Abstract
Despite significant progress in the clinical application of antibody drug conjugates (ADCs), novel cleavage strategies that provide improved selectivity are still needed. Near-IR light could provide a targetable, biocompatible external stimulus to initiate drug release. Here we report the first approach using near-IR light to cleave a small molecule from a biomacromolecule, which we apply to the problem of ADC linkage. Our method uses a recently developed near-IR uncaging reaction that exploits the photochemical reactivity of C4’-N-dialkylamine heptamethine cyanines. This communication describes the synthesis of bioconjugatable cyanine-small molecule photocages, bioconjugation and 690 nm light-mediated cleavage from the anti-EGFR antibody panitumumab, and initial in vitro and in vivo evaluation. These studies provide the critical chemical underpinning from which to develop this near-IR cleavable linker strategy.
Keywords: Drug Delivery, Cyanines, Antibodies, Conjugation, Fluorescence
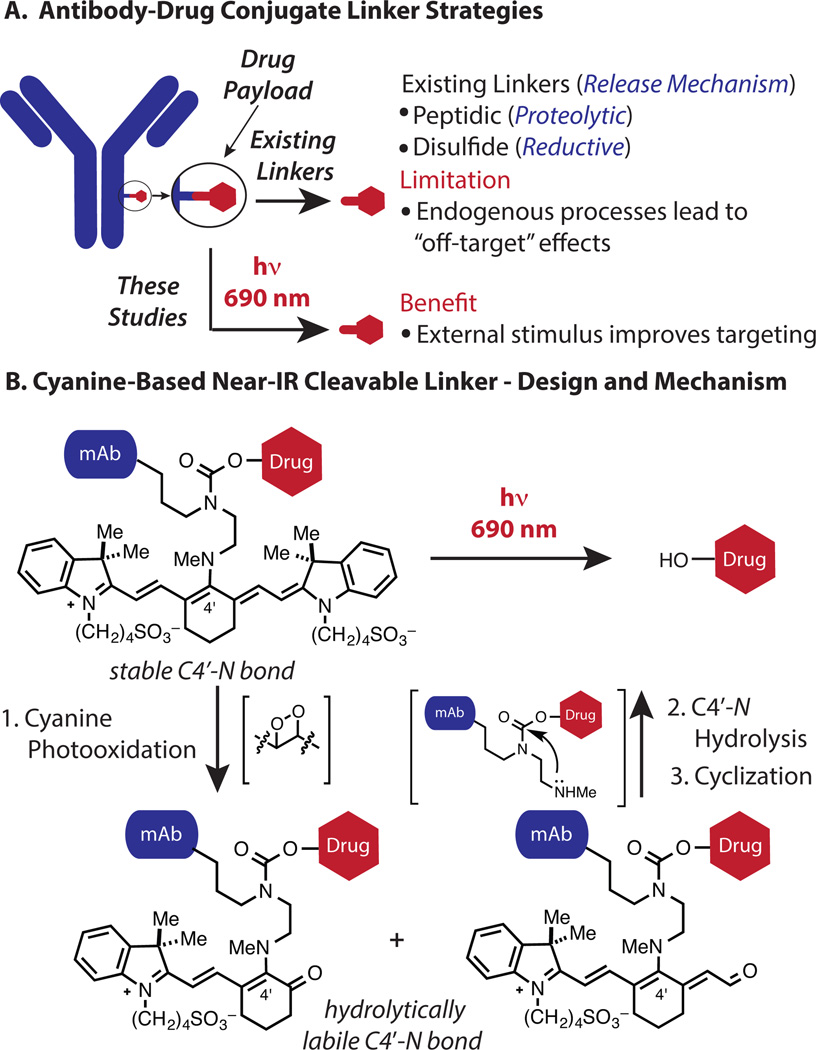
The recent success of antibody-drug conjugates (ADCs) has validated the benefits of combining macromolecule and small molecule therapeutics.[1–4] Within this exciting area, a remaining challenge is to identify linker strategies that provide improved spatiotemporal control of the pivotal drug release step. Existing approaches (Figure 1A) rely on endogenous reactions with little inherent tumor selectivity.[5–6] As a consequence, undesirable release in circulation can be a significant issue.[7–8] Moreover, benign tissue uptake of the antibody, through either antigen-specific or antigen-independent mechanisms, can lead to off-target drug release.[9–10] An appealing solution would be to develop antibody-drug cleavage chemistry that relies on an external stimulus which can be applied in a site-specific fashion.
