Abstract
Background
Atherosclerosis seriously threats human health. Homocysteine is an independent risk factor closely related to DNA methylation. MTHFR C667T loci polymorphism is closely associated with homocysteine level. This study aimed to investigate the relationship among MTHFR C667T loci polymorphism, genome-wide methylation, and atherosclerosis.
Material/Methods
Blood sample was collected from 105 patients with coronary atherosclerosis and 105 healthy controls. Pyrosequencing methylation was used to detect LINE-1 methylation level. Polymerase chain reaction-restriction enzyme fragment length polymorphism (PCR-RFLP) was used to test MTHFR.
Results
LINE-1 methylation level in the patient group was significantly lower than in the controls (t=5.007, P<0.001). MTHFR C667T genotype distribution presented marked differences in the 2 groups. TT genotype carriers had significantly increased risk of atherosclerosis (OR=3.56, P=0.009). Three different genotypes of MTHFR C667T loci showed different LINE-1 methylation level between the 2 groups (P<0.01). LINE-1 methylation level in TT and CT genotype carriers was obviously lower than in CC genotype carriers (P<0.05).
Conclusions
MTHFR C667T loci polymorphism may affect atherosclerosis by regulating genome methylation level.
MeSH Keywords: 4-Nitroquinoline-1-oxide; Atherosclerosis; Multilevel Analysis; Polymorphism, Single-Stranded Conformational
Background
Atherosclerosis (AS) is one of the most common chronic diseases, and is also the pathological basis of many cardiovascular diseases, such as stroke and coronary heart disease. Incidence of AS has been steadily increasing due to changing lifestyles, and the morbidity of cardiovascular and cerebrovascular diseases caused by AS has also increased. More newly diagnosed cases are in young people. AS has become a major killer that seriously threatens health and quality of life.
Methylenetetrahydrofolatereductase (MTHFR) is a key enzyme to catalyze 5, 10-methylene tetrahydrofolic acid to 5-methyl tetrahydrofolic acid, while the latter is the methyl donor of homocysteine (Hcy). Thus, MTHFR is also the key enzyme to regulate Hcy metabolism. It has been found that MTHFR C667T loci polymorphism is associated with enzyme activity. For example, enzyme activity is reduced by 60% in TT genotype carriers [1], leading to Hcy and methylation processes that affect Hcy level in the blood [2–5]. Because Hcy is an independent risk factor for AS [6], MTHFRC667T loci gene polymorphism may also be correlated with AS occurrence. The present study aimed to explore the relationship between MTHFRC667T loci gene polymorphisms and AS susceptibility by conducting a case-control study.
In DNA methylation the methyl of S-adenosylmethionine transfers to the fifth carbon of cytosine to form 5-methyl cytosine under DNA methyltransferase activity. As a common epigenetic modification, DNA methylation abnormality is closely related to multiple diseases such as tumors [7]. DNA methylation is also related to AS [8]. Genome-wide methylation level was lower in AS patients [9] and affects atherosclerotic plaque formation in ApoE−/− mice [10,11]. Studies found that vascular smooth muscle cell genome DNA methylation level was regulated by Hcy [12]. Thus, we speculated that Hcy may affect AS occurrence by influencing the genome-wide methylation level. Hcy level was closely associated with MTHFR C667T loci polymorphism, and genome-wide methylation may also be related to it. This study tried to clarify the relationship between MTHFR gene polymorphism and genome-wide methylation level by detecting MTHFR C667T loci genotype and the LINE-1 methylation level to represent the whole genome [13,14].
Material and Methods
Objects
We enrolled 105 patients with coronary atherosclerosis in the First Affiliated Hospital of Xi’an Jiaotong University between July 1, 2012 and January 1, 2015. No patients received treatment before blood sampling. There were 67 males and 38 females with average age 60.14±2.56 years old. We selected 105 healthy subjects at the same period as controls, including 64 males and 41 females, with mean age 59.68±3.02 years old. Sex and age showed no significant difference between the 2 groups (P>0.05). Exclusion criteria of patients with coronary atherosclerosis includes: 1) severe liver and renal disease; 2) cardiovascular and cerebrovascular diseases, such as hematencephalon, cerebral embolism, cardiac failure, and myocardial infarction; and 3) familial hyperlipidemia. This study was approved by the Institutional Review Board of First Affiliated Hospital of Xi’an Jiaotong University. All patients provided written informed consent for blood sampling.
