Abstract
Background
Eosinophils (EOS) have been associated with prognosis of patients with coronary artery disease, and those who showed plenitudinous coronary collateral circulation (CCC) often have good clinical consequences. However, the relationship between EOS and CCC was seldom reported.
Objective
To investigate the relationship between EOS and CCC development in patients with unstable angina pectoris (UAP).
Methods
The study population consisted of 502 consecutive patients with UAP who underwent coronary angiography and coronary stenosis ≥80%. CCC was graded according to the Rentrop grading system of 0–3. Rentrop grades of 0 and 1 indicated low-grade CCC group, whereas grades 2 and 3 indicated high-grade CCC group.
Results
The EOS was significantly higher in the high-grade CCC group compared with the low-grade CCC group. In multiple logistic regression analysis, EOS (odds ratio: 1.969; 95% confidence interval [CI]: 1.210–3.3205; P=0.006) and neutrophil count (odds ratio: 0.757; 95% CI: 0.584–0.981; P=0.035) were predictors of high-grade CCC development. EOS of >0.12×109/L could independently predict high-grade CCC with 72.5% sensitivity and 58.4% specificity (area under the curve: 0.681; 95% CI: 0.632–0.729).
Conclusion
EOS were associated with high-grade CCC in patients with UAP with coronary stenosis ≥80%. Increased EOS count may play an important role in the development of CCC in patients with UAP.
Keywords: unstable angina pectoris, coronary collateral circulation, eosinophils, coronary artery disease
Introduction
When a coronary artery is occluded, the collateral or anastomosis vessels gradually open, carrying blood into the ischemic or infarcted myocardium. These vessels were defined as coronary collateral circulation (CCC).1 CCC can maintain the blood supply, reduce the myocardial infarction area, protect heart function, avoid ventricular aneurysm formation, and influence the prognosis of patients with acute coronary syndrome (ACS).2–5 The complex mechanisms in the development of CCC are still not clear. Monocytes, neutrophils, lymphocytes, and vascular growth factors (such as vascular endothelial growth factor, fibroblast growth factor, and transforming growth factor β [TGF-β]) in CCC formation play an important role,6 but they cannot fully explain the mechanisms of CCC formation.
Eosinophils (EOS) is one form of leukocytes. Few studies have addressed the relation between EOS and coronary artery disease (CAD). Jiang et al7 reported that the decreased EOS percentage suggested serious myocardial damage and EOS played an important role in thrombosis in patients with ACS. Toor et al8 showed that EOS was a novel biomarker for risk stratification of patients with CAD, which was initially associated with reduced mortality but after 6 months with increased mortality. EOS was a significant source of TGF-β1, which suggested that it might be able to modulate the acute phase and innate inflammatory response.9 At present, there is no research on the relation between EOS and CCC.
In this study, we hypothesized that there was a relation between EOS count and CCC. We tested this hypothesis in Chinese people with unstable angina pectoris (UAP).
Methods
Study design
The study was a cross-sectional, observational, retrospective, and single-center design.
Patients
The study population consisted of 502 patients with UAP who underwent coronary angiography (CAG) in Beijing Mentougou District Hospital from January 1, 2008, to December 31, 2014. UAP was defined by chest discomfort or angina equivalent, electrocardiographic ST-segment depression or prominent T-wave inversion and without elevated cardiac biomarkers.10
Patients with acute myocardial infarction with or without ST-segment elevation, hepatic dysfunction (serum alanine aminotransferase >120 U/L), renal dysfunction (serum creatinine >133 μmol/L), a history of percutaneous coronary intervention or coronary artery bypass grafting, a history of chronic obstructive pulmonary disease, a history of blood transfusion in a month, acute inflammation, a history of trauma or surgery in 2 weeks, hematological disease, cancer, autoimmune disease, thrombocytopenia, a history of allergies to contrast medium, and coronary artery stenosis <80% were excluded. Baseline data, including sex, age, body mass index, hypertension, diabetes mellitus (DM), dyslipidemia, smoking status, relevant medication, left ventricular ejection fraction, heart rate, systolic blood pressure, and diastolic blood pressure, were obtained from the patient’s medical records. All patients were evaluated by hematological indices, such as glucose, serum creatinine, lipids, creatine kinase MB, and high-sensitivity C-reactive protein (hs-CRP). Hypertension was defined if the individual had a history of hypertension or was taking antihypertension medications or as a blood pressure ≥140/90 mmHg at least three times. DM was defined as glycated hemogobin A1c ≥6.5% or fasting plasma glucose level ≥7.0 mmol/L or using antidiabetic drugs. The study was approved by the Beijing Tiantan Hospital Ethical Committee. All patients signed written informed consent.
