ABSTRACT
Simple high-throughput procedures were developed for the direct analysis of glyphosate [N-(phosphonomethyl)glycine] and aminomethylphosphonic acid (AMPA) in human and bovine milk and human urine matrices. Samples were extracted with an acidified aqueous solution on a high-speed shaker. Stable isotope labeled internal standards were added with the extraction solvent to ensure accurate tracking and quantitation. An additional cleanup procedure using partitioning with methylene chloride was required for milk matrices to minimize the presence of matrix components that can impact the longevity of the analytical column. Both analytes were analyzed directly, without derivatization, by liquid chromatography tandem mass spectrometry using two separate precursor-to-product transitions that ensure and confirm the accuracy of the measured results. Method performance was evaluated during validation through a series of assessments that included linearity, accuracy, precision, selectivity, ionization effects and carryover. Limits of quantitation (LOQ) were determined to be 0.1 and 10 µg/L (ppb) for urine and milk, respectively, for both glyphosate and AMPA. Mean recoveries for all matrices were within 89–107% at three separate fortification levels including the LOQ. Precision for replicates was ≤7.4% relative standard deviation (RSD) for milk and ≤11.4% RSD for urine across all fortification levels. All human and bovine milk samples used for selectivity and ionization effects assessments were free of any detectable levels of glyphosate and AMPA. Some of the human urine samples contained trace levels of glyphosate and AMPA, which were background subtracted for accuracy assessments. Ionization effects testing showed no significant biases from the matrix. A successful independent external validation was conducted using the more complicated milk matrices to demonstrate method transferability.
KEYWORDS: Glyphosate, aminomethylphosphonic acid, AMPA, human milk, bovine milk, human urine, mass spectrometry
Introduction
Glyphosate, N-(phosphonomethyl)glycine, is a broad-spectrum herbicide that inhibits 5-enolpyruvylshikimate 3-phosphate synthase (EPSPS), a key enzyme in the shikimate biosynthetic pathway. This process reduces the production of certain amino acids and subsequent protein synthesis affecting plant growth and vitality. Glyphosate has become one of the world's most widely used herbicides due to its effectiveness and its positive environmental and safety profile. [1] Its use with glyphosate tolerant crops offers additional benefits including improved farming practices and environmental sustainability, as well as the displacement of other less favorable herbicide alternatives. [2,3] Aminomethylphosphonic acid (AMPA) is the main environmental degradant of glyphosate and it is formed to a lesser extent in plants. [4] As a result both glyphosate and AMPA are often analyzed together when evaluating potential glyphosate exposure.
While procedures exist for quantifying glyphosate residues in blood and urine, [5–9] there are currently no published methods for milk matrices using instrumentation and techniques that are considered appropriate and acceptable for accurate measurement. The high polarity and ionic nature of glyphosate and AMPA have restricted most methods to more time intensive derivatization-based approaches [5–8] to accommodate the chromatographic phase of the analysis. A simplified direct procedure for human serum developed by Yoshioka et al. [9] utilizes a chloroform protein precipitation with subsequent analysis of the aqueous sample portion by liquid chromatography tandem mass spectrometry (LC-MS/MS). Hydrophilic interaction chromatography (HILIC) with an Obelisc N column was employed to retain both analytes. A comparable approach was successfully extended to more complicated matrices including rice, maize and soybean, [10] but rapid degradation of the Obelisc N column limits its utility and, ultimately, its cost-effectiveness. Similarly, Nagatomi et al. [11] applied a direct detection method for analysis of beer, barley tea, malt, and corn using an ion exchange column, but required extensive cleanup steps prior to LC-MS/MS analysis.
We have developed and validated analytical methods for the selective determination of glyphosate and AMPA in milk and urine. These methods use relatively simple extraction and clean-up procedures along with direct analysis by LC-MS/MS. The chromatographic phase of the analysis employs an inexpensive Bio-Rad Cation-H guard column. Two quantitation ion transitions (precursor-to-product ions) are used to ensure and confirm the accuracy of the measurements. Selectivity and robustness of the two methods were evaluated using several pools of human and bovine milk and several pools of human urine. An additional independent external validation for the more complicated milk matrices was conducted to demonstrate method transferability.