MTHFR C667T loci genotype detection
The Genomic DNA extraction kit (Applied Biosystems) was used to extract DNA from peripheral blood cells according to the manual. DNA purity and content was determined on protein nucleic acid detector. The DNA with absorbance of A260/A280 between 1.8 and 2.0 was used in the subsequent experiment. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used for MTHFR C667T loci genotyping. Target gene was amplified by PCR kit (Takara) at first, and the PCR primers used were [15]: Forward, 5′-TGAAGGAGAAGGTGTCTGCGGGA-3′, Reverse, 5′-AGGACGGTGCGGTGAGAGTG-3′. The cycling conditions consisted of an initial of single cycle of 5 min at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at 55°C, and 60 s at 72°C. PCR products were digested by HinfI restriction enzyme (Takara) at 37°C for 4 h, and the digested products were analyzed by gel electrophoresis. Electrophoresis results are shown in Figure 1.
Figure 1.
Electrophoresis results after enzyme digestion; 1 and 2 are TT genotype, 3 and 4 are CC genotype, and 5 is CT genotype.
LINE-1 promoter DNA methylation level detection
Pyrosequencing was used to detect LINE-1 promoter DNA methylation level. Two μg DNA was modified and purified using EpiTect Bisulfite Kit (Qiagen), according to the instruction manual. Genomic DNA through bisulfite conversion treated by M.SssI methylation transferase was used as positive control (universal methylation, UM). PCR primers used were: Forward, TTTTGAGTTAGGTGTGGGATATA; Reverse, Bio-AAAATCAAAAAATTCCCTTTC; sequencing primer, AGTTAGGTGTGGGATATAGT. The target segment was amplified using the PyroMark PCR kit (Qiagen). The cycling conditions consisted of an initial of single cycle of 15 min at 95°C, followed by 45 cycles of 30 s at 94°C, 30 s at 56°C, and 30 s at 72°C. Five-μl products were identified by agarose gel electrophoresis. One chain of the PCR product was marked by biotin, and it was separated from the unmarked single chain by vacuum preprocessor. The single-chain DNA was sequenced in the PyroMark Q96 ID System (Qiagen). All experiments were performed in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS16.0 software (Chicago, IL). Differences between groups were analyzed using chi-square test, t test, non-condition logistic regression analysis, ANOVA, or covariance analysis, as necessary. The inspection level was α=0.05.
Results
LINE-1 methylation level comparison in the 2 groups
Pyrosequencing was used to detect LINE-1 methylation level (Figure 2). LINE-1 methylation level in the patient group (84.79±1.21%) was obviously lower than in the healthy control (85.85±1.75%) (t=5.10, P<0.001). We used multiple linear regression (Enter method) to exclude possible confounding factors, utilizing LINE-1 methylation level as the dependent variable, and group, age, and sex were used as independent variables in statistical analysis. The results showed that the 2 groups remain significantly different (t=5.007, P <0.001) in terms of LINE-1 methylation levels.
Figure 2.
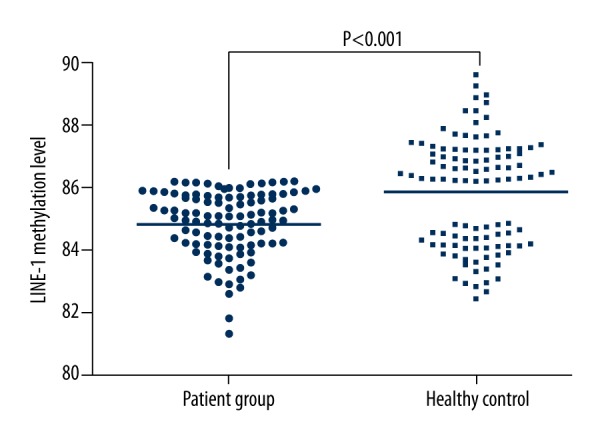
LINE-1 methylation level comparison in the 2 groups.
MTHFR C667T loci genotype distribution and Hardy-Weinberg genetic equilibrium test
Pyro PCR-RFLP was used for MTHFR C667T loci genotyping (Table 1). The chi-square test showed that MTHFR C667T loci allele frequency in controls conformed to Hardy-Weinberg genetic equilibrium (P>0.05), suggesting that enrolled healthy controls represented the general population.
Table 1.
MTHFR C667T loci Hardy-Weinberg genetic equilibrium test.
| Group | Genotype | Allele | χ2 value | P value | |||
|---|---|---|---|---|---|---|---|
| CC Case (%) |
CT Case (%) |
TT Case (%) |
C Case (%) |
T Case (%) |
|||
| Control | 63 (60.0) | 35 (33.3) | 7 (6.7) | 161 (76.7) | 49 (23.3) | 0.49 | 0.48 |
| Patient | 45 (42.9) | 42 (40.0) | 18 (17.1) | 132 (62.9) | 78 (37.1) | 2.16 | 0.14 |
Correlation analysis between MTHFR C667T loci polymorphism and AS susceptibility
The chi-square test was used to compare MTHFR C667T loci genotype and allele distribution in the 2 groups. We found that genotype (χ2=8.48, P=0.014) and allele distribution (χ2=9.49, P=0.002) were both significantly different.
