SUMMARY
Objective
To assess high-risk human papillomavirus (HR HPV) cervical infections and their type distribution among healthy women in Guadeloupe, French West Indies.
Methods
The details of consecutive non-pregnant women who attended cervical cancer screening and had HPV genotyping performed at the largest pathology laboratory on the island from January 1, 2013 to December 31, 2014 were recorded retrospectively. All women with available HPV genotyping results were included in the study.
Results
HR HPV genotyping results for 618 women (median age 42 years) were collected. The overall prevalence rate of HR HPV cervical infection was 36.1% (95% confidence interval (CI) 32.3–40.0%), with the following type distribution: HPV 16 or 18 irrespective of other HPV types, 7.3% (95% CI 5.4–9.6%); other HR HPV types excluding HPV 16 or 18, 28.8% (95% CI 25.3–32.5%). The prevalence rates of overall HR HPV and HR HPV other than 16 or 18 infection increased significantly (p < 0.001) with the severity of cytology grade, from 19.7% for normal cytology to 53.8% in atypical squamous cells of undetermined significance (ASC-US) and 67.7% in low-grade squamous intraepithelial lesions (LSIL).
Conclusion
The high prevalence rate of HR HPV cervical infection with genotypes other than 16 and 18 in Guadeloupe, irrespective of age and the cytology grade, suggests a potential benefit of the new nine-valent HPV vaccine to prevent HPV infection-related cancers in this Caribbean country.
Keywords: High-risk human papillomavirus, Cervical infection, Cytology, Prevalence, Caribbean
1. Introduction
Virus-related cancers are a leading cause of death in Caribbean countries,1,2 and have been identified as an important public health problem by the French West Indies cancer registries. The most common of these viruses associated with cancer are the human papillomavirus (HPV) types designated as ‘high risk’ (HR); these are implicated in 99% of cervical cancer, in 40–80% of anogenital cancers other than cervical, and in approximately 25% of head and neck cancers.3–9 The most prevalent HR HPV types in invasive cervical cancers worldwide are types 16 and 18, which are the primary targets of current HPV vaccination programs.10–12
Although the prevalence of HPV infection and incidence rates of cervical cancer are high in the Caribbean, limited data are available on the prevalence of HR HPV cervical infections among healthy Caribbean women.1,2,10,13–17 In addition, very few studies have been performed to describe the HR HPV type distribution in this population. Investigators of the African Caribbean Cancer Consortium have reported a high prevalence rate of HPV 45 rather than HPV 16 or 18 in Tobago, Jamaica, and Barbados.14–16 No data are available for the French West Indies. The recent US Food and Drug Administration (FDA) approval of a second-generation HPV vaccine that targets five HR HPV types (i.e., 31, 33, 45, 52, and 58) in addition to 16 and 18 warrants the evaluation of HR HPV cervical infections in the whole Caribbean.18 This new prophylactic vaccine may be more appropriate in Caribbean vaccination programs for the prevention of cervical cancer.10,19
Guadeloupe, an overseas department of France, is the largest island of the French West Indies (405 000 inhabitants, mostly Black Caribbean persons of African and European descent). A retrospective study was conducted on the island of Guadeloupe in order to assess the prevalence of cervical HR HPV infections and the type distribution of HR HPV among healthy women in this Caribbean country.
2. Patients and methods
2.1. Data and sample collection
The details of consecutive non-pregnant women who attended cervical cancer screening and had HPV genotyping performed at the largest pathology laboratory on the island from January 1, 2013 to December 31, 2014 were recorded. All cases with available HPV genotyping results of liquid-based cytology samples were included in the study.
Pathology charts were reviewed retrospectively using the computerized database of the laboratory and DIAMIC software. The following data were recorded: resident status to confirm permanent residence on the island of Guadeloupe during the study period, date of birth, date of sampling, and cytology results according to the Bethesda System 2001.20 Samples were classified as atypical squamous cells of undetermined significance (ASC-US), low-grade squamous intraepithelial lesion (LSIL), or high-grade squamous intraepithelial lesion (HSIL).