Materials and methods
Standards and reagents
Analytical standards of glyphosate and AMPA were obtained from Sigma-Aldrich (St Louis, MO, USA). Syntheses of stable isotope labeled internal standards13C3 15N-glyphosate and D2 13C15N-AMPA were performed in-house, although it is noteworthy that comparable stable isotope labeled standards for both glyphosate and AMPA are also commercially available through Cambridge Isotope Laboratories (Andover, MA, USA). The isotopic purity of both labeled reagents was at least 97%, which was adequate to minimize potential interference of the isotopic peaks at the levels measured in these methods. Reagents utilized were HPLC grade acetonitrile (Burdick & Jackson, Muskegon, MI, USA), 85% o-phosphoric acid (VWR, Radnor, PA, USA), 98% formic acid (Sigma-Aldrich), and HPLC grade methylene chloride (EMD Millipore, Billerica, MA, USA). Reagents used can be obtained from a variety of commercial vendors as long as they are of high enough quality for avoiding chromatographic interferences.
Matrices validated
Raw bovine milk was obtained from Genesis Midwest, LLC (Neillsville, WI, USA). Human milk pools were created using milk collected from healthy, lactating women living in the Pullman, WA/Moscow, ID area; all human milk collection procedures were approved by the Washington State University and University of Idaho Institutional Review Boards. Pooled normal human urine was obtained from Innovative Research (Novi, MI, USA). At least three unique pools of each matrix were used for method validation.
Preparation of analytical standards
Stock solutions of analytical reference standards of either glyphosate or AMPA were prepared in water at 1.0 mg/mL (adjusted for purity) and sonicated briefly to ensure complete dissolution. Stability for solutions ranging from 0.05 to 1000 µg/mL of glyphosate and AMPA prepared in water was established to be at least 23 months when stored at approximately 4°C. Mixed glyphosate and AMPA working calibration standard and fortification solutions were prepared by serial dilution of the stock solutions with 0.1% formic acid in water. The calibration standards prepared ranged from 0.0075 to 4.5 µg/mL (corresponding to a final matrix concentration of 5–3000 µg/L or ppb) for analysis of milk samples and from 0.0012 to 24 µg/mL (corresponding to a final matrix concentration of 0.05–1000 µg/L or ppb) for analysis of urine samples. Glyphosate and AMPA fortification solutions consisted of concentrations of 0.015, 0.0375 and 3.75 µg/mL used for milk samples and 0.0024, 0.048 and 19.2 µg/mL used for urine samples. The working internal standard solutions containing both internal standards (D2 13C15N-AMPA and13C3 15N-glyphosate) in 0.1% formic acid in water were prepared from 1.0 mg/mL stock solutions of each internal standard in water. The concentrations of each internal standard in the working internal standard solutions were 0.05 and 0.25 µg/mL for milk and urine analysis, respectively. Stability of the analytes in working solutions prepared in 0.1% formic acid in water and stored at approximately 4°C was demonstrated for at least 68 days.
Sample preparation
Sample processing
Milk and urine samples were stored at −20°C and thawed prior to use. In order to ensure representative subsamples, both urine and milk matrices were homogenized prior to dispensing by employing an ultrasonic water bath (Branson B-22-4 Ultrasonic Cleaner, Danbury, CT, USA) and applying gentle heating (e.g. 37–40°C).
Extraction of milk matrices
Once homogenized, 150 µL of milk was dispensed into a 1.4 mL polypropylene Matrix tube (Thermo Scientific, Waltham, MA, USA) and placed in the appropriate location of a 96-well plate. For tubes designated as standards, 150 µL of 0.1% formic acid in water was dispensed instead of matrix. The following solutions were then added to the appropriate sample types: 100 µL of 0.1% formic acid in water to test samples and controls, 100 µL of working calibration standard solutions to calibration standards, and 100 µL of fortification solutions to QC samples. Next, 650 µL of the working internal standard solution (0.05 µg/mL D2 13C15N-AMPA and13C3 15N-glyphosate) was added to each tube, including tubes designated for calibration standards. The 96-well plate was sealed with a cap mat (Sun-SRI, Rockwood, TN, USA) and then shaken on a Geno/Grinder 2000 (Spex Sample Prep, Metuchen, NJ, USA) at 1200 cycles per minute for 5 min. The plate was centrifuged using a Sorvall Legend XTR centrifuge (Thermo Scientific) at 6,000 × g for 5 min or until suspended materials were cleared from the liquid column. A 300 µL aliquot of each supernatant was transferred to a clean 96-well plate, being careful to avoid any lipid layer which may have formed on top of the liquid column, followed by addition of 300 µL of methylene chloride. The plate was again sealed with a cap mat, then shaken and centrifuged as above. Approximately 150 µL of the aqueous (top) layer was transferred to a clean 96-well plate for analysis by LC-MS/MS. Alternatively, if the lipid layer formed after the initial centrifugation presents a challenge, the method can be modified to include addition of the methylene chloride from the start.
