Abstract
Objective:
To identify a useful predictor of thyroid-associated ophthalmopathy (TAO) from orbital CT images; to evaluate the orbital fat and extraocular muscle area ratio as a CT-derived measure; and to investigate the correlations between this ratio and the clinical manifestations in mild-to-moderate TAO.
Methods:
Between January 2012 and March 2013, 44 patients with TAO and 23 controls were studied prospectively. All of the patients underwent ophthalmic examinations, including clinical activity score, exophthalmometry, clinical photographs, alternate prism and cover test, duction and version test, Hess screen test, binocular single vision test, thyroid function tests and orbital CT. The cross-sectional areas of the four rectus muscles, superior oblique muscle, optic nerve and total orbit area were calculated in the coronal view 6 mm posterior from the posterior pole of globe.
Results:
The cross-sectional area measured on orbital CT showed increased orbital fat in patients with TAO and an increased orbital fat to total orbit area ratio (fat/orbit) in TAO with retraction and proptosis. There were significant correlations between fat/orbit and margin reflex distance 1 (p = 0.022), margin reflex distance 2 (p = 0.013) and the exophthalmometric value (p = 0.007).
Conclusion:
The orbital fat to total orbit area ratio (fat/orbit) is a useful diagnostic index in mild-to-moderate TAO.
Advances in knowledge:
The orbital CT offers a useful diagnostic index in TAO.
INTRODUCTION
Thyroid-associated ophthalmopathy (TAO) is characterized by oedema and inflammation of the extraocular muscles and increased orbital connective tissue and fat.1 The muscle cells are normal until the late stage of ophthalmopathy, when they can become atrophic or fibrotic.2 The clinical symptoms and signs of TAO result mechanically from the discrepancy between the increased volume of the swollen orbital tissues and the fixed volume of the body orbit.3
Most individuals with TAO have evidence of both extraocular muscle and orbital adipose tissue involvement. However, some patients appear to have either extraocular muscle enlargement or orbital fat proliferation.4,5
The clinical features of TAO vary from mild grittiness of the eyes to severe diplopia, loss of vision and disfiguring proptosis.1,6 In mild-to-moderate TAO, there is controversy about whether orbital CT is necessary.
We sought a useful predictor of TAO from orbital CT images and evaluated the correlations between orbital fat and extraocular muscle area ratios, as CT-derived measures, to investigate the clinical manifestations of mild-to-moderate TAO.
METHODS AND MATERIALS
This was a prospective cross-sectional study. From January 2012 to March 2013, we collected data on 134 eyes from 67 patients (16 males and 51 females) evaluated for proptosis at the oculoplasty service of Bundang CHA Medical Center, Seongnam, Republic of Korea. Patients were excluded from the study if they had previously received any steroids, immunosuppressive therapy, radiotherapy or surgical decompression. The mean age at the time of imaging was 40.3 ± 14.6 years. Thyroid function tests included TSH, T3, fT4, IGF-1, and TSH-R, Tg, anti-TPO and TS antibodies (Ab). Body mass index (BMI) was calculated from body weight (in kilograms) and height (in centimetres). The ophthalmic examinations included the clinical activity score (CAS),7 exophthalmometry, clinical photographs, alternate prism and cover test, duction and version test, Hess screen test and binocular single vision test.
Mild TAO was defined as lid lag, lid retraction, lagophthalmos and proptosis alone or in varying combinations. Moderate TAO was defined as persistent lid retraction, lid lag, proptosis and soft tissue change with swelling and myopathy. Upper lid retraction was defined as a margin reflex distance 1 (MRD1) >4.5 mm or a bilateral MRD1 difference >2.0 mm from photographs calculated using ImageJ (National Institutes of Health, Bethesda, MD). Similarly, lower lid retraction was defined as a margin reflex distance 2 (MRD2) >4.5 mm or a bilateral MRD2 difference >2.0 mm. Proptosis was defined as Hertel >17.0 mm or bilateral Hertel difference >2.0 mm measured using Hertel exophthalmometry. Restrictive strabismus was defined as the angle deviation on alternate prism and cover testing with fixation targets at 5 and 1/3 m with refractive correction in place or limitation of eyeball movement in the duction and version test (−1 to −4).
