Abstract
The retinal circulation is a potential marker of cerebral vascular disease because it shares origin and drainage with the intracranial circulation and because it can be directly visualized using ophthalmoscopy. Cross sectional and cohort studies have demonstrated associations between chronic retinal and cerebral vascular disease, acute retinal and cerebral vascular disease and chronic retinal vascular disease and acute cerebral vascular disease. In particular, certain qualitative features of retinopathy, retinal artery occlusion and increased retinal vein caliber are associated with concurrent and future cerebrovascular events. These associations persist after accounting for confounding variables known to be disease-causing in both circulations, which supports the potential use of retinal vasculature findings to stratify individuals with regards to cerebral vascular disease risk.
Keywords: arteries, veins, retina, stroke
Introduction
The retinal circulation is of significant interest as a marker of vascular disease because its arterioles and venules (henceforth referred to as microvasculature) can be directly visualized via ophthalmoscopy (fig. 1). Due to its shared origin and drainage with the cerebrovascular circulation, it is of particular interest as a marker cerebral vascular disease.(1) The retinal arterioles and venules that course across the inner retinal surface to form this visible vascular network originate from the central retinal artery and drain to the central retinal vein. These central retinal vessels enter and leave the eye through the center of the optic nerve. The central retinal artery originates from the internal carotid artery via the ophthalmic artery and the central retinal vein drains either directly or indirectly via the superior ophthalmic vein to the cavernous sinus. The retinal circulation carries a minority of the blood supply to the eye and is responsible for carrying nutrients and oxygen to, while removing waste and carbon dioxide from, the inner retina which includes the retinal ganglion cells that comprise the optic nerve. The fractional oxygen extraction in the retinal circulation is high. In contrast, the majority of the blood supply to the retina is carried via the posterior ciliary arteries to the choroidal circulation, which supplies the retinal pigment epithelium and outer sensory retina. It has a comparatively low oxygen extraction fraction. The retinal circulation is unique compared to other vascular beds in the human body in that it does not have autonomic innervation, and therefore is controlled by local and circulating factors such as local metabolic demands, blood pressure, oxygen and carbon dioxide levels.(2)
Figure 1.
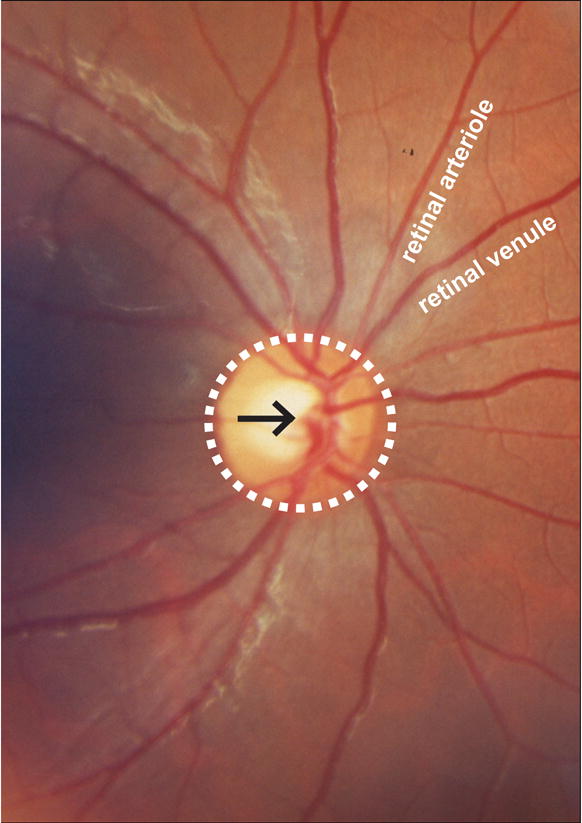
Photographic image of the normal retinal circulation. Central retinal artery and vein (black arrow) pass through the optic nerve head (white dashed circle) and branch sequentially to supply (retinal arterioles) and drain (retinal venules) the inner retinal surface.
The purpose of this manuscript is to review the literature regarding retinal vessel abnormalities as a marker of cerebral vascular disease. A distinction is made between acute disease, characterized by, typically symptomatic, discrete events such as retinal vessel occlusion or cerebral ischemic stroke, and chronic disease, characterized by, typically asymptomatic, progressive features such as vascular retinopathy or cerebral white matter changes attributed to ischemia. Because common variables of demographics, medical history and vital signs may confound such associations, this review preferentially presents data of associations that have been adjusted for such confounders, as these best indicate what additional information can be gleaned from analysis of the retinal circulation.(1) Much of the literature comes from population based cohort and cross-sectional studies. These are summarized in table 1, which is referenced throughout the text.
Table 1.