Extraction of urine matrices
For urine samples, 600 µL was dispensed into each tube for matrix samples and 600 µL of 0.1% formic acid for tubes designated as standards. The following solutions were then added to the appropriate sample types: 25 µL of 1.3% formic acid in water to test samples and controls, 25 µL of working calibration standard solutions to calibration standards, and 25 µL of fortification solutions to QC samples. Finally, 25 µL of the working internal standard solution (0.25 µg/mL D2 13C15N-AMPA and13C3 15N-glyphosate) was added to each tube, including tubes designated for calibration standards. As with milk samples, the 96-well plate was sealed with a cap mat, shaken at 1200 cycles per minute for 5 min, and then centrifuged at 6,000 × g for 5 min or until suspended materials from the liquid column were cleared. Approximately 150 µL of the supernatant was transferred to a clean 96-well plate for analysis by LC-MS/MS.
Analytical methods
Instrumentation employed for the LC-MS/MS determination of glyphosate and AMPA consisted of a Shimadzu (Columbia, MD, USA) Prominence 20A HPLC system (including autosampler and column oven) coupled to a AB Sciex (Framingham, MA, USA) API 5500 triple-quadrupole mass spectrometer via a Turbo-V electrospray (ESI) source. Connections from injection valve to column and from column to mass spectrometer were made using PEEK tubing in order to reduce interaction of the analytes with metal surfaces. Individual methods were developed for detection of the analytes in milk and urine matrices, which employed slightly different conditions.
In both methods, separation of analytes was accomplished using a Bio-Rad (Hercules, CA, USA) Cation-H guard column (30 mm × 4.6 mm) maintained at 40°C. Mobile phases for both methods consisted of 0.1% formic acid in water as mobile phase A and acetonitrile as mobile phase B. The LC effluent was diverted from the mass spectrometer to waste for the first 1 min of the run to avoid fouling the source from non-retained components.
For analysis of milk samples, separation was performed isocratically using 80% mobile phase A and utilizing a flow ramp from an initial flow rate of 500–1000 µL/min from 0 to 4 min, and then held constant until 9 min. At this point, the LC effluent was again diverted to waste and the flow rate was decreased back to 500 µL/min over the course of 0.5 min while at the same time 0.1% phosphoric acid (mobile phase C) was introduced at a flow rate of 300 µL/min (for a total flow of 800 µL/min). These conditions were maintained from 9.5 to 11.5 min to aid in washing the column. The column was then returned to initial conditions and allowed to re-equilibrate for 2 min.
For urine analysis, a gradient separation was utilized, which started with 80% mobile phase A and increased to 100% A from 1.0 to 2.5 min. The same flow ramp from 500 to 1000 µL/min from 0 to 4 min was also utilized and was then held until 7 min, at which point it reverted to the 500 µL/min flow rate. At this time the LC effluent was again diverted to waste and an additional flow of 0.2% phosphoric acid (mobile phase C) was applied at a flow rate of 500 µL/min from 7 to 10 min to aid in washing the column. The column was then returned to initial conditions and allowed to re-equilibrate for 2.5 min.
Injection volumes were 5 μL for milk samples and 20 μL for urine samples. After each injection, the injector needle was washed using 0.2% phosphoric acid in water.