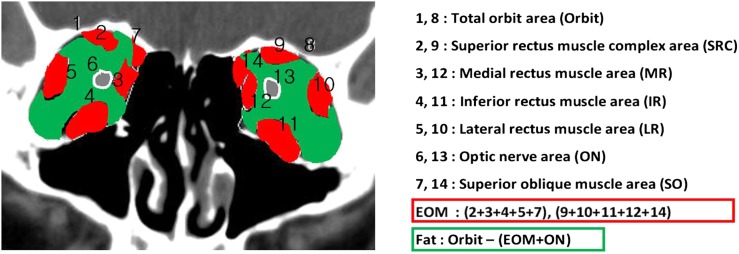
Orbital CT with 2-mm cuts was obtained in all cases. The cross-sectional areas of the four rectus muscles, superior oblique muscle, optic nerve and orbit were measured in the coronal view 6 mm posterior to the posterior pole of the globe CT image with soft tissue windows. We chose the CT slice with the largest cross-sectional area of the four rectus muscles, superior oblique muscle, optic nerve and orbit. The areas of each CT slice were determined by marking the margin of the rectus muscle and the areas calculated automatically with the Syngo® software (2007; Argus, Siemens) (Figure 1). The cross-sectional area of the extraocular muscles was determined as the sum of the cross-sectional areas of each of the four rectus muscles and the superior oblique muscle. The cross-sectional area of orbital fat was determined as the difference in the orbit area and the extraocular muscles and optic nerve. Three ratios were calculated between the cross-sectional areas of the (1) extraocular muscles and orbital fat, (2) extraocular muscles and orbital area and (3) orbital fat and orbital area.
Figure 1.
Method used to calculate the areas of orbital tissues using the Syngo® software (2007; Argus, Siemens). EOM, extraocular muscle.
We assessed each eye as eye manifestations could be asymmetrical. Each eye per subject was evaluated for upper lid retraction, lower lid retraction and proptosis. But, if the patients presented strabismus, both eyes were considered for bilateral motility limitation that causes binocular diplopia and fusional insufficiency.
Statistical analysis was carried out using the SPSS® v. 18.0 (IBM Corp., New York, NY; formerly, SPSS Inc., Chicago, IL). Fisher's exact test, the Mann–Whitney U test, Kruskal–Wallis test and Pearson's correlation were used. All p-values are reported as corrected p-values, and p < 0.01 was considered to indicate statistical significance. Linear regression was used after significant Pearson's correlation results were identified.
RESULTS
Of the 88 eyes from 44 patients with TAO, 12 were males and 32 were females. The 46 eyes from 23 controls comprised 4 males and 19 females. There were no significant differences of female-to-male ratio in TAO and control groups (p = 0.074). There were also no significant differences in mean age (p = 0.414) or BMI (p = 0.806). The mean MRD1 was 3.86 ± 1.51 mm in the TAO group and 3.15 ± 1.10 mm in the controls (p = 0.140). Regarding proptosis, the Hertel value was 15.58 ± 3.65 in the TAO group and 15.16 ± 3.42 in the controls (p = 0.682). There were 10 patients with strabismus in the TAO group (p = 0.705) (Table 1).
Table 1.
Demographic characteristics of the patients
| Characteristic | Thyroid-associated ophthalmopathy (n = 44) | Control group (n = 23) | p-value |
|---|---|---|---|
| Gender, male : female (n) | 12 : 32 | 4 : 19 | 0.082a |
| Mean age (years) | 39.57 ± 14.11 | 43.78 ± 15.90 | 0.414b |
| Body mass index (kg m−2) | 23.17 ± 3.40 | 23.29 ± 2.86 | 0.806b |
| TSH (μIU ml−1) | 2.11 (0.35–5.5) | – | |
| T3 (ng ml−1) | 0.96 (0.6–1.81) | – | |
| fT4 (ng dl−1) | 1.01 (0.89–1.76) | – | |
| TSH-R Ab (U l−1) | 1.24 (0.00–1.75) | – | |
| TS Ab (%) | 36.4 (−) | – | |
| Tg Ab (IU ml−1) | 28.2 (10–124.2) | – | |
| Anti-TPO (IU ml−1) | 7.2 (5–13.6) | – | |
| IGF-1 (ng ml−1) | 287.4 (149.1–332.3) | – | |
| Clinical activity (score) | 2.21 ± 1.08 | – | |
| Margin reflex distance 1 (mm) | 3.86 ± 1.51 | 3.15 ± 1.10 | 0.140b |
| Exophthalmometry (mm) | 15.58 ± 3.65 | 15.16 ± 3.42 | 0.682b |
| Strabismus (n) | 10 | – | 0.705b |
Ab, antibodies.