Figure 1.
(A) Overview (B) Design and mechanism of a cyanine-based near-IR light cleavable ADC.
Light in the near-IR range (650–900 nm) has unique potential in this context. These wavelengths exhibit significant tissue penetration, minimal toxicity, and, moreover, are clinically validated for both diagnostic and therapeutic applications.[11] Near-IR fluorescence imaging is routine in laboratory and certain clinical contexts. Innovative applications, such as methods to optically define tumor margins during surgery, are being developed and clinically translated.[12–13] Light-based therapeutic modalities using phototoxic small molecules have an extensive history in the treatment of cancer and skin disorders.[14–15] Recent advances, including antibody-based photoimmunotherapy (PIT) strategies, are providing new therapeutic avenues in this area.[16–17]
We recently introduced a near-IR uncaging strategy using the heptamethine cyanine fluorophore scaffold as the caging component.[18] The reaction sequence reveals a secondary amine through photooxidation of the cyanine polyene followed by C4′-N bond hydrolysis. A final urea-forming cyclization releases the pendant phenol (Figure 1B). The photooxidative C-C cleavage process entails reaction of cyanine-photosensitized singlet oxygen with the polyene to form thermally labile dioxetane intermediates.[19–20]
Here we apply this cyanine photocaging strategy in the first approach to use near-IR light to cleave a small molecule from a protein or other biological macromolecule.[21] There have been several recent advances in the area of light-mediated drug delivery, including steps toward using tissue-compatible longer wavelengths.[22–34] The strategy reported here has promising characteristics: initiation with 690 nm light, the traceable emissive properties of the cyanine scaffold, and the use of broadly-employed monoclonal antibodies. Below is described the synthesis of the bioconjugatable cyanine photocage, characterization of the uncaging reaction, and cellular evaluation. In vivo imaging of the resulting antibody conjugate confirms its excellent tumor uptake and that the fluorescent signal can be depleted with external irradiation.
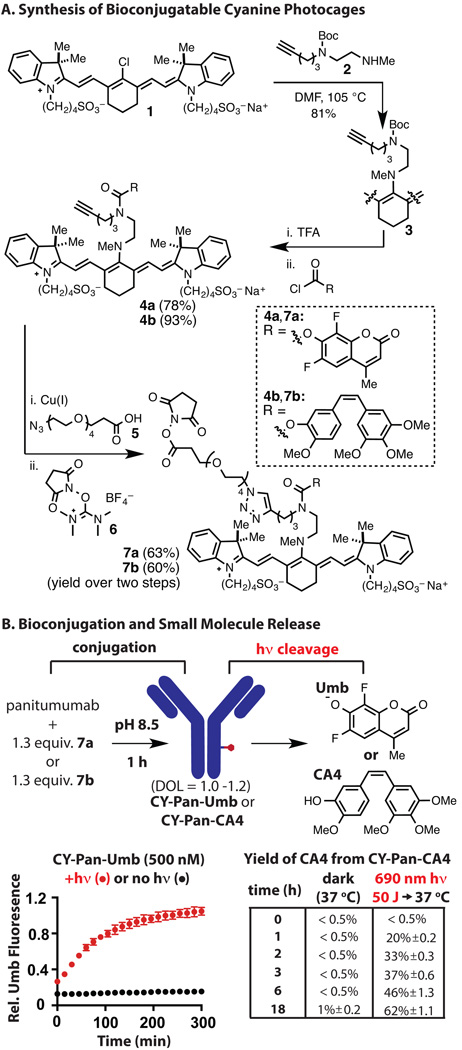
We have prepared bioconjugatable variants of caged 6,8-difluoro-4-methyl-umbelliferone (Umb), a useful fluorescence reporter, and combretastatin A4 (CA4), a potent inhibitor of microtubule polymerization.[35–36] Our linker strategy uses the carbamate functional group as the antibody attachment point. This design ensures that small molecule release from the antibody is the last step in the light-initiated reaction sequence. The synthesis of the NHS esters commenced from commercially available IR-783 (1, Figure 2A). Compound 2, available in four steps from commercial materials, undergoes C4’-substitution in high yield (81%) to afford 3.