Large studies of retinal and cerebral vascular disease
| study | location | inclusion criteria | race | year of retina imaging | no. subjects with retina imaging | follow up post retina imaging (years) |
|---|---|---|---|---|---|---|
| Cohort | ||||||
| Beaver Dam Eye Study | USA | 43–84 yrs | white | 1988–90 | 4926 | 5, 10 |
| Rotterdam Study | Netherlands | ≥ 55 yrs | NR | 1990–93 | 6780** | 11.5 |
| Blue Mountain Eye Study | Australia | ≥ 49 yrs | NR | 1992–94 | 3654 | 5 |
| Atherosclerosis Risk in Communities | USA | 45–64 yrs | mixed | 1993–95* | 10358** | 3.5,11 |
| Cardiovascular Health Study | USA | ≥ 65 yrs | mixed | 1997–98* | 5888** | 5 |
| Singapore Malay Eye Study | Singapore | 40–80 | Malay | 2004–06 | 3280 | 3–5 |
| Cross Sectional | ||||||
| Multicenter Retina Stroke Study | Australia, Singapore | acute stroke | mixed | 2005–07 | 1565 | N/A |
NR not reported,
cohort study initiation preceded retinal imaging substudy,
retinal imaging study was performed on a subpopulation of a larger study
Methods of retinal vessel analysis
The unique visibility of the retinal vascular bed facilitates its study in human disease. The most straightforward analysis of the retinal vasculature is qualitative pattern analysis, similar to that used in clinical ophthalmic care (Fig 2). One common pattern is diabetic retinopathy, which, in the non-proliferative version, is characterized by visible micro aneurysms and flame or blot hemorrhages. Proliferative diabetic retinopathy is characterized by growth of new vessels and retinal tissue edema. A second common pattern is hypertensive retinopathy which is characterized by arteriole narrowing, changes in the venule caliber and path at points where the arterioles cross (AV nicking) and changes in the arteriolar central light reflex (copper wire and silver wire arterioles). Advanced hypertensive retinopathy is associated with findings similar to non-proliferative diabetic retinopathy as well as focal retinal ischemia, which causes areas of retinal whitening (cotton wool spots). Retinal arteriole and retinal venule occlusions can be diagnosed from visual inspection of the fundus based on downstream retinal whitening due to decreased perfusion and upstream hemorrhages due to high venous pressures respectively. The occlusions can also be visualized as truncated vessels using fluorescein angiography, in which the retinal vessels and retinal perfusion are visualized using an intravenously delivered dye. A challenge in using these descriptive findings in a research setting is inter-observer variability, which can be substantial, even when using high quality photographs.(3)
Figure 2.
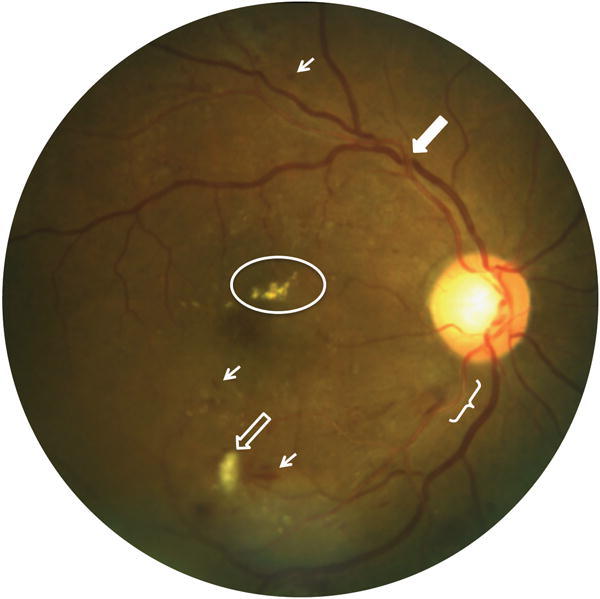
Photographic image of qualitative retinal circulation abnormalities characteristic of diabetic and hypertensive retinopathy. Bracket shows enhanced central arteriolar light reflex (copper wiring). Hollow arrow shows cotton wool spot, indicative of retinal infarct. Solid arrow shows focal narrowing of the venule at the site of arteriolar crossing (AV nicking). Oval shows hard exudates due to lipid deposition. Small arrows show retinal hemorrhages.
Quantitative methods of examination of the retinal vascular system offer better reliability. These can be applied to static images acquired using conventional ophthalmoscopic photography as well as other modern imaging techniques such as scanning laser ophthalmoscopy (SLO), which provides an en face image similar to a photograph, or optical coherence tomography (OCT), which provides cross sectional images. Manual and semi-automated analyses can be applied to static images to quantify arteriole and venule diameters, as well as the ratio between them. En face images also permit analysis of vessel tortuosity,(4) fractal dimension and branching angles.(5) Focal thinning of retinal layers, as can be measured with OCT, may be a marker of prior vascular events causing ischemia. These analysis methods require high quality images and specialized analysis capabilities, which may not be available in the clinical setting.
In contrast to retinal vessel assessments at a single point in time, video fundoscopic imaging allows measurement of retinal vessel changes, both spontaneous(6) and provoked(7). For example, the latency and magnitude of retinal vessel diameter changes occurring during light flicker stimulation of the retina captures effects of changing local metabolic demands,(8) and may be a marker of endothelial function.(9) Changes in retinal vessel size following systemic perturbations such as exercise,(10) hypoxia and hypercapnea have also been used to study retinal vessel autoregulation in response to metabolic changes. Oximetry quantifies oxygen saturation of the blood in the retinal vessels based on reflection of different wavelengths of light.(11) Various technologies have been used to quantify blood flow in the retinal circulation including Doppler OCT(12) and laser speckle flowgraph.(13) These techniques are currently limited to the research sphere and most have not yet been applied to study of retinal and cerebral vascular disease associations.