2.2. HPV testing and genotyping
The genotyping analysis of liquid-based cytology specimens collected in PreservCyt (Hologic Corp.) was performed independently from the histopathological examination, using a semi-genotyping kit (Cobas 4800 HPV test, Roche Diagnostics). This qualitative multiplex assay provides specific genotyping of HPV 16 and 18 while concurrently detecting the other HR HPV types (i.e., 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) in a pooled analysis using amplification of the target DNA by PCR and nucleic acid hybridization.
2.3. Statistical analysis
Results were recorded as numbers and frequencies. The prevalence rates of HPV infection were calculated with 95% confidence intervals (CIs). Age was described using the median and interquartile range (Q1–Q3). Stratification according to age was performed to analyze the HR HPV type distribution by cytology grade within each age group: 18–24, 25–29, 30–39, 40–49, 50–64, and ≥65 years. Cross-tabulations were analyzed by Chi-square test or Fisher’s exact test when appropriate. Comparisons of prevalence rates with other countries were based on the Chi-square test.
For all analyses, two-sided p-values of less than 0.05 were considered statistically significant. Data management and the statistical analysis were performed using SPSS v.17.0 software (SPSS Inc., Chicago, IL, USA).
2.4. Ethical considerations
This retrospective study was approved by the Ethics Committee for Non-interventional Research of Rouen University Hospital (registered number E2015–37).
3. Results
A total of 618 consecutive HR HPV genotyping results from 618 women were collected. The median age of the study population was 42 years (Q1–Q3 32–52 years). The overall prevalence rate of HR HPV cervical infection was 36.1% (95% CI 32.3–40.0%). The distribution of HR HPV types was as follows: HPV 16 or 18 irrespective of other HPV types, 7.3% (95% CI 5.4–9.6%); other HR HPV types excluding HPV 16 and 18, 28.8% (95% CI 25.3–32.5%).
Cytology results were available for 592 of the 618 cases and were classified as normal for 447 women, ASC-US for 106 women, LSIL for 31 women, and HSIL for eight women.
Interestingly, the overall prevalence rate of HR HPV cervical infection increased significantly (p < 0.001) with the severity of cytology grade from normal to LSIL, as did the prevalence rate of HR HPV other than 16 or 18 infection. These varied from 25.1% (95% CI 21.1–29.3%) and 19.7% (95% CI 16.1–23.7%) for normal cytology to 65.1% (95% CI 55.2–74.1%) and 53.8% (95% CI43.8–53.5%) in ASC-US and 77.4% (95% CI 58.9–90.4%) and 67.7% (95% CI 48.6–83.3%) in LSIL (Table 1). The HSIL group was not considered for the analysis because of its small size (n = 8).
Table 1.
Prevalence of HR HPV cervical infections by cytology grade
| Cytology grade
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal (n = 447)
|
ASC-US (n = 106)
|
LSIL (n = 31)
|
p-Value | |||||||
| n | % | 95% CI | n | % | 95% CI | n | % | 95% CI | ||
| Overall HR HPV | 112 | 25.1 | 21.1–29.3 | 69 | 65.1 | 55.2–74.1 | 24 | 77.4 | 58.9–90.4 | <0.001 |
| HR HPV 16 and/or 18 irrespective of other HR HPV types | 24 | 5.4 | 3.5–7.9 | 12 | 11.3 | 6.0–18.9 | 3 | 9.7 | 2.0–25.8 | 0.060 |
| Other HR HPV excluding 16 and 18 | 88 | 19.7 | 16.1–23.7 | 57 | 53.8 | 43.8–63.5 | 21 | 67.7 | 48.6–83.3 | <0.001 |
HR, high-risk; HPV, human papillomavirus; ASC-US, atypical squamous cell of undetermined significance; LSIL, low-grade squamous intraepithelial lesion; CI, confidence interval.