Fisher's exact test.
Mann–Whitney U test.
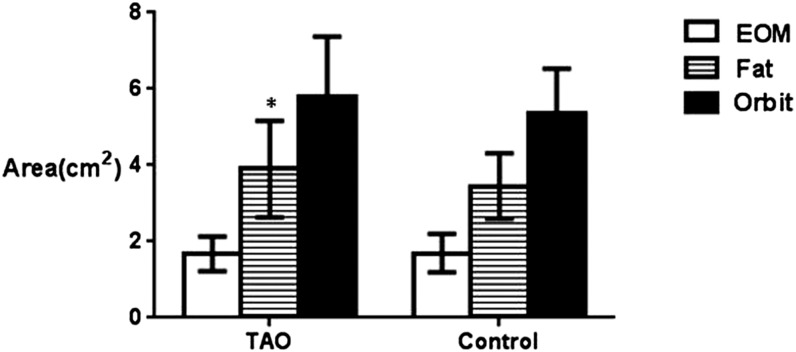
There were no significant differences in the cross-sectional areas of the extraocular muscle (1.68 ± 0.49 vs 1.69 ± 0.51, p = 0.731) and total orbit area (5.81 ± 1.65 vs 5.37 ± 1.16, p = 0.082) except orbital fat area (3.90 ± 1.32 vs 3.45 ± 0.85, p = 0.017) in the TAO group and the controls (Figure 2).
Figure 2.
The cross-sectional area of orbital fat, extraocular muscles and the total orbit in patients with thyroid-associated ophthalmopathy (TAO) and controls [TAO (n = 44) and control (n = 23)]. The areas of each CT slice were determined by marking the margin of the rectus muscle and the areas calculated automatically with the Syngo® software (2007; Argus, Siemens); *p < 0.01 compared with controls (Mann–Whitney U test). EOM, extraocular muscle.
According to the presence of upper lid retraction, the cross-sectional areas of the extraocular muscle and orbital fat using orbital CT in patients with TAO were significantly different, particularly the superior rectus muscle complex area (0.39 ± 0.13 vs 0.34 ± 0.13, p = 0.012) and orbital fat area (4.28 ± 1.32 vs 3.48 ± 1.16, p = 0.002) between TAO with and without upper lid retraction. There were significant differences in the superior rectus muscle complex area (0.39 ± 0.13 vs 0.32 ± 0.11, p = 0.003) and orbital fat area (4.28 ± 1.32 vs 3.45 ± 1.18, p = 0.000) between the TAO group with upper lid retraction and the control group.
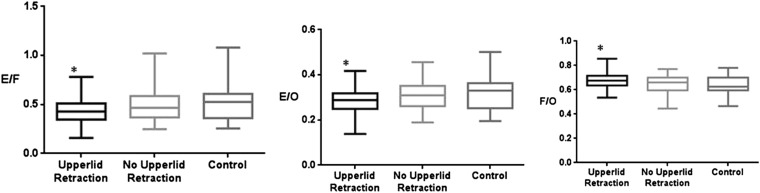
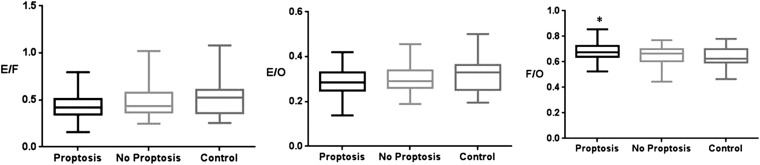
There were significant differences in the cross-sectional area ratios of the extraocular muscles to orbital fat area (0.43 ± 0.17 vs 0.49 ± 0.18, p = 0.006), extraocular muscles to total orbit area (0.28 ± 0.06 vs 0.31 ± 0.05, p = 0.008) and orbital fat to total orbit area (0.68 ± 0.08 vs 0.63 ± 0.06, p = 0.009) between the TAO group with upper lid retraction and the control group (Figure 3).