[37] Initial studies on the Boc removal, carbamate formation sequence revealed that many conditions provided a ~1:1 mixture of two distinct N-linked cyanine products. Further analysis revealed these to be constitutional isomers comprising the desired C4’-N-linkage, 4, and the isomer arising from the intermediate diamine equilibrating through C4’ addition/elimination sequences prior to carbamate formation (Figure S1). Of note, intramolecular electrophilic reactivity involving the C4’ position of heptamethine cyanines has been observed elsewhere.[38–39] It was ultimately determined that deprotection of the Boc group in neat TFA, aqueous bicarbonate quench at −10 °C, and addition of the respective chloroformate to the cooled mixture provided 4a,b in excellent yields (78% and 93%, respectively). Copper-catalyzed (3+2) cycloaddition of 4a,b with azido-PEG4-acid 5 and transformation of the acid to the NHS ester with TSTU (6) provided the fully functionalized cyanines 7a,b in high yield. As a negative control, we also prepared a variant of 7 where CA4 and Umb were replaced with phenol (see SI).
Figure 2.
Synthesis of conjugates and characterization of 690 nm-initiated drug release. (A) Synthesis of 7a,b. (B) Conjugation of 7a,b to Pan, uncaging to yield fluorescent Umb, and yield of CA4 in the presence and absence of 690 nm irradiation (50 J).
The efficient preparation of 7a,b allowed us to investigate the antibody conjugation and cleavage chemistry. We have used panitumumab (Pan), a clinical monoclonal antibody to human epidermal growth factor receptor (EGFR). Pan was incubated with 1.3 equivalents of NHS ester 7a,b in pH 8.5 buffer to provide the CY-Pan-Umb and CY-Pan-CA4 conjugates after size-exclusion chromatography (Figure S2). The purity of both conjugates was confirmed by SDS-PAGE (Figure S3). A solution of CY-Pan-Umb in pH 7.4 PBS was irradiated with 50 J of 690 nm light (33 min at 25 mW/cm2) administered from a convenient LED source at 25 °C, or maintained in the dark, and then Umb fluorescence was monitored while heating at 37 °C (Figure 2B). The Umb fluorescence increase was only observed in the irradiated sample with a half-life for the release reaction (t1/2) of 67 minutes. Suggesting this process will tolerate the acidic environment of the lysosome, adjusting the pH to 5.0 only moderately reduces the rate of release (t1/2 = 105 min, Figure S4). CY-Pan-CA4 was similarly irradiated with 50 J of 690 nm light or not, and then the yield of CA4 was monitored by HPLC following incubation (without additional irradiation) at 37 °C. The irradiated sample provided quite useful yields of free CA4, whereas the unirradiated sample provided only negligible quantities of the free drug (Figure 2B). Notably, incubation of CY-Pan-CA4 in human plasma at 37 °C also led to minimal release of CA4 (<1%) after 72 h (Figure S5). We also examined the absorption and fluorescence emission of the conjugate and the consequence of irradiation on the latter. The CY-Pan-CA4 conjugate maintains the near-IR optical properties of the carboxylic acid precursor to 7b (λabs = 700 nm, λem = 795 nm, Figure S6). Irradiation using 690 nm light reduced the emission of CY-Pan-CA4 (Figure S7), with little remaining fluorescence after the 50 J of light fluence used for drug release. This result suggests that irradiation-induced loss of emission could serve as useful marker for drug release.