Relationship between chronic retinal & cerebral microvascular disease
Both the retina and brain manifest microvascular disease in the setting of vascular risk factors such as diabetes and hypertension. This disease can be chronic or acute. In the retina, chronic disease takes the form of the features that characterize diabetic and hypertensive retinopathy (see above), and these can be directly visualized on the ophthalmoscopic exam or images. In the brain chronic disease takes the form of small vessel ischemic disease that is studied in vivo using white matter changes visualized using magnetic resonance imaging (MRI). White matter changes are typically studied because there is no convenient MRI correlate of focal chronic ischemic pathology in the cerebral grey matter. Grey matter atrophy is a non-specific finding that was not associated retinal vessel changes in a single study.(14)
MRI measures of cerebral white matter disease are associated with categorical features of retinal microvascular disease in both cross sectional and longitudinal study designs. In a cross sectional study of 179 patients with known cerebrovascular, cardiovascular or peripheral arterial disease, 108 (60%) had cerebral small vessel disease as assessed by white matter changes on MRI and 154 (86%) had retinal artery abnormalities with one or more of focal arteriolar narrowing, altered AV crossings, arteriolar sclerosis, or vessel tortuosity on retinal photographs as evaluated by expert clinicians.(3) Retinal vessel abnormalities were more frequent in those with MRI changes (92%) than those without (77%). Similar observations were made in a sub-study of the Atherosclerosis Risk in Communities (ARIC) cohort study (Table 1).(15) 1684 participants had both brain MRI and retinal photography. White matter lessions were associated with any retinopathy as well as individual features of micro aneurysm, exudate, hemorrhage (OR 2.5, 95% CI 1.5–4.0), AV nicking (OR 2.1, 95% CI 1.4–3.2), and focal arteriolar narrowing (OR 2.1, 95% CI 1.4–3.1). For brain white matter lesions felt to represent MRI defined stroke, retinal features of AV nicking focal arteriolar narrowing and hemorrhage were associated with the outcome only in persons without hypertension.(16) A follow up study incorporated MRI results 10 years later in 830 subjects. They found associations between increasing volume of white matter disease and any baseline retinopathy, AV nicking, focal arteriolar narrowing and retinal hemorrhage, but not micro aneurysms.(17) The association of retinal and brain microvascular changes after adjusting for vascular risk factors suggests that there may be a sub-population that is more susceptible to microvascular disease and that retinal vessel analysis may identify such individuals. Some authors have hypothesized that there is a continuum of disease with retinal blood vessel changes occurring earlier than MRI cerebral tissue changes in individuals with systemic vascular disease.(3)
Similar associations between chronic retina and brain microvascular changes have been observed in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), an inherited arteriopathy caused by mutations in the Notch 3 gene. Liu et al reported diffuse retinal arteriolar narrowing, defined based on caliber in relation to venules and arteriolar wall reflection, in 15 of 15 patients with CADASIL compared with 2 of 15 age matched control subjects. The presence of retinal arteriolar narrowing was significantly correlated with degree of white matter abnormalities in these CADASIL patients.(18) Cavallari et al reported decreased retinal vessel fractal dimension (i.e. less complex vessel branching patterns) in 10 CADASIL patients compared to 10 aged matched controls.(19) These reports suggest that retinal vessels can be affected in, and therefore may have a role as a marker for, inherited neurovascular diseases.
Two cross sectional studies of chronic retinal vascular disease and cerebral macrovascular disease (i.e. narrowing of large arteries) have moved beyond vascular structure assessments to assess vascular ability to provide blood flow. Since flow is difficult to measure non-invasively surrogate measurements are used. Elevated blood velocity, assessed using Doppler ultrasound, is an indicator of vessel narrowing and suggests limitations in blood flow. Blood vessel reactivity assesses vessel diameter change directly or indirectly based on blood velocity measurement, following a metabolic stimulus and thereby assesses a blood vessel’s ability to dilate to meet to demand for increased blood flow. Intracranial large artery disease (i.e. macrovascular disease) was assessed using transcranial Doppler ultrasonography in 499 acute stroke patients as part of the Multicenter Retina Stroke (MCRS) study (Table 1). Severe intracranial arterial disease, defined by increased flow velocity in the middle cerebral, anterior cerebral or internal carotid arteries, was associated with enhanced retinal arteriolar central light reflex (OR 1.83, 95% CI 1.97–3.16), but not focal arteriolar narrowing or AV nicking.(20) There is a possibility that this observed association between retinal microvascular structural disease and cerebral macrovascular functional limitations, may be causal (i.e. large artery narrowing contributing to retinal vascular disease by limited ocular blood supply).