Figure 3.
The cross-sectional area ratio of orbital fat and extraocular muscles to the total orbit in patients with thyroid-associated ophthalmopathy according to upper lid retraction [upper lid retraction (n = 24), no upper lid retraction (n = 20), control (n = 23)]. Upper lid retraction: a margin reflex distance 1 (MRD1) >4.5 mm or a bilateral MRD1 difference >2.0 mm from photographs calculated using ImageJ (National Institutes of Health, Bethesda, MD); *p < 0.01 compared with controls (Kruskal–Wallis test). E/F, extraocular muscles/orbital fat; E/O, extraocular muscles/total orbital area; F/O, orbital fat/total orbital area.
According to the presence of lower lid retraction, the cross-sectional areas of the extraocular muscle and orbital fat using orbital CT in TAO patients were significantly different, particularly orbital fat area (4.20 ± 1.32 vs 3.50 ± 1.20, p = 0.004) between the TAO group with and without lower lid retraction. There were significant differences in orbital fat area (4.20 ± 1.32 vs 3.48 ± 1.18, p = 0.003) between the TAO group with lower lid retraction and the control group.
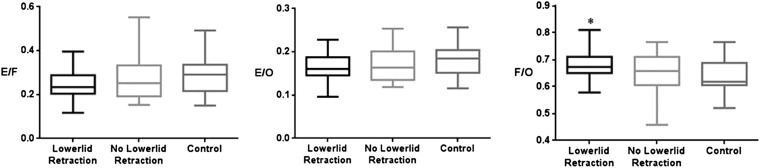
Regarding the presence of lower lid retraction, the cross-sectional area ratio of orbital fat to the total orbit area in patients with TAO was significant (0.68 ± 0.08 vs 0.61 ± 0.10, p = 0.008) between the TAO group with lower lid retraction and the control group (Figure 4).
Figure 4.
The cross-sectional area ratio of orbital fat and extraocular muscles to the total orbit in patients with thyroid-associated ophthalmopathy according to lower lid retraction [lower lid retraction (n = 21), no lower lid retraction (n = 23), control (n = 23)]. Lower lid retraction: a margin reflex distance 2 (MRD2) >4.5 mm or a bilateral MRD2 difference >2.0 mm from photographs calculated using ImageJ (National Institutes of Health, Bethesda, MD); *p < 0.01 compared with controls (Kruskal–Wallis test). E/F, extraocular muscles/orbital fat; E/O, extraocular muscles/total orbital area; F/O, orbital fat/total orbital area.
Regarding the presence of proptosis, the cross-sectional areas of the extraocular muscle and orbital fat using orbital CT in patients with TAO were significantly different, particularly the superior rectus muscle complex area (0.43 ± 0.15 vs 0.34 ± 0.10, p = 0.007), superior oblique muscle (0.17 ± 0.07 vs 0.13 ± 0.04, p = 0.005) and orbital fat area (4.42 ± 1.34 vs 3.59 ± 1.12, p = 0.011) between the TAO group with and without proptosis. There were significant differences in the superior rectus muscle complex area (0.43 ± 0.15 vs 0.33 ± 0.10, p = 0.005), superior oblique muscle (0.17 ± 0.07 vs 0.15 ± 0.06, p = 0.003) and orbital fat area (4.42 ± 1.34 vs 3.45 ± 0.86, p = 0.008) between the TAO group with proptosis and the control group.
The cross-sectional area ratio of orbital fat to the total orbit area in patients with TAO according to proptosis was significant (0.68 ± 0.06 vs 0.64 ± 0.07, p = 0.016) between the TAO group with proptosis and the control group (Figure 5).
Figure 5.