Laboratory analyses
Fasting blood samples were collected from all patients. White blood cell count, neutrophil count, lymphocyte count, EOS count, hemoglobin, platelet count, and mean platelet volume were performed by a blood counter (Sysmex XN-1000, Sysmex Corporation, Kobe, Japan). Serum glucose, serum creatinine, lipids, creatine kinase MB, and hs-CRP were measured by a blood counter (Olympus chemistry analyzer AU640, Olympus Corporation, Tokyo, Japan).
CAG and grading of coronary collaterals
CAG was performed through the right femoral artery or right radial artery in all patients using a standard Judkins technique. Coronary arteries were shown in the right and left anterior oblique views with cranial and caudal positions. Injection of contrast medium (Iodixanol, Visipaque; GE Healthcare Ireland, Cork, Ireland) was carried out by manual bolus. CAD was identified as stenosis of the major coronary artery of at least 80%. The coronary angiograms were analyzed by two experienced cardiologists blinded to the study. CCC was graded according to the Rentrop classification as follows: grade 0= no visible collaterals, grade 1= the filling of the side branch via collateral vessels without visualization of the epicardial segment, grade 2= the partial filling of epicardial coronary artery, and grade 3= the complete filling of epicardial coronary artery.11 Patients were divided into low-grade CCC group (grades 0 and 1) and high-grade CCC group (grades 2 and 3) according to the Rentrop classification.
Statistical analysis
Statistical analysis was performed using the SPSS software for Windows Version 13.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as the mean ± standard deviation, while noncontinuous variables were expressed as percentage. Baseline characteristics of patients were compared using the mean of independent-samples t-test for continuous variables and the chi-square test for categorical variables. Multivariate, stepwise backward conditional logistic regression analysis was used to identify the independent predictors of the presence of CCC using variables showing marginal association with it on univariable testing. Receiver-operating characteristic analyses were used to detect the cutoff values of EOS and model in the prediction of CCC; sensitivity and specificity at that point were determined. P-value <0.05 was considered statistically significant.
Results
The baseline data
The study population consisted of 502 patients with UAP (age: 60.1±10.9 years, male ratio: 32.9%), of which 308 patients (61.4%) had Rentrop grade 0, 45 cases (9.0%) had grade 1, 86 cases (17.1%) had grade 2, and 63 cases (12.5%) had grade 3. There were no significant differences in sex, age, body mass index, medical history, relevant medication, ejection fraction, heart rate, systolic blood pressure, diastolic blood pressure, white blood cell count, hemoglobin, platelet count, mean platelet volume, neutrophil count, lymphocyte count, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglycerides, glucose, creatinine, and creatine kinase MB except for EOS count and hs-CRP between the two groups (P<0.001 and P=0.018, respectively; Table 1).
Table 1.