Table 2 shows the HR HPV type distribution by country (i.e., Guadeloupe, France, developed regions, and Tobago) for normal cytology; data were obtained from Heard et al.,21 the World Health Organization/Institut Català d’Oncologia (WHO/ICO) 2010 report,10 and a secondary analysis of data published previously from the Tobago study performed by Ragin et al.14 The overall prevalence rate of cervical HR HPV infection, as well as the prevalence rate of HR HPV other than 16 or 18 infection, was significantly higher in Guadeloupe than in France and in developed regions (p < 0.001). Conversely, the crude prevalence of HPV 16 and/or 18 cervical infection was not significantly different between Guadeloupe and other countries. Interestingly, data from the Caribbean island of Tobago were of the same order of magnitude as those recorded in Guadeloupe.
Table 2.
HR HPV type distribution by country for normal cytology
| Guadeloupe (French West Indies)
|
France (Heard et al., 2013)
|
Developed regions (WHO/ICO 2010)
|
Tobago (Ragin et al., 2007)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | p-Valuea | % | 95% CI | p-Valuea | % | 95% CI | p-Valuea | |
| HR HPVb | 25.1 | 21.1–29.3 | 13.7 | 11.7–15.6 | <0.001 | 10.3 | 10.2–10.4 | <0.001 | 20.6 | 15.3–26.8 | 0.192 |
| HPV 16 and/or 18c | 5.4 | 3.5–7.9 | 3.9 | 2.8–5.1 | 0.089 | 3.6 | 3.5–3.7 | 0.017 | 2.5 | 0.8–5.6 | 0.089 |
| HR HPV other than 16 and/or 18d | 19.7 | 16.1–23.7 | 9.7 | 8.0–11.4 | <0.001 | 6.0 | 5.9–6.1 | <0.001 | 18.1 | 13.1–24.1 | 0.616 |
HR, high-risk; HPV, human papillomavirus; WHO/ICO, World Health Organization/Institut Català d’Oncologia; CI, confidence interval.
Comparison to Guadeloupe.
At least one HR HPV type among 16, 18, and other HR types (i.e., 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68).
HPV 16 and/or 18 irrespective of other HR HPV types.
Other HR HPV types, excluding HPV 16 and 18.
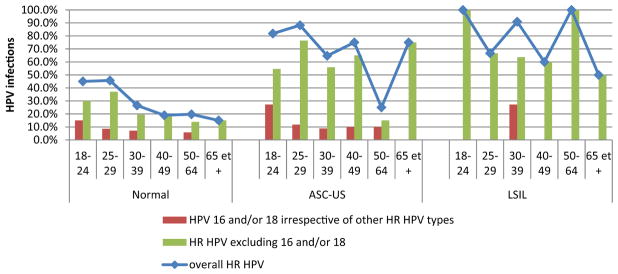
Figure 1 presents the crude prevalence of overall HR HPV, HPV 16 and/or 18, and HR HPV other than 16 or 18 cervical infections by grade of cytology for each age group. Regardless of the cytology grade, the overall rate of HR HPV cervical infection decreased with age for women older than 29 years, except for the age group ≥65 years. The largest drop was observed in ASC-US for women aged 50–64 years (i.e., overall HR HPV of 25% (95% CI 8.7–49.1), HPV 16 and/or 18 of 10% (95% CI 1.2–31.7), HR HPV other than 16 or 18 of 15% (95% CI 3.2–37.9) versus 74% (p < 0.001), 12% and 62% (p < 0.001), respectively, for younger women). Interestingly, whatever the age group, the cervical infection rate of HR HPV other than 16 or 18 was always higher than the corresponding rate of HPV 16 and/or 18 infection for each cytology grade from normal to LSIL. This was particularly obvious for the latter cytology grade, for which the rate of HPV 16 and/or 18 cervical infection was 0% in all age groups except for women aged 30–39 years (i.e., 27.3%).
Figure 1.
Crude prevalence of overall HR HPV, HPV 16 and/or 18, and HR HPV other than 16 or 18 cervical infections by grade of cytology for each age group.