Another small study directly compared microvascular function in the retina with macrovascular function in the brain.(21) The investigators measured retinal vascular reactivity by assessing change in retinal vessel diameter before and during light flicker stimulation. They measured cerebrovascular reactivity with transcranial Doppler measurement of middle cerebral artery blood velocity during breathholding to induce hypercarbia, a known stimulant of vascular muscular relaxation. 12 subjects with cerebral microvascular disease, as assessed by white matter changes on MRI, were compared with 14 control subjects. The white matter disease group had larger retinal veins, impaired retinal arteriole reactivity, impaired retinal venule reactivity and impaired cerebral macrovascular reactivity compared with control subjects. Furthermore, cerebral macrovascular reactivity was correlated with retinal microvascular reactivity (Pearson correlation coefficient, r=0.45), and retinal arteriole:venule diameter ratio (r=0.69). Further study is necessary to determine if assessments of retinal vasculature reactivity, which can be measured with commercially available devices, have a role in screening for or risk stratifying cerebral vascular disease.
Relationship between acute retinal & cerebral vascular events
Both the retina and brain manifest vascular disease in the form of acute events. In the retina this takes the form of central or branch, retinal artery or retinal vein occlusions (RAO, RVO). In the brain this takes the form of strokes, either ischemic or hemorrhagic. Associations between acute retinal events and acute cerebral vascular disease have clinical and epidemiological implications with regards to optimizing clinical care with the aim of reducing the substantial morbidity and mortality of stroke.
There is cross-sectional evidence to support that retinal emboli are often associated with simultaneous asymptomatic cerebral ischemia. In patients with acute symptomatic retinal emboli, one study demonstrated MRI findings of acute cerebral infarct (i.e. focal diffusion restriction) in 17of 55 (31%) of patients with acute retinal artery occlusion (RAO) on fluorescein angiography.(22) In a separate prospective study, 8/33 (24%) of patients with acute symptomatic RAO had focal diffusion restriction on brain MRI.(23) These studies suggest that simultaneous emboli to retinal and brain circulations are common, most likely due to a shared cardiac or large vessel source. They demonstrate that subclinical brain ischemia is common in the setting of acute retinal ischemia and justify managing acute or transient retinal ischemia as one would a cerebral stroke or transient ischemic attack. This recommendation is included in current stroke and transient ischemic attack guidelines, which recommend brain imaging and hospitalization for high risk patients presenting with acute retinal ischemia due to the high short term risk of cerebral infarct and need for prompt identification of modifiable risk factors for future stroke.(24) (28)
Further reinforcement of retinal and brain ischemia sharing a common etiology in at risk patients is found in studies of populations at high risk of cardio-embolic disease. In a longitudinal study of 87,177 individuals with non-valvular atrial fibrillation with 285,547 person years of follow up, RAO and RVO were more common in individuals known to be at higher risk of embolism based on risk stratification scores. They remained independently associated with the combined outcome of cerebral or peripheral embolism after controlling for other risk factors (HR 1.38, 95% CI 1.08–1.79 RAO, HR 1.25, 95% CI 1.02–1.54 RVO).(25) Thus RAO and RVO may be sentinel ischemic events that may further risk stratify individuals already known to be at risk for embolic events.
Other studies support retinal arterial events as risk factors for future stroke in a broader population. In the Beaver Dam Eye Study (BDES) (Table 1), retinal emboli had a significantly elevated hazard ratio for stroke related death after adjusting for confounding variables (HR 2.61, 95% CI 1.12–6.08).(26) A distinction was not made between ischemic and hemorrhagic stroke. A study of 350 patients in the Taiwan National Health Insurance Database with RVO did not find increased risk of stroke after adjustment for covariates.(27)
Shared pathophysiology consisting of anterior circulation embolic events, offers an explanation for the observed relationships between retinal arterial emboli and concurrent and future cerebral ischemia. The associations between RVO and stroke and between retinal events and hemorrhagic stroke are less certain both epidemiologically and pathophysiologically. Though guidelines advise emergent management in cases of acute retinal arterial events, the degree to which testing or intervention prompted by retinal events alters future cerebral vascular disease incidence, morbidity or mortality has not been established and needs further study.
Relationship between chronic retinal microvascular disease and acute cerebral vascular events
Chronic retinal vascular changes may be risk factors for stroke. As they are more prevalent than the acute retinal events discussed above, this relationship has received a lot of attention in large cohort studies (Table 1). The discussion below is divided by type of retinal marker studied: qualitative, retinal vessel caliber, and other quantitative markers.
Qualitative retinal vascular markers
In the Atherosclerosis Risk in Communities (ARIC) study, retinopathy (micro aneurysms or hemorrhages) and AV nicking at baseline were associated with incident ischemic strokes during 3.5 years of follow up.(29) The combination of qualitative retinal markers and brain white matter disease on MRI had a stronger association with incident stroke than either alone (RR 19.8, 95% CI 6.4–61.3 for both vs. 2.7 (0.9–7.9) for cerebral white matter lesions and 4.0 (1.3–12.2) for retinopathy).(15) Moderate or severe hypertensive retinopathy at baseline was associated with incident stroke including in those whose blood pressure was well controlled (HR 2.37, 95% CI 1.39–4.02).(30) In the Blue Mountain Eye Study (BMES), retinopathy at baseline in non-diabetics was associated with both stroke death and incident stroke, moderate retinal AV nicking at baseline was associated with stroke death, and focal retinal arteriolar narrowing at baseline was associated with incident stroke or transient ischemic attack during 5 years of follow up (RR range 1.6 – 3.5).(31) Ischemic and hemorrhagic events were not distinguished from each other. The Singapore Malay Eye Study (SMES) also reported as association between baseline retinopathy and incident stroke during 4 years of follow up and developed models that improved upon those based on vascular risk factors by incorporating retinal vascular parameters.(5) These models improved classification of stroke outcome by 10%.