Characteristics of the patients at baseline
| Characteristics | Low-grade CCC group (n=353) | High-grade CCC group (n=149) | P-value |
|---|---|---|---|
| Male sex, no (%) | 121 (34.3) | 44 (29.5) | 0.301 |
| Age, yr | 60.4±11.1 | 60.7±10.6 | 0.763 |
| Body mass index, kg/m2 | 25.3±3.6 | 25.0±3.7 | 0.412 |
| Medical history, no (%) | |||
| Hypertension | 194 (55.0) | 84 (56.4) | 0.770 |
| Diabetes mellitus | 112 (31.7) | 51 (34.2) | 0.585 |
| Dyslipidemia | 119 (33.7) | 48 (32.2) | 0.745 |
| Current smoker | 171 (48.4) | 67 (45.0) | 0.476 |
| Relevant medication, no (%) | |||
| Aspirin | 79 (22.8) | 40 (26.8) | 0.329 |
| Statin | 44 (12.5) | 25 (16.8) | 0.200 |
| Beta-blocker | 42 (11.9) | 15 (10.1) | 0.555 |
| ACE inhibitor or ARB | 81 (22.9) | 31 (20.8) | 0.599 |
| Ejection fraction, % | 55.4±6.5 | 56.7±6.8 | 0.061 |
| Heart rate, bpm | 75.5±12.3 | 74.2±11.3 | 0.285 |
| Systolic blood pressure, mmHg | 134.3±21.8 | 137.8±23.2 | 0.120 |
| Diastolic blood pressure, mmHg | 74.2±12.2 | 76.1±12.5 | 0.127 |
| White blood cell count, ×109/L | 9.0±2.6 | 8.8±2.2 | 0.381 |
| Hemoglobin, g/L | 136.4±15.2 | 135.4±22.4 | 0.615 |
| Platelet count, ×109/L | 192.4±50.1 | 196.9±54.6 | 0.392 |
| Mean platelet volume, fL | 9.9±0.9 | 9.8±0.9 | 0.069 |
| Neutrophil count, ×109/L | 6.5±2.4 | 6.3±1.8 | 0.165 |
| Lymphocyte count, ×109/L | 1.9±0.7 | 1.9±0.6 | 0.744 |
| Eosinophil count, ×109/L | 0.11±0.03 | 0.14±0.03 | <0.001 |
| Total cholesterol, mmol/L | 4.3±0.9 | 4.4±0.9 | 0.880 |
| LDL cholesterol, mmol/L | 2.8±0.7 | 2.8±0.8 | 0.955 |
| HDL cholesterol, mmol/L | 1.1±0.3 | 1.1±0.2 | 0.797 |
| Triglycerides, mmol/L | 1.8±1.0 | 1.8±0.9 | 0.986 |
| Glucose, mmol/L | 6.6±2.6 | 6.6±2.6 | 0.911 |
| Creatinine, μmol/L | 89.7±15.8 | 89.9±12.6 | 0.902 |
| Peak CKMB, U/L | 431.7±463.6 | 352.6±455.8 | 0.078 |
| hs-CRP, mg/L | 16.1±4.9 | 15.1±4.4 | 0.018 |
Note: Plus or minus values are mean ± SD.
Abbreviations: CCC, coronary collateral circulation; yr, year; ACE, angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CKMB, creatine kinase MB; hs-CRP, high-sensitivity C-reactive protein; SD, standard deviation.
Logistic regression analysis
A multivariate logistic regression analysis showed that EOS count (odds ratio: 1.969; 95% confidence interval [CI]: 1.210–3.205; P=0.006) and neutrophil count (odds ratio: 0.757; 95% CI: 0.584–0.981; P=0.035) were the independent predictors of the presence of CCC (Table 2). EOS count of >0.12×109/L could independently predict the presence of high-grade CCC with 72.5% sensitivity and 58.4% specificity (area under the curve [AUC]: 0.681; 95% CI: 0.632–0.729) (Figure 1). The result of receiver-operating characteristic curve analysis for the multivariate model for high-grade CCC was AUC: 0.719 and 95% CI: 0.669–0.768 with 81.9% sensitivity and 54.0% specificity (Figure 2).
Table 2.
Multivariate logistic regression analysis of variables related to CCC development (method = backward stepwise [Wald])
| Category | B | SE | Wald | OR (95% CI) | P-value |
|---|---|---|---|---|---|
| Systolic blood pressure | 0.203 | 0.110 | 3.448 | 1.225 (0.989–1.519) | 0.063 |
| Eosinophil count | 0.678 | 0.249 | 7.428 | 1.969 (1.210–3.205) | 0.006 |
| Neutrophil count | −0.279 | 0.132 | 4.439 | 0.757 (0.584–0.981) | 0.035 |
| Peak CKMB | −0.218 | 0.112 | 3.770 | 0.804 (0.645–1.002) | 0.052 |
| hs-CRP | −0.224 | 0.116 | 3.746 | 0.799 (0.637–1.003) | 0.053 |
Abbreviations: CCC, coronary collateral circulation; SE, standard error; OR, odds ratio; CI, confidence interval; CKMB, creatine kinase MB; hs-CRP, high-sensitivity C-reactive protein.
Figure 1.