4. Discussion
This study is the first report of HR HPV cervical infection among healthy women from the general population of the French West Indies. Moreover this is the first study describing the HR HPV distribution type among age groups and cervical cytology grades in healthy Caribbean women.
A high overall HR HPV cervical infection crude prevalence of 36.1% (95% CI 32.3–40.0) was demonstrated. This high prevalence rate is in accordance with those of all previous Caribbean studies, in which the crude prevalence of HR HPV cervical infection has varied between 35.4% in Tobago2,14 and 70.8% in Jamaica.15
As in previous worldwide studies and in the largest French study, it was found that the crude prevalence of HR HPV cervical infection was highest in young women, decreasing in women of older age,10,21,22 but increasing slightly in women ≥65 years of age compared to women aged 50–64 years.21
The main result of this study is the very high prevalence rate of HR HPV infection with genotypes other than 16 and 18 in Guadeloupe, irrespective of age and the cytology grade from normal to LSIL. The higher prevalence rate of infections caused by HR HPV types other than 16 or 18 compared to the rate for HPV 16 and/or 18 has been highlighted in previous studies from developed regions,10,22 as well as in the large French series published by Heard et al.,21 but this was not of the same order of magnitude as in the present study. Indeed, in the present study, the crude prevalence of HR HPV cervical infections with genotypes other than 16 or 18 was found to vary from 19.7% (95% CI 16.1–23.7%) for normal cytology to 53.8% (95% CI 43.8–53.5%) in ASC-US and 67.7% (95% CI 48.6–83.3%) in LSIL. These results are in accordance with those of a previous study in Tobago, which demonstrated a crude prevalence rate of 18.1% (95% CI 13.1–24.1%) for infection with HR HPV types other than 16 or 18 versus 2.5% for infection with HPV 16 and/or 18 in healthy women with normal cytology (Table 2).14
The recent worldwide study by Serrano et al., including 10 575 invasive cervical cancer cases from 48 countries except the Caribbean, was performed in order to estimate the relative contribution of the nine HPV types targeted in the second-generation HPV vaccine; this study confirmed the variation in relative contribution of HPV 16/18 among geographical regions.19 Thus the recently FDA-approved nine-valent HPV vaccine, which covers five additional HR HPV types (i.e., 31, 33, 45, 52, and 58) to those covered by current HPV vaccines, could help to increase protection against HPV infection-related cancers, particularly in regions with high prevalence rates of HR HPV cervical infection with genotypes other than 16 or 18, such as the Caribbean. This is supported by the recent report on 131 invasive cervical cancers from Martinique (French West Indies), which showed a slightly heterogeneous distribution of HR HPV types: HPV 16 (47%), HPV 35 (14%), HPV 18 (10%), HPV 33 (8%), HPV 45 (6%), and HPV 51 (6%).23
As with all retrospective studies, this study has various possible biases. To limit the selection bias due to the single-center design, all consecutive HPV genotyping results registered in the largest pathology laboratory of Guadeloupe, which handles more than 10 000 Pap smears per year from women living in all parts of the island, were included exhaustively. The absence of major selection bias in this series is supported by the similarities in clinical features (i.e., median age 42 years) and in the crude prevalence of overall HR HPV, HR HPV other than 16 or 18, and HPV 16 or 18 infection in this series compared to those found in previous Caribbean studies.14–16
In conclusion, the present study showed a high prevalence rate of HR HPV cervical infection of 36.1% in healthy women from Guadeloupe, attributable to a high rate of infection with HR HPV genotypes other than 16 or 18 (i.e., 28.8%). These findings suggest that larger Caribbean studies should be performed in order to assess the potential impact of the new HPV vaccine on the Caribbean HPV ecology and related cervical lesions. This second-generation HPV vaccine might potentially be helpful in the development of an appropriate Caribbean vaccination program for the prevention of cervical cancer, which is a current public health problem in this region.
Footnotes
Nadège Cordel and Camille Ragin are members of the African Caribbean Cancer Consortium..
Funding: None.
Ethical approval: E2015–37.
Conflict of interest: None.