The cross-sectional area ratio of orbital fat and extraocular muscles to the total orbit in patients with thyroid-associated ophthalmopathy according to proptosis [proptosis (n = 19), no proptosis (n = 25), control (n = 23)]. Proptosis: Hertels over 17.0 mm or bilateral Hertel difference >2.0 mm measured using Hertel exophthalmometry; *p < 0.01 compared with controls (Kruskal–Wallis test). E/F, extraocular muscles/orbital fat; E/O, extraocular muscles/total orbital area; F/O, orbital fat/total orbital area.
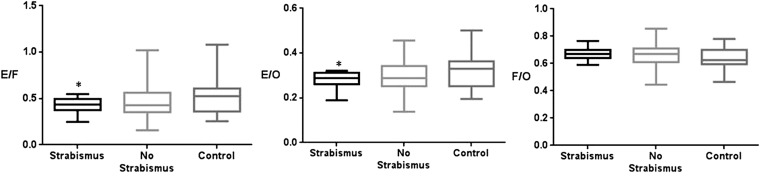
Regarding the presence of strabismus, the cross-sectional areas of the extraocular muscle and orbital fat using orbital CT were significantly different, particularly orbital fat area (4.28 ± 0.95 vs 3.80 ± 1.32, p = 0.007) between the TAO group with strabismus and without strabismus. There were significant differences in the orbital fat area (4.28 ± 1.32 vs 3.45 ± 0.85, p = 0.001) between the TAO group with strabismus and the control group.
There were significant differences in the cross-sectional area ratios of the extraocular muscles to orbital fat area (0.42 ± 0.08 vs 0.51 ± 0.16, p = 0.005) and the extraocular muscles to total orbit area (0.28 ± 0.03 vs 0.31 ± 0.06, p = 0.007) between the TAO group with strabismus and the control group (Figure 6).
Figure 6.
The cross-sectional area ratio of orbital fat and extraocular muscles to the total orbit in patients with thyroid-associated ophthalmopathy according to strabismus [strabismus (n = 10), no strabismus (n = 34), control (n = 23)]. Strabismus: the angle deviation on alternate prism and cover testing with fixation targets at 5 and 1/3 m with refractive correction in place or limitation of eyeball movement in the duction and version test (−1 to −4); *p < 0.01 compared with controls (Kruskal–Wallis test). E/F, extraocular muscles/orbital fat; E/O, extraocular muscles/total orbital area; F/O, orbital fat/total orbital area.
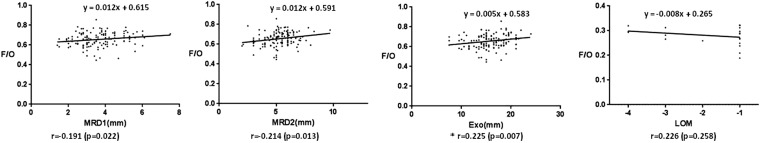
The correlation between the cross-sectional area ratio of orbital fat to total orbit and the clinical manifestations in patients with TAO was significant for MRD1 (r = −0.191, p = 0.022), MRD2 (r = −0.214, p = 0.013) and the exophthalmometric value (r = 0.225, p = 0.007) (Figure 7).
Figure 7.
The correlation between the cross-sectional area ratio of orbital fat to total orbit and clinical measures including margin reflex distance 1 (MRD1), margin reflex distance 2 (MRD2), grade of exophthalmos (Exo) using Hertel, grade of limitation of eye movement (LOM) in patients with thyroid-associated ophthalmopathy patients; *p < 0.01 (Pearson's correlation). F/O, orbital fat/total orbital area.
DISCUSSION
Thyroid orbitopathy occurs most commonly in middle-aged females. Female preponderance in TAO was also noted in this study, and there were no significant differences of female-to-male ratio in the TAO and control groups.