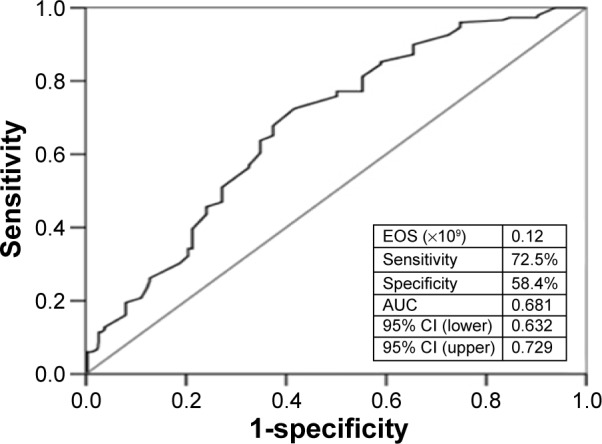
ROC curves for EOS value in the prediction of high-grade coronary collateral circulation.
Note: AUC =0.681 (0.632–0.729).
Abbreviations: ROC, receiver-operating characteristic; EOS, eosinophil; AUC, area under the curve; CI, confidence interval.
Figure 2.
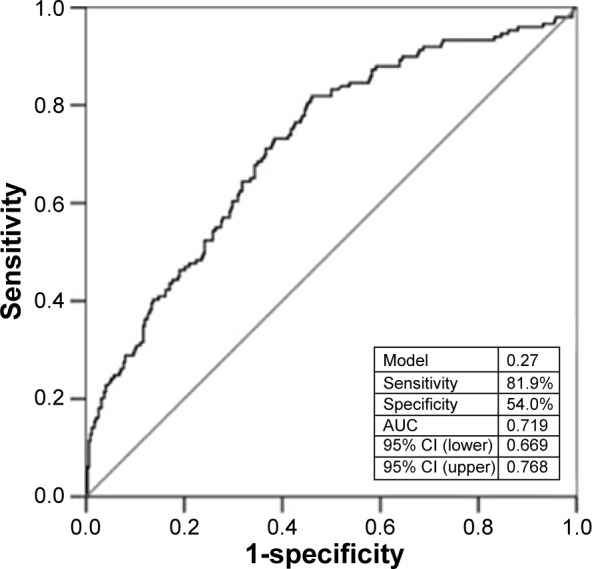
ROC curves for multivariate logistic regression model in the prediction of high-grade coronary collateral circulation.
Note: AUC =0.719 (0.669–0.768).
Abbreviations: ROC, receiver-operating characteristic; AUC, area under the curve; CI, confidence interval.
Discussion
The aim of this study was to investigate the relationship between EOS count and CCC development, which revealed that increased EOS count was associated with high-grade CCC in patients with UAP.
CCC between the epicardial coronary arteries in a normal human heart was not visible on normal angiography. However, when epicardial coronary arteries stenosis exceeds 80%, the pressure within the CCC will decrease and consequently CCC might be visible.12 The process of CCC formation included vasculogenesis, angiogenesis, and artery vessel formation.4 Many factors affected the formation of CCC, such as the duration and/or severity of coronary artery stenosis, endothelial dysfunction, shear stress, endogenous mediators, hypertension, DM, dyslipidemia, smoking, and drugs.1,4,6,13–17 The endogenous mediators included vascular endothelial growth factor, TGF-α or -β, fibroblast growth factor, nitric oxide, inflammatory markers, and neurohumoral factors in angiogenesis.6 Moreover, the inflammatory cells, including monocytes, neutrophils, lymphocytes, and nature killer cells, secreted proinflammatory enzymes and cytokines that were supposed to play a role in the formation of CCC.6
Inflammation was a key feature of CCC development. However, few studies had addressed the relation between EOS and CAD. Jiang et al7 reported that the decreased EOS percentage suggested serious myocardial damage, and EOS played an important role in thrombosis in patients with ACS. Verdoia et al18 reported that higher EOS levels were not independently associated with the prevalence and extent of CAD in patients undergoing CAG but appeared confounded by their link with major cardiovascular risk factors. These studies did not involve the relationship between EOS and CCC development. Our study showed that EOS in high-grade CCC group were obviously higher than that of low-grade CCC group. EOS played an important role in the regulation of inflammatory response among patients with UAP.