Non-conditional logistic regression analysis was used to analyze the correlation between MTHFR C667T loci polymorphism and AS susceptibility (Table 2). Compared with CC carriers, TT genotype carriers showed obviously higher AS susceptibility (OR=3.6, P=0.008). AS susceptibility in T allele carriers was 1.94 times that in C allele carrier (95%CI=1.27~2.97, P=0.002). To exclude the influence of age and sex on the incidence, they were listed in the non-conditional logistic regression covariates. The results show that there was a significant increase in the risk of AS in MTHFR C667T locus TT genotype compared with CC genotype (OR=3.56, P=0.009). The risk of AS with T allele was 1.92 times that with C allele (95% CI=1.25~2.94, P=0.003).
Table 2.
Correlation analysis between MTHFR C667T loci polymorphism and AS susceptibility.
| Variable | OR (95%CI) Before correction |
P Before correction |
OR (95%CI) After correction |
P After correction |
|---|---|---|---|---|
| CT/CC | 1.68 (0.93–3.03) | 0.085 | 1.67 (0.92–3.03) | 0.093 |
| TT/CC | 3.60 (1.39–9.34) | 0.008* | 3.56 (1.37–9.27) | 0.009* |
| CT+TT/CC | 2.00 (1.16–3.46) | 0.013* | 1.98 (1.14–3.46) | 0.016* |
| T/C | 1.94 (1.27–2.97) | 0.002* | 1.92 (1.25–2.94) | 0.003* |
The relationship between MTHFR C667T loci polymorphism and LINE-1 methylation level
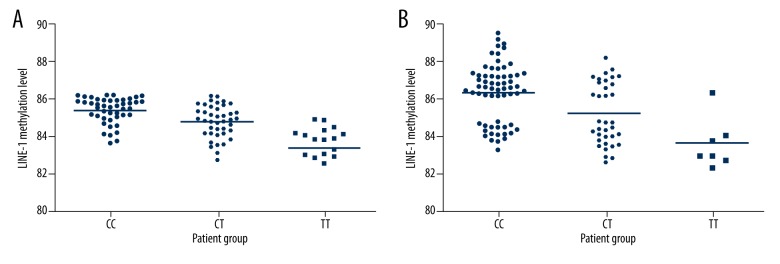
ANOVA was adopted to compare LINE-1 methylation level in different genotypes. We found that LINE-1 was correlated with MTHFR C667T loci polymorphism (Table 3 and Figure 3). LSD test also showed that LINE-1 methylation level in TT and CT genotype carriers were markedly lower than that in CC genotype carriers (P<0.05). After excluding the possible interference, such as sex and age, covariance analysis showed that LINE-1 methylation level still presented obvious differences among different MTHFR C667T loci genotypes (P<0.05).
Table 3.
LINE-1 methylation level comparison in different MTHFR C667T loci genotype.
| Genotype | Control | Control | ||||
|---|---|---|---|---|---|---|
| LINE-1 (%) | F | P | LINE-1 (%) | F | P | |
| TT | 83.37±1.52a,b | 27.81 | <0.001 | 83.70±1.35a,b | 12.98 | <0.001 |
| CT | 84.74±0.96a | 85.25±1.64a | ||||
| CC | 85.40±0.68 | 86.41±1.57 | ||||
P<0.05 compared with CC;
P<0.05 compared with CT.
Figure 3.
LINE-1 methylation distribution in different genotypes.
Discussion
AS, caused by a variety of genetic factors and environmental factors, is a chronic inflammation and autoimmune process. Its pathogenesis has not been fully elucidated, and mainly includes multiple theories, such as lipid infiltration, homocysteine, and endothelial injury reaction. Hcy is a type of sulfur-containing amino acid. High Hcy hyperlipidemia is an independent risk factor of cardiovascular disease [16,17]. Hcy level is closely related to DNA methylation level [17–19], and it can affect atherosclerosis occurrence and development by regulating DNA methylation level [20]. MTHFR is a key enzyme in regulating Hcy. Therefore, MTHFR gene polymorphism may affect DNA methylation level and further influence atherosclerosis morbidity.