Detection and quantitation of glyphosate and AMPA were performed using multiple reaction monitoring via collision-induced dissociation, for which the fragmentation mechanisms and resulting ions have been previously elucidated. [12] The ion source was operated in negative ion mode with a spray voltage of −4,500 V and a temperature of 600°C. Nitrogen was used for all source and collision gases, which were set as follows (in arbitrary units): Curtain gas, 15; Collision gas, 6; Gas 1, 80; and Gas 2, 60. Two precursor-product ion transitions for each analyte and each stable label internal standard were monitored and used for quantitation to ensure the selectivity of the method. Each ion transition was carried out at unit resolution (Q1 and Q3) using scan times of 150 ms. Precursor/product ion transitions were as follows: glyphosate, m/z 168/63 (primary transition) and m/z 168/79 (secondary transition);13C3 15N-glyphosate, m/z 172/63 and m/z 172/79; AMPA, m/z 110/63 (primary transition) and m/z 110/79 (secondary transition); and D2 13C15N-AMPA, m/z 114/63 and m/z 114/79. Data were acquired and processed using AB Sciex Analyst software.
Results and discussion
Accuracy and fit of calibration curve
Linearity of the method was tested using analytical standard solutions of glyphosate and AMPA and employing stable isotope labeled internal standards. Calibration curves for milk analysis used ten standard levels covering a range from 0.0075 to 4.5 µg/mL (corresponding to final matrix concentrations of 5–3,000 µg/L) for both glyphosate and AMPA. A total of twelve standard levels ranging from 0.0012 to 24 µg/mL (corresponding to final matrix concentrations of 0.05–1000 µg/L) were used in the urine method. A linear fit with 1/x weighting provided coefficients of determination (R 2) values >0.999 for both transitions of glyphosate and AMPA in all matrices. All calibration curve points were within ±20% of their respective nominal concentrations.
Limit of quantitation and detection
The limit of quantitation (LOQ) for glyphosate and AMPA in each method was established through fortifications of matrix samples. Two ion transitions (precursor-to-product ions) were quantitated for both glyphosate and AMPA. The LOQ for each analyte (per matrix) was set as the lowest fortification concentration tested in which both ion transitions had mean accuracies between 70 and 120% and precision ≤20% relative standard deviation (RSD), based on at least five replicates. The raw bovine milk and human milk matrices obtained for validation did not contain any glyphosate or AMPA at the levels being evaluated. However, the urine samples purchased for method validation did contain trace amounts of the analytes of interest. Therefore, background subtraction was used in order to calculate accuracy of the fortifications when determining the LOQ. The LOQ was determined to be 10 μg/L (ppb) for both ion transitions of glyphosate and AMPA in milk matrices and 0.10 μg/L (ppb) for both ion transitions of glyphosate and AMPA in urine. Example chromatograms showing a typical response for the more sensitive primary ion transition for glyphosate and AMPA in a control sample and one fortified at the LOQ can be found in Figures 1 and 2 for human milk and urine, respectively. Improvements in the LOQ for urine were realized through enhancements in the chromatography method as well as the ability to limit the amount of dilution required during sample preparation. Milk required more dilution in order to prevent fouling of the HPLC column and maintain good recoveries, especially at the LOQ. Various sample cleanup techniques were tested for milk, such as heating, liquid-liquid partitioning with various solvents, and protein precipitation. It was determined that partitioning with methylene chloride afforded the best combination of glyphosate and AMPA recovery coupled with column longevity.
Figure 1.
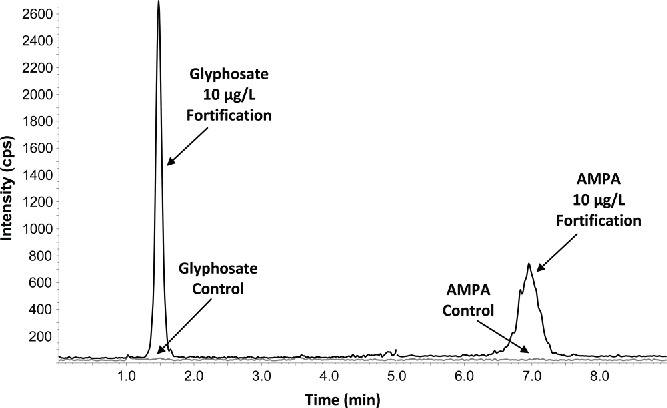
Representative chromatogram of a control human milk sample and subsequent fortification of glyphosate and AMPA at LOQ of 10 μg/L using the primary quantitation transitions.
Figure 2.