References
- 1.Philipps AA, Jacobson JS, Magai C, Condsedine N, Mehler N, Neugut AI. Cancer incidence and cancer mortality in the Caribbean. Cancer Invest. 2007;25:476–83. doi: 10.1080/07357900701359841. [DOI] [PubMed] [Google Scholar]
- 2.Ragin C, Edwards R, Heron DE, Kuo J, Wentzel E, Gollin SM, Taioli E. Prevalence of cancer-associated viral infections in healthy Afro-Caribbean populations: a review of the literature. Cancer Invest. 2008;26:936–47. doi: 10.1080/07357900801975280. [DOI] [PubMed] [Google Scholar]
- 3.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 4.Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30S:F55–70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 5.Alemany L, Saunier M, Alvarado-Cabrero I, Quirós B, Salmeron J, Shin HR, et al. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer. 2015;136:98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannweiler S, Sygulla S, Winter E, Regauer S. Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53 but lack p16ink4a over-expression. J Am Acad Dermatol. 2013;69:73–81. doi: 10.1016/j.jaad.2012.12.973. [DOI] [PubMed] [Google Scholar]
- 7.de Sanjosé S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, et al. Worldwide human papillomavirus attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur J Cancer. 2013;49:3450–61. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Alemany L, Saunier M, Tinoco L, Quirós B, Alvarado-Cabrero I, Alejo M, et al. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur J Cancer. 2014;50:2846–54. doi: 10.1016/j.ejca.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Syrjanen S, Lodi G, von Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17(Suppl 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 10.WHO/ICO Information Centers on HPV and Cervical Cancer. 3. WHO/ICO; 2010. Human papillomavirus and related cancers. [Google Scholar]
- 11.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2013;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz N, Manalastas RJ, Pitisuttihum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi: 10.1016/S0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 13.Ragin C, Watt A, Markovic N, Bunker C, Edwards R, Eckstein S, et al. Comparisons of high-risk cervical HPV infections in Caribbean and US populations. Infect Agents Cancer. 2009;4(Suppl 1):S9. doi: 10.1186/1750-9378-4-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragin C, Wheeler V, Wilson J, Bunker C, Gollin S, Patrick A, Taioli E. Distinct distribution of HPV types among cancer-free Afro-Caribbean women from Tobago. Biomarkers. 2007;12:510–22. doi: 10.1080/13547500701340384. [DOI] [PubMed] [Google Scholar]
- 15.Watt A, Garwood D, Jackson M, Younger N, Ragin C, Smikle M, et al. High-risk and multiple human papillomavirus (HPV) infections in cancer-free Jamaican women. Infect Agents Cancer. 2009;4(Suppl 1):S11. doi: 10.1186/1750-9378-4-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward JM, Schmalenberg K, Antonishyn NA, Levett PN, Guittens-St Hilaire M. Human papillomavirus genotype distribution and risk factors analysis among a pre-vaccination population in Barbados. 5th International African–Caribbean Cancer Consortium Conference; 2014. [Google Scholar]
- 17.Cordel N, Tressières B, Bonnecarrere L. Squamous cell carcinomas in Afro-Caribbean women. J Am Acad Dermatol. 2012;67:788–9. doi: 10.1016/j.jaad.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 18.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 19.Serrano B, de Sanjosé S, Tous S, Quiros B, Muñoz N, Bosch X, Alemany L. Human papillomavirus attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur J Cancer. 2015;51:1732–41. doi: 10.1016/j.ejca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 21.Heard I, Tondeur L, Arowas L, Falguières M, Demazoin MC, Favre M. Human papillomavirus distribution in organised cervical cancer screening in France. PLoS One. 2013;8:e79372. doi: 10.1371/journal.pone.0079372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Munoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 23.Dos Santos G, Michel M, Ekindi M, Jouanelle-Sulpicy C, Dorival MJ, Warter A, Cesaire R. Human papillomavirus (HPV) genotype distribution in invasive cervical cancers in Martinique (French West Indies). Eurogyn Congress; February 2015. [Google Scholar]