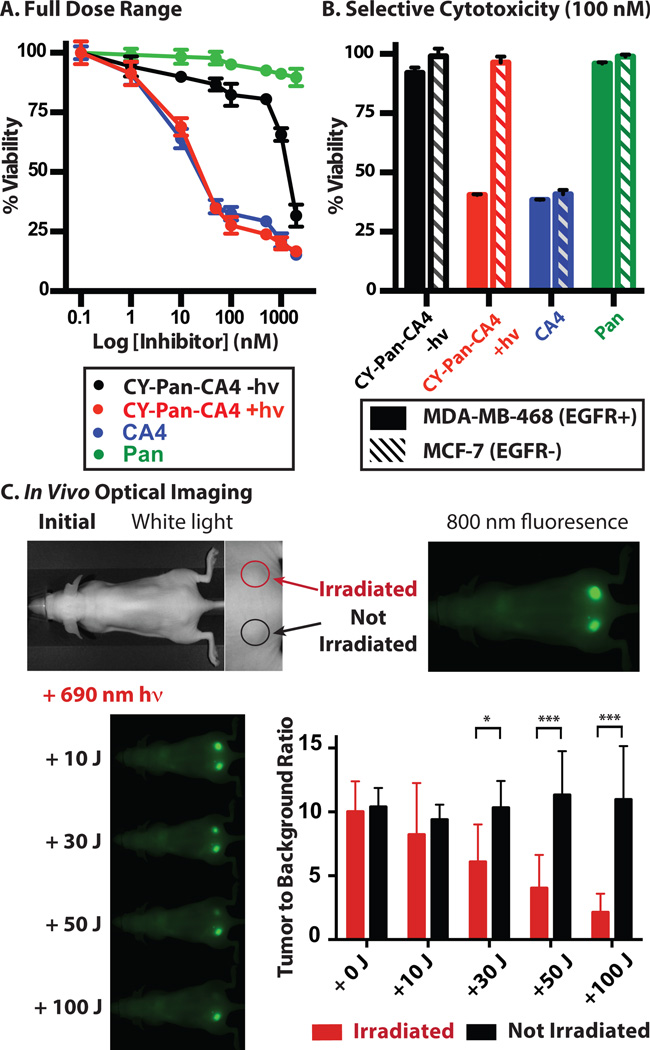
We characterized the CY-Pan-CA4 conjugate in an in vitro context. Fluorescence confocal microscopy using EGFR+ (MDA-MB-468) and EGFR− (MCF-7) cells revealed that only the former exhibited characteristic antibody labeling (Figure S8). This specific cellular labeling was also confirmed using fluorescence activated cell sorting (FACS, Figure S9). Together, these results indicate that the binding specificity of Pan is preserved in the immunoconjugate. We next assessed whether CY-Pan-CA4 elicits a cytotoxic effect in these same cell lines in a light- and antigen-dependent fashion. We first determined cell viability with continuous exposure to a wide concentration range of CY-Pan-CA4 to examine the full biological effect of cleaved vs. uncleaved conjugate. Irradiation of cells in the presence of CY-Pan-CA4 with 30 J of 690 nm light led to a growth inhibitory activity (IC50 = 16 nM) that nearly matched that of CA4 alone (IC50 = 11 nM) (Figure 3A). By contrast, the absence of irradiation significantly diminished this growth inhibitory effect (IC50 = 1.1 µM), providing additional evidence for the high dark stability of the conjugated form. Finally, as expected, the antibody alone had no effect on cell viability over the concentration range examined (IC50 > 2 µM). We also evaluated the internalized and cell-surface bound antibody fraction. MDA-MB-468 (EGFR+) and MCF-7 (EGFR−) cells were incubated with CY-Pan-CA4 (100 nM) for 24 h, the media was replaced, irradiation was carried out as above, and cell viability was evaluated. A significant reduction in cell viability was observed only upon 690 nm irradiation in the EGFR+ cell line, with little effect in either the EGFR− cell line or in the absence of irradiation (Figure 3B). Finally, no effect on viability was apparent using a version of the antibody conjugate that releases only biologically inactive phenol, indicating that the observed cytotoxicity is solely a consequence of drug release (Figure S10).