Analyses of stroke subtypes have attempted to link retinal pathology with specific stroke pathology and this has been most illuminating for lacunar ischemic stroke. In 11 year follow up data from ARIC, focal retinal arterial narrowing and AV nicking were associated with incident lacunar stroke, while retinopathy was associated with incident thrombotic and cardio-embolic infarcts but not lacunar infarcts.(32) Kwa et al reported an association between retinal exudates and lacunar infarcts, but not cerebral white matter disease on MRI.(3) The Multicenter retina stroke (MCRS) study reported focal arteriolar narrowing, AV nicking and enhanced arteriolar light reflex to be associated with concurrent lacunar infarct in non-diabetics.(33) It has been proposed that these data support similar pathophysiology underlying retinal vascular disease and the cerebral arteriolar disease that leads to lacunar infarcts, with similar pathophysiology leading to vascular wall changes in the retina, manifesting as nicking and light reflex changes, and in the brain, manifesting as lacunar infarcts.
Many of the cohort studies either have not distinguished between hemorrhagic and ischemic cerebral events, or do not report subgroup data for hemorrhagic cerebral events. This may relate to the relatively small incidence of hemorrhagic versus ischemic strokes in these studies. In the MCRS study, which is cross sectional in design, deep intracerebral hemorrhage (ICH) was compared with ischemic stroke. Severe AV nicking and severe focal arteriolar narrowing were associated with deep ICH.(34) Retinal micro-aneurysms, hemorrhages and cotton wool spots were associated with lobar ICH as opposed to deep ICH or cerebral infarct in non-diabetics.(35) The authors proposed that the differences in arteriolar wall findings, namely AV nicking and arteriolar narrowing, in the retina of patients with deep ICH, versus retinal microaneurysms, hemorrhages and cotton wool spots in lobar ICH and cerebral infarct reflect the different cerebral vascular pathologies that underlie these distinct cerebral vascular events.
Retinal vessel diameter as a marker
Of the quantitative retinal vascular markers, retinal artery and vein diameter are the most widely studied. One challenge in measuring this marker is calibration of retinal photographs. This was initially addressed through normalizing artery to vein diameters (AVR) or arterial length to diameter (LDR) to obtain dimensionless ratios. Initial studies using this methodology including 3 year ARIC follow up and BDES either did not find associations or found attenuated association between stroke and AVR or artery LDR.(29, 36) Subsequently, retinal vessel diameter measurements have been normalized to the optic nerve head diameter, which allowed separate analysis of artery and vein dimensions and revealed positive associations as discussed below. Another challenge in the population study of retinal vascular dimensions is variations in retinal vascular anatomy between individuals. This has been surmounted through calculation of index artery and vein diameter ratios, typically the central retinal artery and vein equivalents (CRAE, CRVE). These are calculated for each eye based on diameters of vessels surrounding the optic disc.
Multiple cohort studies have examined the association between baseline retinal vessel diameters and future stroke, with the most consistent result being an association with larger retinal vein diameters and future stroke. The Cardiovascular Health Study (CHS) reported an association between largest retinal vein quartile vs. smallest retinal vein quartile and stroke, but not between artery diameter and stroke.(37) Similarly, the Rotterdam study found associations between vein diameter and any stroke or ischemic stroke, but not retinal artery diameter and stroke.(38) A separate analysis of the Rotterdam cohort, with longer term follow up, confirmed the association of larger retinal vein diameter with stroke. They also reported a trend for increasing risk of ICH with narrower retinal artery caliber and an association between wider retinal veins and lobar hemorrhage, but not deep hemorrhage.(39) The ARIC study reported retinal artery diameter to be inversely associated with risk of ischemic stroke and retinal vein diameter to be directly associated with lacunar stroke.(32) The SMES found retinal vein diameter to be associated with incident stroke.(5) Some of the variability in findings has been attributed to age and race/ethnicity differences in the study populations.
A Meta-analysis of individuals without diabetes in Australian Diabetes, Obesity and Lifestyle study, BDES, BMES, ARIC study, CHS and Rotterdam study evaluated the clinical impact of retinal vessel changes on stroke risk category. Based on this large analysis of 20,798 persons with 945 incident strokes, both infarct & hemorrhagic, they found an elevated pooled hazard ratio for a 20 micron increase in retinal vein diameter and a non-significant hazard ratio for 20 micron decrease in retinal arterial diameter. 10% of individuals on the borderline of traditionally defined stroke risk categories were reclassified up or down categorically with the additional information of retinal vessel caliber. However, changes in individual risk on a continuous scale were small.(40)
Cross-sectional studies, the most prominent being the MCRS study, have increased the sample size of stroke subjects compared to those in cohort studies by studying the stroke population directly. They reported AVR to be associated with lacunar infarct, and retinal vein widening to be associated with lacunar infarct in non-diabetics. Retinal artery narrowing was not significant.(33) In a substudy of individuals with carotid ultrasound assessement of extracranial large vessel disease, retinal vein diameter was larger ipsilateral to carotid stenosis > 75%. This association held in the subset of patients in whom the carotid stenosis led to cerebral stroke and suggests that asymmetric retinal vein widening may be a marker of internal carotid artery stenosis, with its associated stroke risk.(41) Another MCRS study of deep ICH found narrower retinal artery diameters and wider vein diameters than in non-lacunar ischemic stroke.(34) A different cross sectional study of 557 ischemic stroke patients and an equal number of demographically matched controls found that stroke patients were more likely to have decreased retinal artery diameter and increased retinal vein diameter. In analysis of stroke subtypes retinal vein diameter was not different between subjects with cardioembolic stroke and controls.(42)
Other quantitative retinal vascular markers
Semi-automated image analysis techniques have been leveraged to quantify other aspects of retinal vasculature anatomy. Tortuosity quantifies the frequency with which a vessel crosses a low dimensional spline. Branching, also called optimality, measures the extent to which the vessel bifurcations deviate from what theoretically optimizes blood flow. Fractal dimension quantifies the complexity of the vascular branching pattern.