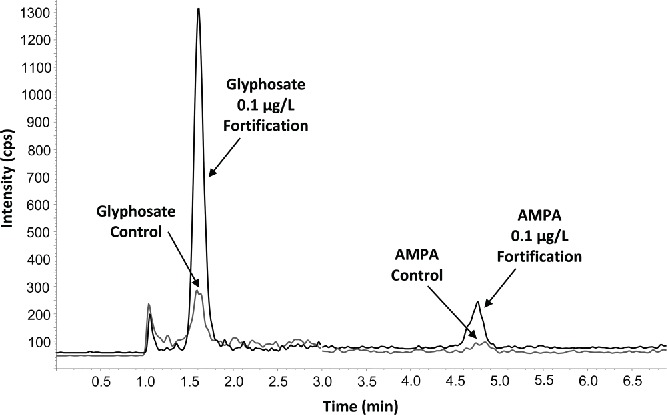
Representative chromatogram of a control human urine sample and subsequent fortification of glyphosate and AMPA at LOQ of 0.1 μg/L using the primary quantitation transitions.
The limit of detection (LOD) for each transition of glyphosate and AMPA for all matrices was estimated using the standard deviation (STDEV) of recovery measurements for replicate samples fortified at the LOQ and applying Equation 1.
| (1) |
In this equation, t 0.99 is the one-tailed t test value at the 99% confidence interval for N−1 degrees of freedom, where N is the number of replicates. The LOD and LOQ values for each matrix can be found in Table 1.
Table 1.
LOQ and LOD values for glyphosate and AMPA in milk and urine matrices.
| Matrix | Analyte (precursor/product ions) | LOQ (µg/L, ppb) | LOD (µg/L, ppb) |
|---|---|---|---|
| Bovine milk | Glyphosate primary (168/63) | 10 | 0.58 |
| Glyphosate secondary (168/79) | 10 | 1.9 | |
| AMPA primary (110/63) | 10 | 0.64 | |
| AMPA secondary (110/79) | 10 | 1.7 | |
| Human milk | Glyphosate primary (168/63) | 10 | 0.92 |
| Glyphosate secondary (168/79) | 10 | 2.8 | |
| AMPA primary (110/63) | 10 | 1.2 | |
| AMPA secondary (110/79) | 10 | 1.6 | |
| Human urine | Glyphosate primary (168/63) | 0.10 | 0.023 |
| Glyphosate secondary (168/79) | 0.10 | 0.038 | |
| AMPA primary (110/63) | 0.10 | 0.033 | |
| AMPA secondary (110/79) | 0.10 | 0.041 |
Accuracy and precision
Assessment of the accuracy and precision of each method was performed through calculation of recoveries for control matrix samples fortified at three different concentration levels, including the LOQ. Both bovine and human milk samples were fortified at 10, 25 and 2,500 μg/L (ppb), with five replicates at each level. Human urine was fortified at 0.1, 2 and 800 μg/L (ppb), with six replicates each. For all three matrix types evaluated, the average recovery values at each fortification level were within the range of 89–107% with precision ≤7.4% RSD for milk and ≤11.4% RSD for urine. A summary of the results can be found in Table 2.
Table 2.
Accuracy and precision results for glyphosate and AMPA in milk and urine matrices.