Figure 3.
In vitro and in vivo analysis of CY-Pan-CA4. (A) Light-dependent (690 nm, 30 J) cytotoxicity of CY-Pan-CA4, CA4, and Pan against MDA-MB-468 cells (continuous dose). (B) Light-dependent (690 nm, 30 J) cytotoxicity of internalized CY-Pan-CA4, CA4, and Pan against MDA-MB-468 and MCF-7 cells (media exchange). (C) Serial fluorescence images of A431 tumors (implanted in both sides of the dorsum) pre- and post-irradiation with various doses of near-IR light at 2 days post-injection. Only the right tumor was irradiated; the left tumor was covered with aluminum foil. *p = 0.03, **p = 0.001, ***p < 0.0001.
CY-Pan-CA4 is likely not suitable to address tumor burden in vivo as the potency of CA4 does not match that of typical ADC payloads.[40] We carried out in vivo imaging studies that sought to assess conjugate stability, tumor localization, and if external irradiation could modulate the fluorescent signal. To examine the biodistribution of the CY-Pan-CA4 conjugate, we used a xenograft tumor model with dorsal A431 (EGFR+) tumors. After tail vein injection of CY-Pan-CA4 (100 µg), we visualized the conjugate using an 800 nm emission channel. Significant selective tumor accumulation was observed, with high tumor-to-background ratios between 1–7 days (maximal between 2–3 days, Figure S11 and S12). To evaluate the effect of irradiation on the fluorescent signal, we implanted mice with two tumors, one in each side of the dorsum, administered CY-Pan-CA4 as above, and, after 2 days, irradiated one of the tumors with increasing light doses from a 690 nm laser (500 mW/cm2). The fluorescence of only the irradiated tumor decreased in a light-dependent manner, with 100 J (3.3 min irradiation) nearly ablating the signal (Figure 3C). Notably, the light doses applied here are similar to or compare favorably to those required for conventional photodynamic therapy-type applications.[41]
These studies are the first to use near-IR light to initiate small molecule/therapeutic protein cleavage. Given the widespread use of near-IR optical tools in clinical and basic science settings, this approach should enable highly targeted, timed delivery of bioactive small molecules in complex settings. Suggesting theranostic applications, the fluorescence signal provides a useful marker for target accumulation and the loss of that signal can indicate drug release.[42] Future inquiries will include alternative drug payloads, as well as the choice of antibody and labeling method - critical issues with all ADC strategies. These conventional ADC optimization efforts will be coupled with insights gained in ongoing structural optimization studies seeking to increase the kinetics and quantum efficiency of the cyanine photocaging reaction.
Supplementary Material
Acknowledgments
We thank Dr. Joseph Barchi for NMR assistance and Dr. James Kelley for mass spectrometric analysis. We thank the Optical Microscopy and Analysis Laboratory (Center for Cancer Research, NCI Frederick) for assistance in obtaining confocal fluorescence images and the CCR Flow Cytometry Core (Cancer and Inflammation Program, Center for Cancer Research, NCI-Frederick) for help with flow cytometry analysis. We thank Mr. James Shaum for synthesis of Umb. This work was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, and the National Cancer Institute, National Institutes of Health.
Contributor Information
Dr. Roger R. Nani, Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, MD.
Dr. Alexander P. Gorka, Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, MD.
Dr. Tadanobu Nagaya, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, Bethesda, MD
Dr. Hisataka Kobayashi, Molecular Imaging Program, Center for Cancer Research, National Cancer Institute, Bethesda, MD
Dr. Martin J. Schnermann, Email: martin.schnermann@nih.gov, Chemical Biology Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, MD.