Cohort studies have shown variable associations between baseline retinal vascular markers and stroke incidence. In the BDES, branching and tortuosity were not associated with stroke or ischemic heart disease deaths in 10 years of follow up.(36) Similarly, the SMES found neither tortuosity, branching nor fractal dimension to be associated with stroke incidence over 4 years of follow up.(5) However, in a case-control analysis of the BMES, decreased fractal dimension was associated with incident stroke over 5 years of follow up.(43)
Cross sectional studies have been more fruitful. A cross sectional study of 557 ischemic stroke patients and an equal number of demographically matched controls found that stroke patients were more likely to have decreased retinal artery and vein fractal dimension and increased retinal artery and vein tortuosity. Branching angles were not different. The relationships persisted in analysis of stroke subtypes.(42) Another study with 166 patients found decreased retinal fractal dimension to be associated with lacunar cerebral infarct.(44) The MCRS confirmed this observation by finding retinal fractal dimension to be inversely associated with lacunar cerebral infarcts, but not with large artery or cardio embolic cerebral infarcts.(45) Though these results are intriguing, the fact that the associations were not present in cohort studies limits their potential application as markers of future stroke risk for use in primary prevention efforts. Their role in predicting stroke outcome or secondary stroke risk is not known.
A recent study used OCT to evaluate for stigmata of prior retinal ischemic events using combined cross-sectional and cohort methodologies. Focal retinal nerve fiber layer defects, thought to represent prior ischemic insults to the retina, were more common in both cross sectional and cohort stroke groups versus a cohort derived control group.(46) This may be a surrogate marker for prior RAO, though guidelines do not currently recommend secondary stroke prevention on this basis.
Future Directions
In the study of retinal vascular and cerebral vascular disease associations, most of the retinal analysis has been based on static images, which is appropriate, since these can be practically obtained and analyzed asynchronously in large sample sizes. Most of the cerebral analysis has been based on clinical events or static MRI. Application of modern retinal imaging techniques to obtain dynamic measurements of retinal vessel diameter, retinal blood flow and retinal oxygen saturation and dynamic cerebral imaging techniques have the potential to allow more detailed study of physiological and pathophysiological associations of these vascular beds. While the technical nature of such studies currently precludes use in large population studies, advances in technology will improve the feasibility of wide scale measurements of these kinds.
Both cross sectional and cohort study designs have provided intriguing insight into relationships between retinal and cerebral vascular disease. Important questions remain regarding the clinical implications of these observations. This needs to go beyond identifying individuals at higher risk of concurrent and future events to studying interventions in order to identify those that improve cerebral vascular morbidity and mortality.
Conclusions
Retinopathy findings, particularly in non-diabetics and non-hypertensive individuals, retinal artery occlusion and increased retinal vein caliber are associated with increased cerebral vascular events both concurrently and in the future. These associations persist after accounting for confounding variables known to be disease causing in both circulations. It is not known whether the relationships identified reflect other confounding variables such as an individual’s susceptibility to vascular disease. Future research is needed do deepen our understanding of these associations on a pathophysiological level and to identify interventions beyond traditional vascular risk factor modification, that, when prompted by identification of retinal vascular disease, reduce cerebral vascular morbidity and mortality.