Why was peripheral EOS count different between the two groups? Did some of EOS deposit in the thrombi or in other places, or were they degraded? In this study, peripheral EOS counts were different in patients with UAP between the two groups. We suspected that part of peripheral EOS might be in the thrombi or in the CCC. The research of Jiang et al7 had shown that EOS played an important role in thrombosis in patients with ACS. Coronary stenotic extension due to acute thrombosis induced a drop of pressure and oxygen saturation in the distal vascular anastomoses. This induced a steep pressure gradient over bridging collateral vessels and a subsequent elevation in fluid shear stress. At the cellular and molecular level, endothelial cells responded to the changes of shear stress with mechanosensors, including transmembrane proteins and glycocalyx, and led to cytoskeletal reorganization and activation of signal transduction pathways. The EOS granule proteins, particularly major basic proteins, potentially contributed to the hypercoagulation.7 Meanwhile, EOS expressed an array of cell surface receptors that were critical to their own survival, recruitment, and activation, such as cytokine receptors (eg, interleukin-5 receptor [IL-5R], IL-4R, IL-13R, granulocyte-macrophage colony-stimulating factor, c-kit), chemokine receptors, complement receptors, immunoglobulin receptors, leukotriene receptors, and receptors for growth factors (eg, TGF-β receptor). More importantly, because of the existence of local autocrine and paracrine regulatory loops through which EOS partly controlled both their local tissue accumulation and effector functions, EOS were also the study sources of the ligands that binded these receptors. EOS played a role in the regulation of tissue immune microenvironment by affecting directly or indirectly immunoregulation of lymphocyte functions, leukocyte survival, activation, migration, and effector functions, and immunoregulation of resident tissue cells.9 Besides that, TGF-β1 that combined with TGF-β receptors was a potent angiogenic factor and promoted microvascular regeneration with other vascular growth factors. When coronary stenotic extension led to severe myocardial ischemia, TGF-β1 synthetized and secreted by endothelial cells and smooth muscle cells inhibited expression of adhesive molecule in endothelial cells, weakened the adhesion between cells and vascular endothelial cells and reduced inflammation, kept unobstructed, activated the endothelial of ischemia area, and promoted microvascular proliferation.19,20 The stimulation to the endothelial cells weakened once CCC was established due to the the pressure step being reduced. The process seemed to be negative feedback. EOS did play an important role in the initiation, generation, and maintenance of CCC. This may explain the reason that EOS count was higher in high-grade CCC group than that of low-grade CCC group.
EOS could independently predict the presence of high-grade CCC with 72.5% sensitivity and 58.4% specificity (AUC: 0.681), and the multivariate model predicted it with 81.9% sensitivity and 54.0% specificity (AUC: 0.719). The latter seemed to be better than the former.
In addition, hs-CRP level in high-grade CCC group was obviously lower than that of low-grade CCC group, but neutrophils were no different between the two groups. Multiple factor regression analysis showed that there was a negative relationship between neutrophils and high-grade CCC, and hs-CRP was not an independent predictor for high-grade CCC in patients with UAP. These results were similar to the research of Ayahan et al.21
Limitations
There were some limitations to our study. First, there was no direct evidence about EOS accumulated in the coronary collateral vessels. Second, this study did not include the patients with acute myocardial infarction in which more supporting evidence may be found. Finally, large sample, prospective, and multicenter studies are needed.