References
- 1.Alley SC, Okeley NM, Senter PD. Curr. Opin. Chem. Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 2.Chari RV, Miller ML, Widdison WC. Angew. Chem. Int. Ed. 2014;53:3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 3.Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, Borzilleri RM. Drug Discov. Today. 2014;19:869–881. doi: 10.1016/j.drudis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Casi G, Neri D. J. Control. Release. 2012;161:422–428. doi: 10.1016/j.jconrel.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Ducry L, Stump B. Bioconjug. Chem. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 6.Flygare JA, Pillow TH, Aristoff P. Chem. Biol. Drug Des. 2013;81:113–121. doi: 10.1111/cbdd.12085. [DOI] [PubMed] [Google Scholar]
- 7.Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, Senter PD, Wahl AF. Clin. Cancer Res. 2005;11:843–852. [PubMed] [Google Scholar]
- 8.Dorywalska M, Strop P, Melton-Witt JA, Hasa-Moreno A, Farias SE, Galindo Casas M, Delaria K, Lui V, Poulsen K, Loo C, Krimm S, Bolton G, Moine L, Dushin R, Tran TT, Liu SH, Rickert M, Foletti D, Shelton DL, Pons J, Rajpal A. Bioconjug. Chem. 2015;26:650–659. doi: 10.1021/bc5005747. [DOI] [PubMed] [Google Scholar]
- 9.Gorovits B, Krinos-Fiorotti C. Cancer Immunol. Immunother. 2013;62:217–223. doi: 10.1007/s00262-012-1369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson HK, Lambert JM. AAPS J. 2012;14:799–805. doi: 10.1208/s12248-012-9386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissleder R. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen QT, Tsien RY. Nat. Rev. Cancer. 2013;13:653–662. doi: 10.1038/nrc3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJG, van der Zee AGJ, Bart J, Low PS, Ntziachristos V. Nat. Med. 2011;17:1315. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 14.Dolmans DE, Fukumura D, Jain RK. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 15.Ethirajan M, Chen YH, Joshi P, Pandey RK. Chem. Soc. Rev. 2011;40:340–362. doi: 10.1039/b915149b. [DOI] [PubMed] [Google Scholar]
- 16.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Nat. Med. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spring BQ, Abu-Yousif AO, Palanisami A, Rizvi I, Zheng X, Mai Z, Anbil S, Sears RB, Mensah LB, Goldschmidt R, Erdem SS, Oliva E, Hasan T. Proc. Natl. Acad. Sci. U S A. 2014;111:E933–E942. doi: 10.1073/pnas.1319493111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorka AP, Nani RR, Zhu J, Mackem S, Schnermann MJ. J. Am. Chem. Soc. 2014;136:14153–14159. doi: 10.1021/ja5065203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorka AP, Nani RR, Schnermann MJ. Org. Biomol. Chem. 2015;13:7584. doi: 10.1039/c5ob00788g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nani RR, Kelley JA, Ivanic J, Schnermann MJ. Chem. Sci. 2015 doi: 10.1039/c5sc02396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.We are only aware of a single prior report of light-mediated ADC cleavage using UV or visible wavelengths, although the structure of the linker was not reported: Gilbert CW, McGowan EB, Seery GB, Black KS, Pegram MDJ. J. Exp. Ther. Oncol. 2003;3:27–35. doi: 10.1046/j.1359-4117.2003.01064.x.