Acknowledgments
Funding: K23 EY 024345 from the National Institutes of Health, Unrestricted Departmental Grant from Research to Prevent Blindness
Footnotes
Disclosures: none
References
- 1.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. Journal of anatomy. 2005;206(4):319–48. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic research. 2000;32(6):249–56. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 3.Kwa VI, van der Sande JJ, Stam J, Tijmes N, Vrooland JL. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59(10):1536–40. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- 4.Kalitzeos AA, Lip GY, Heitmar R. Retinal vessel tortuosity measures and their applications. Experimental eye research. 2013;106:40–6. doi: 10.1016/j.exer.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Cheung CY, Tay WT, Ikram MK, Ong YT, De Silva DA, Chow KY, et al. Retinal microvascular changes and risk of stroke: the Singapore Malay Eye Study. Stroke; a journal of cerebral circulation. 2013;44(9):2402–8. doi: 10.1161/STROKEAHA.113.001738. [DOI] [PubMed] [Google Scholar]
- 6.Bek T, Jeppesen P, Kanters JK. Spontaneous high frequency diameter oscillations of larger retinal arterioles are reduced in type 2 diabetes mellitus. Investigative ophthalmology & visual science. 2013;54(1):636–40. doi: 10.1167/iovs.12-11182. [DOI] [PubMed] [Google Scholar]
- 7.Heitmar R, Blann AD, Cubbidge RP, Lip GY, Gherghel D. Continuous retinal vessel diameter measurements: the future in retinal vessel assessment? Investigative ophthalmology & visual science. 2010;51(11):5833–9. doi: 10.1167/iovs.09-5136. [DOI] [PubMed] [Google Scholar]
- 8.Kotliar KE, Lanzl IM, Schmidt-Trucksass A, Sitnikova D, Ali M, Blume K, et al. Dynamic retinal vessel response to flicker in obesity: A methodological approach. Microvascular research. 2011;81(1):123–8. doi: 10.1016/j.mvr.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Lim M, Sasongko MB, Ikram MK, Lamoureux E, Wang JJ, Wong TY, et al. Systemic associations of dynamic retinal vessel analysis: a review of current literature. Microcirculation (New York, NY: 1994) 2013;20(3):257–68. doi: 10.1111/micc.12026. [DOI] [PubMed] [Google Scholar]
- 10.Jeppesen P, Gregersen PA, Bek T. The age-dependent decrease in the myogenic response of retinal arterioles as studied with the Retinal Vessel Analyzer. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2004;242(11):914–9. doi: 10.1007/s00417-004-0945-4. [DOI] [PubMed] [Google Scholar]
- 11.Harris A, Dinn RB, Kagemann L, Rechtman E. A review of methods for human retinal oximetry. Ophthalmic surgery, lasers & imaging: the official journal of the International Society for Imaging in the Eye. 2003;34(2):152–64. [PubMed] [Google Scholar]
- 12.Wang Y, Fawzi AA, Varma R, Sadun AA, Zhang X, Tan O, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Investigative ophthalmology & visual science. 2011;52(2):840–5. doi: 10.1167/iovs.10-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiga Y, Shimura M, Asano T, Tsuda S, Yokoyama Y, Aizawa N, et al. The influence of posture change on ocular blood flow in normal subjects, measured by laser speckle flowgraphy. Current eye research. 2013;38(6):691–8. doi: 10.3109/02713683.2012.758292. [DOI] [PubMed] [Google Scholar]
- 14.Ikram MK, de Jong FJ, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, et al. Retinal vascular calibers associate differentially with cerebral gray matter and white matter atrophy. Alzheimer disease and associated disorders. 2013;27(4):351–5. doi: 10.1097/WAD.0b013e31829344ed. [DOI] [PubMed] [Google Scholar]
- 15.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. Jama. 2002;288(1):67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 16.Cooper LS, Wong TY, Klein R, Sharrett AR, Bryan RN, Hubbard LD, et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke; a journal of cerebral circulation. 2006;37(1):82–6. doi: 10.1161/01.STR.0000195134.04355.e5. [DOI] [PubMed] [Google Scholar]
- 17.Hanff TC, Sharrett AR, Mosley TH, Shibata D, Knopman DS, Klein R, et al. Retinal microvascular abnormalities predict progression of brain microvascular disease: an atherosclerosis risk in communities magnetic resonance imaging study. Stroke; a journal of cerebral circulation. 2014;45(4):1012–7. doi: 10.1161/STROKEAHA.113.004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Wu Y, Xie S, Luan XH, Yuan Y. Retinal arterial abnormalities correlate with brain white matter lesions in cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy. Clinical & experimental ophthalmology. 2008;36(6):532–6. doi: 10.1111/j.1442-9071.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 19.Cavallari M, Falco T, Frontali M, Romano S, Bagnato F, Orzi F. Fractal analysis reveals reduced complexity of retinal vessels in CADASIL. PloS one. 2011;6(4):e19150. doi: 10.1371/journal.pone.0019150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Silva DA, Manzano JJ, Woon FP, Liu EY, Lee MP, Gan HY, et al. Associations of retinal microvascular signs and intracranial large artery disease. Stroke; a journal of cerebral circulation. 2011;42(3):812–4. doi: 10.1161/STROKEAHA.110.589960. [DOI] [PubMed] [Google Scholar]