Conclusion
In conclusion, EOS was associated with and could independently predict high-grade CCC in patients with UAP with epicardial coronary arteries stenosis >80%.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34(34):2674–2682. doi: 10.1093/eurheartj/eht195. [DOI] [PubMed] [Google Scholar]
- 2.Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992;327:1825–1831. doi: 10.1056/NEJM199212243272601. [DOI] [PubMed] [Google Scholar]
- 3.Billinger M, Kloos P, Eberli FR, Windecker S, Meier B, Seiler C. Physiologically assessed coronary collateral flow and adverse cardiac ischemic events: a follow-up study in 403 patients with coronary artery disease. J Am Coll Cardiol. 2002;40(9):1545–1550. doi: 10.1016/s0735-1097(02)02378-1. [DOI] [PubMed] [Google Scholar]
- 4.Koerselman J, van der Graaf Y, de jaegere PP, Grobbee DE. Coronary collaterals: an important and underexposed aspect of coronary artery disease. Circulation. 2003;107(19):2507–2511. doi: 10.1161/01.CIR.0000065118.99409.5F. [DOI] [PubMed] [Google Scholar]
- 5.Meier P, Hemingway H, Lansky AJ, Knapp G, Pitt B, Seiler C. The impact of the coronary collateral circulation on mortality: a meta-analysis. Eur Heart J. 2012;33(5):614–621. doi: 10.1093/eurheartj/ehr308. [DOI] [PubMed] [Google Scholar]
- 6.Hakimzadeh N, Verberne HJ, Siebes M, Piek JJ. The future of collateral artery research. Curr Cardiol Rev. 2014;10(1):73–86. doi: 10.2174/1573403X113099990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang P, Wang DZ, Ren YL, Cai JP, Chen BX. Significance of eosinophil accumulation in the thrombus and decrease in peripheral blood in patients with acute coronary syndrome. Coron Artery Dis. 2015;26(2):101–106. doi: 10.1097/MCA.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toor IS, Jaumdally R, Lip GY, Millane T, Varma C. Eosinophil count predicts mortality following percutaneous coronary intervention. Thromb Res. 2012;130(4):607–611. doi: 10.1016/j.thromres.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen EA, Taranova AG, Lee NA, Lee JJ. Eosinophils: singularly destructive effector cells or purveyors of immunoregulation? J Allergy Clin Immunol. 2007;119(6):1313–1320. doi: 10.1016/j.jaci.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(23):e179–e347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–592. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 12.Traupe T, Gloeler S, de Marchi SF, Werner GS, Seiler C. Assessment of the human coronary collateral circulation. Circulation. 2010;122(12):1210–1220. doi: 10.1161/CIRCULATIONAHA.109.930651. [DOI] [PubMed] [Google Scholar]
- 13.Weyers JJ, Schwartz SM, Minami E, et al. Effects of cell grafting on coronary remodeling after myocardial infarction. J Am Heart Assoc. 2013;2(3):e000202. doi: 10.1161/JAHA.113.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiter MS, van Golde JM, Schaper NC, Stehouwer CD, Huijberts MS. Diabetes impairs arteriogenesis in the peripheral circulation: review of molecular mechanisms. Clin Sci (Lond) 2010;119(6):225–238. doi: 10.1042/CS20100082. [DOI] [PubMed] [Google Scholar]
- 15.Mouquet F, Cuilleret F, Susen S, et al. Metabolic syndrome and collateral vessel formation in patients with documented occluded coronary arteries: association with hyperglycaemia, insulin-resistance, adiponectin and plasminogen activator inhibitor-1. Eur Heart J. 2009;30(7):840–849. doi: 10.1093/eurheartj/ehn569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dincer I, Ongun A, Turhan S, Ozdol C, Kumbasar D, Erol C. Association between the dosage and duration of statin treatment with coronary collateral development. Coron Artery Dis. 2006;17(6):561–565. doi: 10.1097/00019501-200609000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Ren J, Li H, Prior BM, Yang HT. Angiotensin converting enzyme inhibition enhances collateral artery remodeling in rats with femoral artery occlusion. Am J Med Sci. 2008;335(3):177–187. doi: 10.1097/MAJ.0b013e318142b978. [DOI] [PubMed] [Google Scholar]
- 18.Verdoia M, Schaffer A, Cassetti E, et al. Absolute eosinophils count and the extent of coronary artery disease: a single centre cohort study. J Thromb Thrombolysis. 2015;39(4):459–466. doi: 10.1007/s11239-014-1120-3. [DOI] [PubMed] [Google Scholar]
- 19.Hinkel R, Trenkwalder T, Kupatt C. Molecular and cellular mechanisms of thymosin β4-mediated cardioprotection. Ann N Y Acad Sci. 2012;1269:102–109. doi: 10.1111/j.1749-6632.2012.06693.x. [DOI] [PubMed] [Google Scholar]
- 20.Pezzini A, Del Zotto E, Giossi A, Volonghi I, Costa P, Padovani A. Transforming growth factor β signaling perturbation in the Loeys–Dietz syndrome. Curr Med Chem. 2012;19(3):454–460. doi: 10.2174/092986712803414286. [DOI] [PubMed] [Google Scholar]
- 21.Ayahan S, Ozturk S, Erdem A, et al. Hematological parameters and coronary collateral circulation in patients with stable coronary artery disease. Exp Clin Cardiol. 2013;18(1):e12–e15. [PMC free article] [PubMed] [Google Scholar]


