3 Gy less radiation than with IMRT, respectively. It is obvious that decreasing the doses to the auditory organs as low as possible is the key to avoid long-term hearing loss. Our study demonstrated that the VMAT-S could better protect the cochlea, vestibule and middle ear function.
Besides the hearing apparatus doses, cisplatin-based chemoradiotherapy also resulted in hearing loss. The studies of Chan et al29 showed significantly more hearing loss after chemoradiotherapy than after RT. Some studies reported that a single dose of ≥60 mg m−2 or a total dose of 1050 mg of cisplatin increased incidences of hearing loss.30,31 As the patients in our study had locally advanced disease and received cisplatin-based chemoradiotherapy, the effect of cisplatin to hearing loss could not be evaluated directly. The synergistic ototoxic of radiation and cisplatin has been observed in patients. Therefore, it is necessary to restrict the hearing apparatus dose to minimize hearing loss, especially when chemoradiotherapy is used. There was agreement that a higher radiation dose to the cochlea was significantly associated with more hearing loss. Radiation dose limits to the cochlea, starting from 45 to 60 Gy, were observed to increase the incidence of hearing loss.20,27,29 Petsuksiri et al6 reported that dose limitation to the cochlea would potentially protect hearing loss in patients who received cisplatin chemotherapy. Our studies showed that VMAT-S for locoregionally advanced NPC achieved significant improvements in dose reduction to hearing apparatus compared with IMRT. Therefore, VMAT-S can improve the hearing apparatus sparing.
One of the objectives of VMAT was the capability to deliver treatments in short times. Holt et al32 reported a comparison of IMRT and VMAT for head and neck cancer treatment. In this study, the average delivery time for VMAT was 5.54 min, which was 57.9% shorter than that of IMRT. Speed of delivery is a major advantage of VMAT as it reduces the risk of intrafraction movements. In addition, the shorter treatment time can increase tumour control.33 Zheng et al34 investigate the impact of prolonged fraction delivery times simulating IMRT on cultured NPC cell killing. They found that prolonged fraction delivery times significantly decreased the cell killing in both CNE1 and CNE2 cell lines. The reason may be due to sublethal damage repair during the irradiation.
CONCLUSION
Radiation therapy using VMAT planning can lower the dose to the cochlea, vestibule and middle ears while maintaining good target coverage compared with IMRT. However, the clinical outcomes need further investigation.
FUNDING
The work was supported by grants from the Nature Science Foundation of Anhui Province of China (no. 1408085MH189).
Contributor Information
J Gao, Email: ghdd2006@gmail.com.
T-L Qian, Email: gj11667@126.com.
C-Z Tao, Email: gj1166@126.com.
Y-H Zhang, Email: High11667@yahoo.com.
Y Zhou, Email: High11667@chinaren.com.
J Yang, Email: 536885906@qq.com.
J He, Email: huidi2006@gmail.com.
R Wang, Email: 602957088@qq.com.
P-J Zhou, Email: 602957088@qq.com.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2009; 73: 1326–34. doi: 10.1016/j.ijrobp.2008.07.062 [DOI] [PubMed] [Google Scholar]
- 3.Saleh-Ebrahimi L, Zwicker F, Muenter MW, Bischof M, Lindel K, Debus J, et al. Intensity modulated radiotherapy (IMRT) combined with concurrent but not adjuvant chemotherapy in primary nasopharyngeal cancer—a retrospective single center analysis. Radiat Oncol 2013; 8: 20. doi: 10.1186/1748-717X-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau RM, Leung SF, Kam MK, Cheung KY, Kwan WH, Yu KH, et al. A broadly adaptive array of dose-constraint templates for planning of intensity-modulated radiation therapy for advanced T-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2009; 74: 21–8. doi: 10.1016/j.ijrobp.2008.07.041 [DOI] [PubMed] [Google Scholar]