Clinical and CT measures of TAO were compared. All of the CT measures of extraocular muscle enlargement were correlated significantly, with the muscle area and total muscle volume being the most highly correlated. The only clinical measures that correlated significantly with optic neuropathy were limited ocular motility and perhaps proptosis, confirming a prior report.8
Although many clinical signs are of value in diagnosing the orbital involvement in TAO, the limitation of motility and optic nerve changes need to be evaluated regularly once a baseline orbital CT had been performed. Regarding the imaging studies of orbital soft tissue change in TAO, there are some advantages and disadvantages between CT and MRI. Orbital MRI has direct multiplanar imaging, absence of ionizing radiation, better contrast resolution, lack of bone artefact, visualization of bone marrow and usefulness for demyelinating disease. But orbit CT scan takes less time of examination, good ability to visualize the fat and the extraocular muscle as well as cortical bone/calcium, more suitable patients, less certain image artefacts, lower cost of scan units, low cost of examinations than does MRI. Traditional ultrasound test can define the internal echo in the extraocular muscles but hardly measure the size of the muscles or fat.
Using sensitive diagnostic imaging techniques such as ultrasonography, CT or MRI, the orbital soft tissues are found to be abnormal in nearly 75% of the patients with TAO.9 The volume changes indicate inflammation and the immune reaction of orbit tissues. According to some, but not all, authors, the degree of orbit muscle and fat volume changes are independent of the clinical activity and severity of TAO.7,10,11 This can be explained not only by differences in age, gender and orbit anatomic variability, but also by the severity of the TAO.
We used an accurate, computer-assisted technique to measure the area of the extraocular muscles (EOM). By subtracting this value from the total orbit area, it is possible to obtain an indirect measurement of the other orbit components in the immediate retro-globe coronal section. We feel that extraocular muscle width is strictly correlated with the EOM volume.12
Chang et al13 reported a correlation between the total muscle areas and CAS in 22 cases with TAO, and Kvetny et al14 reported a correlation between CAS and EOM volume by using MRI in 15 patients with active TAO, while a correlation between CAS and orbital fat volume has not been reported. Le Moli et al15 investigated a correlation between the extraocular muscles to total orbit area ratio and CAS by calculating from CT scan coronal area in 32 patients with TAO. The extraocular muscles to total orbit area ratio decreased after 18 months in most patients with TAO.
In our study, there were no significant differences in the cross-sectional areas of the extraocular muscle and total orbit area, but there was a significant difference in the cross-sectional area of orbital fat in patients with TAO. The orbital fat to total orbit area ratio increases in most patients with mild-to-moderate TAO, and there are positive direct correlations between the orbital fat to total orbit area ratio and MRD1, MRD2 and exophthalmometric values. This positive correlation between the orbital fat to total orbit area ratio increment and typical features of TAO, indicates that the most clinical manifestations of active TAO occur in association with periorbital fat volume increase. There was also a positive correlation between the limitation of motion of eye movement and the ratio of extraocular muscle to total orbit area which was referred to a correlation factor of CAS in previous studies.13–15 Based on these results, we support the concept that TAO is classified into two distinct types, orbital fat dominant TAO which presents the clinical features like upper lid retraction, lower lid retraction and proptosis and extraocular muscle dominant TAO which presents clinical features like the limitation of motion of the extraocular muscle, diplopia and strabismus.
There are some limitations to this study. First, the orbital volume that we measured was the extraocular orbital volume 6 mm posterior to the globe. We could not calculate the anterior extent of the orbital volume using our technique and so might have underestimated the orbital volume change. Second, one observer (HCK) made the clinical measurements. Another observer (SWY) made the radiological measurements using CT scan. We did not assess for interobserver and intraobserver variability for measurement. It is true that this may be a limitation of the study. Third, the method we used to calculate the orbital volume approximated only the orbital volume; true integration would produce a more accurate orbital volume measurement. 67 cases are sufficient to perform a statistical analysis. Nevertheless, an additional study of a larger population must be performed before orbital CT can be used to diagnose mild-to-moderate TAO.
In conclusion, the orbital fat to total orbit area ratio (fat/orbit) is a useful diagnostic factor in mild-to-moderate TAO.
Contributor Information
H C Kim, Email: gonasa@naver.com.
S W Yoon, Email: jansons@cha.ac.kr.
H Lew, Email: eye@cha.ac.kr.