| Matrix | Analyte (precursor/product ion) (amu) | Fortification level (µg/L, ppb) | N | Individual recoveries (%) | Average recovery (%) | STDEV (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| Bovine milk | Glyphosate primary (168/63) | 10 | 5 | 95, 100, 102, 100, 98 | 99 | 2.5 | 2.5 |
| 25 | 5 | 98, 99, 102, 103, 94 | 99 | 3.5 | 3.6 | ||
| 2,500 | 5 | 97, 98, 97, 99, 98 | 98 | 0.8 | 0.8 | ||
| Glyphosate secondary (168/79) | 10 | 5 | 101, 108, 113, 105, 106 | 107 | 4.4 | 4.1 | |
| 25 | 5 | 95, 91, 92, 93, 101 | 94 | 4.1 | 4.4 | ||
| 2,500 | 5 | 101, 97, 106, 99, 105 | 102 | 3.7 | 3.7 | ||
| AMPA primary (110/63) | 10 | 5 | 94, 108, 98, 104, 98 | 101 | 5.4 | 5.4 | |
| 25 | 5 | 97, 85, 96, 101, 100 | 96 | 6.2 | 6.5 | ||
| 2,500 | 5 | 102, 100, 98, 102, 99 | 100 | 1.7 | 1.7 | ||
| AMPA secondary (110/79) | 10 | 5 | 106, 92, 102, 105, 103 | 102 | 5.5 | 5.4 | |
| 25 | 5 | 94, 89, 97, 103, 95 | 96 | 5.1 | 5.3 | ||
| 2,500 | 5 | 101, 99, 101, 104, 102 | 101 | 1.8 | 1.8 | ||
| Human milk | Glyphosate primary (168/63) | 10 | 5 | 100, 95, 100, 102, 99 | 99 | 2.4 | 2.5 |
| 25 | 5 | 100, 99, 96, 99, 98 | 98 | 1.5 | 1.5 | ||
| 2,500 | 5 | 100, 101, 97, 99, 99 | 99 | 1.4 | 1.5 | ||
| Glyphosate secondary (168/79) | 10 | 5 | 96, 109, 91, 105, 104 | 101 | 7.5 | 7.4 | |
| 25 | 5 | 95, 96, 104, 95, 99 | 98 | 3.9 | 3.9 | ||
| 2,500 | 5 | 97, 97, 98, 98, 99 | 98 | 0.7 | 0.7 | ||
| AMPA primary (110/63) | 10 | 5 | 96, 102, 99, 98, 104 | 100 | 3.2 | 3.2 | |
| 25 | 5 | 98, 93, 102, 98, 94 | 97 | 3.8 | 3.9 | ||
| 2,500 | 5 | 95, 96, 99, 96, 96 | 97 | 1.2 | 1.3 | ||
| AMPA secondary (110/79) | 10 | 5 | 103, 102, 99, 95, 106 | 101 | 4.4 | 4.4 | |
| 25 | 5 | 99, 99, 95, 92, 98 | 97 | 3.0 | 3.1 | ||
| 2,500 | 5 | 98, 100, 98, 103, 100 | 100 | 2.1 | 2.2 | ||
| Human urine | Glyphosate primary (168/63) | 0.10 | 6 | 95, 87, 80, 94, 99, 94 | 92 | 6.8 | 7.5 |
| 2 | 6 | 102, 99, 102, 105, 104, 101 | 102 | 2.0 | 2.0 | ||
| 800 | 6 | 93, 94, 94, 102, 96, 96 | 96 | 3.3 | 3.4 | ||
| Glyphosate secondary (168/79) | 0.10 | 6 | 83, 102, 83, 107, 103, 106 | 97 | 11.3 | 11.6 | |
| 2 | 6 | 102, 94, 98, 102, 102, 98 | 99 | 3.2 | 3.2 | ||
| 800 | 6 | 94, 93, 94, 99, 97, 100 | 96 | 3.0 | 3.0 | ||
| AMPA primary (110/63) | 0.10 | 6 | 92, 97, 79, 86, 101, 77 | 89 | 9.7 | 10.9 | |
| 2 | 6 | 101, 99, 99, 97, 97, 99 | 99 | 1.5 | 1.5 | ||
| 800 | 6 | 93, 94, 95, 97, 96, 95 | 95 | 1.5 | 1.6 | ||
| AMPA secondary (110/79) | 0.10 | 6 | 126, 98, 105, 110, 91, 113 | 107 | 12.2 | 11.4 | |
| 2 | 6 | 97, 97, 97, 96, 97, 94 | 96 | 1.0 | 1.0 | ||
| 800 | 6 | 92, 92, 93, 97, 94, 94 | 94 | 1.8 | 2.0 |
Selectivity determination
The selectivity of the method was demonstrated through the analysis of at least three independent sources of matrix determined to be free of both analytes. Both the primary and secondary quantitation ion transitions were used to determine the selectivity of each analyte. No significant interferences (>30% of the LOQ) were observed within the retention window for all glyphosate and AMPA transitions for all three matrices (see Figs. 1 and 2).