- 5.Tuan JK, Ha TC, Ong WS, Siow TR, Tham IW, Yap SP, et al. Late toxicities after conventional radiation therapy alone for nasopharyngeal carcinoma. Radiother Oncol 2012; 104: 305–11. doi: 10.1016/j.radonc.2011.12.028 [DOI] [PubMed] [Google Scholar]
- 6.Petsuksiri J, Sermsree A, Thephamongkhol K, Keskool P, Thongyai K, Chansilpa Y, et al. Sensorineural hearing loss after concurrent chemoradiotherapy in nasopharyngeal cancer patients. Radiat Oncol 2011; 6: 19. doi: 10.1186/1748-717X-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theunissen EA, Bosma SC, Zuur CL, Spijker R, van der Baan S, Dreschler WA, et al. Sensorineural hearing loss in patients with head and neck cancer after chemoradiotherapy and radiotherapy: a systematic review of the literature. Head Neck 2015; 37: 281–92. doi: 10.1002/hed.23551 [DOI] [PubMed] [Google Scholar]
- 8.Low WK, Toh ST, Wee J, Fook-Chong SM, Wang DY. Sensorineural hearing loss after radiotherapy and chemoradiotherapy: a single, blinded, randomized study. J Clin Oncol 2006; 24: 1904–9. doi: 10.1200/JCO.2005.05.0096 [DOI] [PubMed] [Google Scholar]
- 9.Wei Y, Zhou T, Zhu J, Zhang Y, Sun M, Ding X, et al. Long-term outcome of sensorineural hearing loss in nasopharyngeal carcinoma patients: comparison between treatment with radiotherapy alone and chemoradiotherapy. Cell Biochem Biophys 2014; 69: 433–7. doi: 10.1007/s12013-014-9814-x [DOI] [PubMed] [Google Scholar]
- 10.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 2008; 35: 310–17. doi: 10.1118/1.2818738 [DOI] [PubMed] [Google Scholar]
- 11.Lee FK, Yip CW, Cheung FC, Leung AK, Chau RM, Ngan RK. Dosimetric difference amongst 3 techniques: TomoTherapy, sliding-window intensity-modulated radiotherapy (IMRT), and RapidArc radiotherapy in the treatment of late-stage nasopharyngeal carcinoma (NPC). Med Dosim 2014; 39: 44–9. doi: 10.1016/j.meddos.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 12.Uzel EK, Karacam S, Elicin O, Uzel O. Comparison of two different IMRT planning techniques in the treatment of nasopharyngeal carcinoma. Effect on parotid gland radiation doses. Strahlenther Onkol 2013; 189: 552–8. doi: 10.1007/s00066-013-0344-z [DOI] [PubMed] [Google Scholar]
- 13.Ning ZH, Mu JM, Jin JX, Li XD, Li QL, Gu WD, et al. Single arc volumetric-modulated arc therapy is sufficient for nasopharyngeal carcinoma: a dosimetric comparison with dual arc VMAT and dynamic MLC and step-and-shoot intensity-modulated radiotherapy. Radiat Oncol 2013; 8: 237. doi: 10.1186/1748-717X-8-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Commission on Radiation Units and Measurements. Report 50: prescribing, recording, and reporting photon beam therapy. Bethesda, MD: ICRU; 1993. [Google Scholar]
- 15.International Commission on Radiation Units and Measurements. Report 62: prescribing, recording and reporting photon beam therapy (supplement to ICRU report 50). Bethesda, MD: ICRU; 1999. [Google Scholar]
- 16.International Commission on Radiation Units and Measurements. Report 83: prescribing, recording, and reporting photon beam therapy intensity-modulated radiation therapy (IMRT). Bethesda, MD: ICRU; 2010. [Google Scholar]
- 17.Bzdusek K, Friberger H, Eriksson K, Hardemark B, Robinson D, Kaus M. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys 2009; 36: 2328–39. doi: 10.1118/1.3132234 [DOI] [PubMed] [Google Scholar]
- 18.Pacholke HD, Amdur RJ, Schmalfuss IM, Louis D, Mendenhall WM. Contouring the middle and inner ear on radiotherapy planning scans. Am J Clin Oncol 2005; 28: 143–7. doi: 10.1097/01.coc.0000143847.57027.16 [DOI] [PubMed] [Google Scholar]
- 19.Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010; 76: S10–19. doi: 10.1016/j.ijrobp.2009.07.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan CC, Eisbruch A, Lee JS, Snorrason RM, Ten Haken RK, Kileny PR. Prospective study of inner ear radiation dose and hearing loss in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys 2005; 61: 1393–402. doi: 10.1016/j.ijrobp.2004.08.019 [DOI] [PubMed] [Google Scholar]