- 21.Bettermann K, Slocomb JE, Shivkumar V, Lott ME. Retinal vasoreactivity as a marker for chronic ischemic white matter disease? Journal of the neurological sciences. 2012;322(1–2):206–10. doi: 10.1016/j.jns.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 22.Helenius J, Arsava EM, Goldstein JN, Cestari DM, Buonanno FS, Rosen BR, et al. Concurrent acute brain infarcts in patients with monocular visual loss. Annals of neurology. 2012;72(2):286–93. doi: 10.1002/ana.23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. American journal of ophthalmology. 2014;157(6):1231–8. doi: 10.1016/j.ajo.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke; a journal of cerebral circulation. 2009;40(6):2276–93. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 25.Christiansen CB, Lip GY, Lamberts M, Gislason G, Torp-Pedersen C, Olesen JB. Retinal vein and artery occlusions: a risk factor for stroke in atrial fibrillation. Journal of thrombosis and haemostasis: JTH. 2013;11(8):1485–92. doi: 10.1111/jth.12297. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Jensen SC, Moss SE, Meuer SM. Retinal emboli and stroke: the Beaver Dam Eye Study. Archives of ophthalmology. 1999;117(8):1063–8. doi: 10.1001/archopht.117.8.1063. [DOI] [PubMed] [Google Scholar]
- 27.Ho JD, Liou SW, Lin HC. Retinal vein occlusion and the risk of stroke development: a five-year follow-up study. American journal of ophthalmology. 2009;147(2):283–90.e2. doi: 10.1016/j.ajo.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Biousse V. Acute retinal arterial ischemia: an emergency often ignored. American journal of ophthalmology. 2014;157(6):1119–21. doi: 10.1016/j.ajo.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–40. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 30.Ong YT, Wong TY, Klein R, Klein BE, Mitchell P, Sharrett AR, et al. Hypertensive retinopathy and risk of stroke. Hypertension. 2013;62(4):706–11. doi: 10.1161/HYPERTENSIONAHA.113.01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell P, Wang JJ, Wong TY, Smith W, Klein R, Leeder SR. Retinal microvascular signs and risk of stroke and stroke mortality. Neurology. 2005;65(7):1005–9. doi: 10.1212/01.wnl.0000179177.15900.ca. [DOI] [PubMed] [Google Scholar]
- 32.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke; a journal of cerebral circulation. 2010;41(7):1349–55. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindley RI, Wang JJ, Wong MC, Mitchell P, Liew G, Hand P, et al. Retinal microvasculature in acute lacunar stroke: a cross-sectional study. The Lancet Neurology. 2009;8(7):628–34. doi: 10.1016/S1474-4422(09)70131-0. [DOI] [PubMed] [Google Scholar]
- 34.Baker ML, Hand PJ, Liew G, Wong TY, Rochtchina E, Mitchell P, et al. Retinal microvascular signs may provide clues to the underlying vasculopathy in patients with deep intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2010;41(4):618–23. doi: 10.1161/STROKEAHA.109.569764. [DOI] [PubMed] [Google Scholar]
- 35.Baker ML, Hand PJ, Wong TY, Liew G, Rochtchina E, Mitchell P, et al. Retinopathy and lobar intracerebral hemorrhage: insights into pathogenesis. Archives of neurology. 2010;67(10):1224–30. doi: 10.1001/archneurol.2010.249. [DOI] [PubMed] [Google Scholar]
- 36.Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47(5):975–81. doi: 10.1161/01.HYP.0000216717.72048.6c. [DOI] [PubMed] [Google Scholar]
- 37.Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Archives of internal medicine. 2006;166(21):2388–94. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 38.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, et al. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology. 2006;66(9):1339–43. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 39.Wieberdink RG, Ikram MK, Koudstaal PJ, Hofman A, Vingerling JR, Breteler MM. Retinal vascular calibers and the risk of intracerebral hemorrhage and cerebral infarction: the Rotterdam Study. Stroke; a journal of cerebral circulation. 2010;41(12):2757–61. doi: 10.1161/STROKEAHA.110.599084. [DOI] [PubMed] [Google Scholar]
- 40.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. American journal of epidemiology. 2009;170(11):1323–32. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Silva DA, Liew G, Wong MC, Chang HM, Chen C, Wang JJ, et al. Retinal vascular caliber and extracranial carotid disease in patients with acute ischemic stroke: the Multi-Centre Retinal Stroke (MCRS) study. Stroke; a journal of cerebral circulation. 2009;40(12):3695–9. doi: 10.1161/STROKEAHA.109.559435. [DOI] [PubMed] [Google Scholar]
- 42.Ong YT, De Silva DA, Cheung CY, Chang HM, Chen CP, Wong MC, et al. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke; a journal of cerebral circulation. 2013;44(8):2121–7. doi: 10.1161/STROKEAHA.113.001741. [DOI] [PubMed] [Google Scholar]
- 43.Kawasaki R, Che Azemin MZ, Kumar DK, Tan AG, Liew G, Wong TY, et al. Fractal dimension of the retinal vasculature and risk of stroke: a nested case-control study. Neurology. 2011;76(20):1766–7. doi: 10.1212/WNL.0b013e31821a7d7d. [DOI] [PubMed] [Google Scholar]
- 44.Doubal FN, MacGillivray TJ, Patton N, Dhillon B, Dennis MS, Wardlaw JM. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology. 2010;74(14):1102–7. doi: 10.1212/WNL.0b013e3181d7d8b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung N, Liew G, Lindley RI, Liu EY, Wang JJ, Hand P, et al. Retinal fractals and acute lacunar stroke. Annals of neurology. 2010;68(1):107–11. doi: 10.1002/ana.22011. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Li Y, Wang C, Xu L, You QS, Wang YX, et al. Localized retinal nerve fiber layer defects and stroke. Stroke; a journal of cerebral circulation. 2014;45(6):1651–6. doi: 10.1161/STROKEAHA.113.004629. [DOI] [PubMed] [Google Scholar]