Ionization effects
Ionization effects were assessed by comparing the instrument response ratio of the analyte and its associated stable label internal standard in fortifications of diluent (no matrix extract) to fortifications of control matrix extracts. A total of three independent sources were tested for each matrix. No significant differences were observed between the fortified diluent and fortified control matrix extracts for glyphosate and AMPA. Control matrix fortifications were within 6.5% of the diluent spiked samples for both the primary and secondary ion transitions of glyphosate and AMPA across all matrices tested, indicating the methods do not contain significant bias from ionization effects. Since no significant ionization effects or interferences were observed in these matrices it was determined that the use of matrix matched standards is not required for accurate quantitation of glyphosate and AMPA.
Carryover
Carryover was evaluated by placing an appropriate diluent blank directly after the highest concentration calibration standard. All analyte responses (including primary and secondary ion transitions) in the carryover sample were below the detection limit.
Stability of processed samples
In order to establish a period of time for which samples that have been extracted and prepared for analysis may be stored without affecting the accuracy of the determination, a processed set of fortification samples and calibration standards were analyzed, stored at 4°C for several days, and then re-injected. Stability was ascertained by demonstrating that the fortification sample results still met the criteria for accuracy and precision. The processed sample stability was determined to be a minimum of 6, 8 and 10 days for bovine milk, human urine and human milk, respectively.
Transfer and validation by a secondary lab
The method for analysis of glyphosate and AMPA in milk matrices was provided to Covance Laboratories Inc. (Madison, WI, USA) for verification of the transferability of the method and to perform extended stability testing. The Covance facility used similar equipment including an AB Sciex QTrap 5500 triple-quadrupole mass spectrometer for detection. Due to differences in instrumentation, the method at Covance attained a slightly higher LOQ of 25 μg/L (ppb) for both transitions of glyphosate and AMPA in human and bovine milk. Using the same equation as earlier (Equation 1), the LOD for the more sensitive, primary ion transition of glyphosate and AMPA were estimated to be 6 and 9 μg/L (ppb), respectively. Six replicates of three fortification levels, including the LOQ, for both bovine and human milk were analyzed in each of three separately prepared analytical batches. The resulting inter-assay mean accuracies at each fortification level across both matrices fell between 88 and 99% including both transitions of glyphosate and AMPA, with RSD values ≤10.4%. Additionally, there were no interferences, carryover or matrix effects observed for either analyte.
Matrix stability
Stability of the analytes in matrix at various storage conditions was also examined at Covance Laboratories. Aliquots of human milk fortified at 25 µg/L (LOQ) and 2,500 µg/L were stored in either a refrigerator held at approximately 5°C or a freezer maintained at approximately −20°C. Samples maintained in the refrigerator had acceptable recoveries for both analytes after 24 h of storage. Acceptable recoveries were also obtained for samples stored in the freezer for at least 3 months and for aliquots that underwent three freeze/thaw cycles.
Conclusion
Sensitive and selective LC-MS/MS methods for the determination of glyphosate and AMPA in bovine milk, human milk and human urine matrices were developed. Both methods are similar in nature requiring simplified extraction procedures that can be prepared and analyzed in a high-throughput 96-well plate format. The milk method requires an additional cleanup step to sequester and minimize matrix components that can reduce the longevity of the analytical column. Chromatography utilizes a cost-effective Bio-Rad Cation-H guard column where extracts are analyzed directly without the need for derivatization.
The use of two independent quantitation ion transitions for both glyphosate and AMPA ensures the accuracy and provides confirmation of any measured result. Validated recoveries for milk matrices (human and bovine) were within 88–114% with RSD for replicate values ≤7.4% at fortification levels of 10, 25 and 2,500 μg/L (ppb). Recoveries for human urine were between 82 and 127% with RSD values ≤11.4% for replicate fortifications at 0.1, 2 and 800 μg/L (ppb). Due to the presence of trace levels of glyphosate in some human urine samples, background subtraction was employed to adjust recoveries to better assess the accuracy of the procedure. All human and bovine milk samples used in this study were completely free of any detectable levels of glyphosate and AMPA. Assessment of ionization effects in all matrices showed no significant biases present within the procedures to impact the accuracy of measured results.
Transferability of the procedures was demonstrated for the more complicated bovine and human milk matrices. Differences in the sensitivity of the instrumentation used at the second laboratory resulted in a slight increase in the LOQ from 10 to 25 μg/L (ppb). Recoveries and precision were similar to the originating validation over the course of three separate batch runs. Additional stability assessments show analytes are stable in milk for over 24 h at 5°C, 3 months at −20°C and three freeze thaw cycles.