- 22.Broichhagen J, Frank JA, Trauner D. Acc. Chem. Res. 2015;48:1947–1960. doi: 10.1021/acs.accounts.5b00129. [DOI] [PubMed] [Google Scholar]
- 23.Bio M, Rajaputra P, Nkepang G, Awuah SG, Hossion AML, You Y. J. Med. Chem. 2013;56:3936–3942. doi: 10.1021/jm400139w. [DOI] [PubMed] [Google Scholar]
- 24.Nkepang G, Bio M, Rajaputra P, Awuah SG, You Y. Bioconjug. Chem. 2014;25:2175–2188. doi: 10.1021/bc500376j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shell TA, Shell JR, Rodgers ZL, Lawrence DS. Angew. Chem. Int. Edit. 2014;53:875–878. doi: 10.1002/anie.201308816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samanta S, Beharry AA, Sadovski O, McCormick TM, Babalhavaeji A, Tropepe V, Woolley GA. J. Am. Chem. Soc. 2013;135:9777–9784. doi: 10.1021/ja402220t. [DOI] [PubMed] [Google Scholar]
- 27.Helmy S, Leibfarth FA, Oh S, Poelma JE, Hawker CJ, de Alaniz JR. J. Am. Chem. Soc. 2014;136:8169–8172. doi: 10.1021/ja503016b. [DOI] [PubMed] [Google Scholar]
- 28.Carter KA, Shao S, Hoopes MI, Luo D, Ahsan B, Grigoryants VM, Song W, Huang H, Zhang G, Pandey RK, Geng J, Pfeifer BA, Scholes CP, Ortega J, Karttunen M, Lovell JF. Nat. Commun. 2014;5:3546. doi: 10.1038/ncomms4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Q, Huang Q, Li C, Bao C, Liu Z, Li F, Zhu L. J. Am. Chem. Soc. 2010;132:10645–10647. doi: 10.1021/ja103415t. [DOI] [PubMed] [Google Scholar]
- 30.Carling CJ, Viger ML, Huu VAN, Garcia AV, Almutairi A. Chem. Sci. 2015;6:335–341. doi: 10.1039/c4sc02651a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antony LA, Slanina T, Sebej P, Solomek T, Klan P. Org. Lett. 2013;15:4552–4555. doi: 10.1021/ol4021089. [DOI] [PubMed] [Google Scholar]
- 32.Umeda N, Takahashi H, Kamiya M, Ueno T, Komatsu T, Terai T, Hanaoka K, Nagano T, Urano Y. ACS Chem. Biol. 2014;9:2242–2246. doi: 10.1021/cb500525p. [DOI] [PubMed] [Google Scholar]
- 33.Rubinstein N, Liu P, Miller EW, Weinstain R. Chem. Commun. 2015;51:6369–6372. doi: 10.1039/c5cc00550g. [DOI] [PubMed] [Google Scholar]
- 34.Goswami PP, Syed A, Beck CL, Albright TR, Mahoney KM, Unash R, Smith EA, Winter AH. J. Am. Chem. Soc. 2015;137:3783–3786. doi: 10.1021/jacs.5b01297. [DOI] [PubMed] [Google Scholar]
- 35.Nagaiah G, Remick SC. Future Oncol. 2010;6:1219–1228. doi: 10.2217/fon.10.90. [DOI] [PubMed] [Google Scholar]
- 36.Borowiak M, Nahaboo W, Reynders M, Nekolla K, Jalinot P, Hasserodt J, Rehberg M, Delattre M, Zahler S, Vollmar A, Trauner D, Thorn-Seshold O. Cell. 2015;162:403–411. doi: 10.1016/j.cell.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 37.Strekowski L, Lipowska M, Patonay G. J. Org. Chem. 1992;57:4578–4580. [Google Scholar]
- 38.Nani RR, Shaum JB, Gorka AP, Schnermann MJ. Org. Lett. 2015;17:302–305. doi: 10.1021/ol503398f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim SY, Hong KH, Kim DI, Kwon H, Kim HJ. J. Am. Chem. Soc. 2014;136:7018–7025. doi: 10.1021/ja500962u. [DOI] [PubMed] [Google Scholar]
- 40.Anderl J, Faulstich H, Hechler T, Kulke M. Methods Mol. Biol. 1045:51–70. doi: 10.1007/978-1-62703-541-5_4. 203. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z. Technol. Cancer Res. Treat. 2005;4:283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rai P, Mallidi S, Zheng X, Rahmanzadeh R, Mir Y, Elrington S, Khurshid A, Hasan T. Adv. Drug Deliv. Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.