REFERENCES
- 1.Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev 2000; 21: 168–99. [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP. Graves' disease. N Engl J Med 2000; 343: 1236–48. doi: 10.1056/NEJM200010263431707 [DOI] [PubMed] [Google Scholar]
- 3.Anderson RL, Tweeten JP, Patrinely JR, Garland PE, Thiese SM. Dysthyroid optic neuropathy without extraocular muscle involvement. Ophthalmic Surg 1989; 20: 568–74. [PubMed] [Google Scholar]
- 4.Giaconi JA, Kazim M, Rho T, Pfaff C. CT scan evidence of dysthyroid optic neuropathy. Ophthal Plast Reconstr Surg 2002; 18: 177–82. doi: 10.1097/00002341-200205000-00005 [DOI] [PubMed] [Google Scholar]
- 5.Carter KD, Frueh BR, Hessburg TP, Musch DC. Long-term efficacy of orbital decompression for compressive optic neuropathy of Graves' eye disease. Ophthalmology 1991; 98: 1435–42. doi: 10.1016/S0161-6420(91)32115-8 [DOI] [PubMed] [Google Scholar]
- 6.Bahn RS, Heufelder AE. Pathogenesis of Graves' ophthalmopathy. N Engl J Med 1993; 329: 1468–75. doi: 10.1056/NEJM199311113292007 [DOI] [PubMed] [Google Scholar]
- 7.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 1997; 47: 9–14. doi: 10.1046/j.1365-2265.1997.2331047.x [DOI] [PubMed] [Google Scholar]
- 8.Feldon SE, Muramatsu S, Weiner JM. Clinical classification of Graves' ophthalmopathy. Identification of risk factors for optic neuropathy. Arch Ophthalmol 1984; 102: 1469–72. doi: 10.1001/archopht.1984.01040031189015 [DOI] [PubMed] [Google Scholar]
- 9.Kirsch E, Hammer B, von Arx G. Graves' orbitopathy: current imaging procedures. Swiss Med Wkly 2009; 139: 618–23. doi: smw-12741 [DOI] [PubMed] [Google Scholar]
- 10.Kaspar M, Archibald C, De BA, Li AW, Yamada M, Chang CH, et al. Eye muscle antibodies and subtype of thyroid-associated ophthalmopathy. Thyroid 2002; 12: 187–91. doi: 10.1089/105072502753600115 [DOI] [PubMed] [Google Scholar]
- 11.Nagy EV, Toth J, Kaldi I, Damjanovich J, Mezosi E, Lenkey A, et al. Graves' ophthalmopathy: eye muscle involvement in patients with diplopia. Eur J Endocrinol 2000; 142: 591–7. doi: 10.1530/eje.0.1420591 [DOI] [PubMed] [Google Scholar]
- 12.Feldon SE, Lee CP, Muramatsu SK, Weiner JM. Quantitative computed tomography of Graves' ophthalmopathy. Extraocular muscle and orbital fat in development of optic neuropathy. Arch Ophthalmol 1985; 103: 213–15. doi: 10.1001/archopht.1985.01050020065021 [DOI] [PubMed] [Google Scholar]
- 13.Chang TC, Huang KM, Hsiao YL, Tzeng SS, Kao S. Relationships of orbital computed tomographic findings and activity scores to the prognosis of corticosteroid therapy in patients with Graves' ophthalmopathy. Acta Ophthalmol Scand 1997; 75: 301–4. doi: 10.1111/j.1600-0420.1997.tb00779.x [DOI] [PubMed] [Google Scholar]
- 14.Kvetny J, Puhakka KB, Røhl L. Magnetic resonance imaging determination of extraocular eye muscle volume in patients with thyroid‐associated ophthalmopathy and proptosis. Acta Ophthalmol Scand 2006; 84: 419–23. doi: 10.1111/j.1600-0420.2005.00617.x [DOI] [PubMed] [Google Scholar]
- 15.Le Moli R, Pluchino A, Muscia V, Regalbuto C, Luciani B, Squatrito S, et al. Graves' orbitopathy: extraocular muscle/total orbit area ratio is positively related to the Clinical Activity Score. Eur J Ophthalmol 2012; 22: 301–8. doi: 10.5301/ejo.5000018 [DOI] [PubMed] [Google Scholar]