Acknowledgments
The authors would like to thank Albert White, Xiaohong Feng and Hongyue Guo at Monsanto Company for method development and validation work. The authors would also like to acknowledge Covance Laboratories Inc., Madison, WI, USA for the independent laboratory validation and matrix stability work.
References
- Franz J.E., Mao M.K., Sikorski J.A. Glyphosate: A unique global herbicide. American Chemical Society: Washington, DC; [Google Scholar]
- Dill G.M., CaJacob C.A., Padgette S.R. Glyphosate-resistant crops: Adoption, use and future considerations. Pest Manag. Sci. 2008;(4):326–331. doi: 10.1002/ps.1501. [DOI] [PubMed] [Google Scholar]
- Duke S.O., Powles S.B. Glyphosate. A once-in-a-century herbicide. Pest Manag. Sci. 2008;(4):319–325. doi: 10.1002/ps.1518. [DOI] [PubMed] [Google Scholar]
- Duke S.O. Glyphosate degradation in glyphosate-resistant and-susceptible crops and weeds. J. Agr. Food Chem. 2010;(11):5835–5841. doi: 10.1021/jf102704x. [DOI] [PubMed] [Google Scholar]
- Hori Y., Fujisawa M., Shimada K., Hirose Y. Determination of the herbicide glyphosate and its metabolite in biological specimens by gas chromatography-mass spectrometry. A case of poisoning by Roundup® herbicide. J. Anal. Toxicol. 2003;(3):162–166. doi: 10.1093/jat/27.3.162. [DOI] [PubMed] [Google Scholar]
- Motojyuku M., Saito T., Akieda K., Otsuka H., Yamamoto I., Inokuchi S. Determination of glyphosate, glyphosate metabolites, and glufosinate in human serum by gas chromatography–mass spectrometry. J. Chromatogr. B. 2008;(2):509–514. doi: 10.1016/j.jchromb.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Saito T., Miura N., Namera A., Oikawa H., Miyazaki S., Nakamoto A., Inokuchi S. Mixed-mode C–C18 monolithic spin-column extraction and GC-MS for simultaneous assay of organophosphorus compounds, glyphosate, and glufosinate in human serum and urine. Forensic Toxicol. 2012;(1):1–10. [Google Scholar]
- Zouaoui K., Dulaurent S., Gaulier J., Moesch C., Lachâtre G. Determination of glyphosate and AMPA in blood and urine from humans: About 13 cases of acute intoxication. Forensic Sci. Int. 2013;(1):e20–e25. doi: 10.1016/j.forsciint.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Yoshioka N., Asano M., Kuse A., Mitsuhashi T., Nagasaki Y., Ueno Y. Rapid determination of glyphosate, glufosinate, bialaphos, and their major metabolites in serum by liquid chromatography–tandem mass spectrometry using hydrophilic interaction chromatography. J. Chromatogr. A. 2011;(23):3675–3680. doi: 10.1016/j.chroma.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Botero-Coy A.M., Ibáñez M., Sancho J., Hernández F. Direct liquid chromatography–tandem mass spectrometry determination of underivatized glyphosate in rice, maize and soybean. J. Chromatogr. A. 2013:157–165. doi: 10.1016/j.chroma.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Nagatomi Y., Yoshioka T., Yanagisawa M., Uyama A., Mochizuki N. Simultaneous LC-MS/MS analysis of glyphosate, glufosinate, and their metabolic products in beer, barley tea, and their ingredients. Biosci. Biotechnol. Biochem. 2013;(11):2218–2221. doi: 10.1271/bbb.130433. [DOI] [PubMed] [Google Scholar]
- Goodwin L., Startin J.R., Goodall D.M., Keely B.J. Negative ion electrospray mass spectrometry of aminomethylphosphonic acid and glyphosate: Elucidation of fragmentation mechanisms by multistage mass spectrometry incorporating in-source deuterium labelling. Rapid Commun. Mass Spectrom. 2004;(1):37–43. doi: 10.1002/rcm.1264. [DOI] [PubMed] [Google Scholar]