- 21.Wang SZ, Li J, Miyamoto CT, Chen F, Zhou LF, Zhang HY, et al. A study of middle ear function in the treatment of nasopharyngeal carcinoma with IMRT technique. Radiother Oncol 2009; 93: 530–3. doi: 10.1016/j.radonc.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 22.Feuvret L, Noel G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys 2006; 64: 333–42. doi: 10.1016/j.ijrobp.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 23.Vanetti E, Clivio A, Nicolini G, Fogliata A, Ghosh-Laskar S, Agarwal JP, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009; 92: 111–17. doi: 10.1016/j.radonc.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 24.Lu SH, Cheng JC, Kuo SH, Lee JJ, Chen LH, Wu JK, et al. Volumetric modulated arc therapy for nasopharyngeal carcinoma: a dosimetric comparison with TomoTherapy and step-and-shoot IMRT. Radiother Oncol 2012; 104: 324–30. doi: 10.1016/j.radonc.2011.11.017 [DOI] [PubMed] [Google Scholar]
- 25.Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P, et al. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys 2010; 76: S50–7. doi: 10.1016/j.ijrobp.2009.04.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuur CL, Simis YJ, Lansdaal PE, Hart AA, Schornagel JH, Dreschler WA, et al. Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol 2007; 25: 3759–65. doi: 10.1200/JCO.2006.08.9540 [DOI] [PubMed] [Google Scholar]
- 27.Bhandare N, Antonelli PJ, Morris CG, Malayapa RS, Mendenhall WM. Ototoxicity after radiotherapy for head and neck tumours. Int J Radiat Oncol Biol Phys 2007; 67: 469–79. doi: 10.1016/j.ijrobp.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 28.Wakisaka H, Yamada H, Motoyoshi K, Ugumori T, Takahashi H, Hyodo M. Incidence of long-term ipsilateral and contralateral ototoxicity following radiotherapy for nasopharyngeal carcinoma. Auris Nasus Larynx 2011; 38: 95–100. doi: 10.1016/j.anl.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 29.Chan SH, Ng WT, Kam KL, Lee MC, Choi CW, Yau TK, et al. Sensorineural hearing loss after treatment of nasopharyngeal carcinoma: a longitudinal analysis. Int J Radiat Oncol Biol Phys 2009; 73: 1335–42. doi: 10.1016/j.ijrobp.2008.07.034 [DOI] [PubMed] [Google Scholar]
- 30.Rademaker-Lakhai JM, Crul M, Zuur L, Baas P, Beijnen JH, Simis YJ, et al. Relationship between cisplatin administration and the development of ototoxicity. J Clin Oncol 2006; 24: 918–24. doi: 10.1200/JCO.2006.10.077 [DOI] [PubMed] [Google Scholar]
- 31.Zuur CL, Simis YJ, Lansdaal PE, Hart AA, Rasch CR, Schornagel JH, et al. Risk factors of ototoxicity after cisplatin-based chemo-irradiation in patients with locally advanced head-and-neck cancer: a multivariate analysis. Int J Radiat Oncol Biol Phys 2007; 68: 1320–5. doi: 10.1016/j.ijrobp.2007.01.042 [DOI] [PubMed] [Google Scholar]
- 32.Holt A, Van Gestel D, Arends MP, Korevaar EW, Schuring D, Kunze-Busch MC, et al. Multi-institutional comparison of volumetric modulated arc therapy vs. intensity-modulated radiation therapy for head-and-neck cancer: a planning study. Radiat Oncol 2013; 8: 26. doi: 10.1186/1748-717X-8-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu X, Lofroth PO, Karlsson M, Zackrisson B. The effect of fraction time in intensity modulated radiotherapy: theoretical and experimental evaluation of an optimisation problem. Radiother Oncol 2003; 68: 181–7. doi: 10.1016/S0167-8140(03)00165-8 [DOI] [PubMed] [Google Scholar]
- 34.Zheng XK, Chen LH, Wang WJ, Ye F, Liu JB, Li QS, et al. Impact of prolonged fraction delivery times simulating IMRT on cultured nasopharyngeal carcinoma cell killing. Int J Radiat Oncol Biol Phys 2010; 78: 1541–7. doi: 10.1016/j.ijrobp.2010.07.005 [DOI] [PubMed] [Google Scholar]