Our results show that atherosclerosis patients presented lower genome-wide methylation level than in healthy controls, which is consistent with previous reports [9,21]. Our results further confirm that atherosclerosis occurrence is closely related to genome-wide methylation level reduction and that MTHFR C667T loci polymorphism was associated with AS occurrence. TT genotype carriers presented higher AS risk than CC genotype carriers, indicating that TT genotype was susceptible to AS. Szabo et al. [22] found that MTHFR C667T loci TT and CT genotypes in AS patients accounted for a higher proportion than controls, which is consistent to our results.
Studies have demonstrated that AS occurrence was associated with DNA methylation level, such as ER-α [18] and TFPI-2 [23]. This study further confirmed that genome-wide methylation level reduction was related to AS. Furthermore, LINE-1 methylation level in MTHFR C667T loci TT and CT genotype carriers was lower than in CC genotype carriers, suggesting that LINE-1 methylation level was associated with MTHFR C667T loci polymorphism. Bozovic et al. [24] found that LINE-1 methylation level in the mothers of Down syndrome children carrying MTHFR C667T loci TT and CT genotypes was lower than those of CC genotype carriers. Burghardt et al. [25] also reported that LINE-1 methylation level in TT carriers was lowest among the patients with schizophrenia. Together with the abovementioned study results, we confirmed that LINE-1 methylation level was associated with MTHFR C667T loci polymorphism.
Conclusions
Our results suggest that AS occurrence is associated with MTHFR C667T loci polymorphism and LINE-1 methylation level, and that MTHFR C667T loci polymorphism may affect AS through regulating genome-wide methylation level. Because MTHFR C667T loci polymorphism may affect Hcy level [5], MTHFR C667T loci polymorphism may affect genome-wide methylation level through mediating Hcy level. However, we did not detect Hcy level in the subjects, and this needs further investigation. Our sample size was not large, so the above reasoning remains to be further validated by expanding sample size. Our study provides a theoretical basis for AS mechanism investigation by analyzing the relationship between genome-wide methylation and MTHFR C667T loci polymorphism.
Footnotes
Source of support: This study was supported by the Key Program of the National Natural Science Foundation of China (NSFC, No. 30830051) and the “13115” Scientific and Technological Innovation Project of Shanxi Province, China (2008ZDKG-62)
References
- 1.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–13. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 2.Watkins D, Rosenblatt DS. Inborn errors of cobalamin absorption and metabolism. Am J Med Genet C Semin Med Genet. 2011;157C:33–44. doi: 10.1002/ajmg.c.30288. [DOI] [PubMed] [Google Scholar]
- 3.Montjean D, Benkhalifa M, Dessolle L, et al. Polymorphisms in MTHFR and MTRR genes associated with blood plasma homocysteine concentration and sperm counts. Fertil Steril. 2011;95:635–40. doi: 10.1016/j.fertnstert.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 4.Karpati I, Balla J, Szoke G, et al. [Frequency of hyperhomocysteinemia in hemodialysis patients with folic acid supplementation]. Orv Hetil. 2002;143:1635–40. [PubMed] [Google Scholar]
- 5.Gecene M, Tuncay F, Borman P, et al. Atherosclerosis in male patients with ankylosing spondylitis: the relation with methylenetetrahydrofolate reductase (C677T) gene polymorphism and plasma homocysteine levels. Rheumatol Int. 2013;33:1519–24. doi: 10.1007/s00296-012-2552-8. [DOI] [PubMed] [Google Scholar]
- 6.Ansari R, Mahta A, Mallack E, Luo JJ. Erratum: hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2015;11:106. doi: 10.3988/jcn.2015.11.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paska AV, Hudler P. Aberrant methylation patterns in cancer: a clinical view. Biochem Med (Zagreb) 2015;25:161–76. doi: 10.11613/BM.2015.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guardiola M, Vallve JC, Zaina S, Ribalta J. [Epigenetics in atherosclerosis]. Clin Investig Arterioscler. 2015 doi: 10.1016/j.arteri.2015.04.002. [Epub ahead of print] [in Spanish] [DOI] [PubMed] [Google Scholar]
- 9.Greissel A, Culmes M, Napieralski R, et al. Alternation of histone and DNA methylation in human atherosclerotic carotid plaques. Thromb Haemost. 2015;114:390–402. doi: 10.1160/TH14-10-0852. [DOI] [PubMed] [Google Scholar]
- 10.Hiltunen MO, Turunen MP, Hakkinen TP, et al. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med. 2002;7:5–11. doi: 10.1191/1358863x02vm418oa. [DOI] [PubMed] [Google Scholar]
- 11.Lund G, Andersson L, Lauria M, et al. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem. 2004;279:29147–54. doi: 10.1074/jbc.M403618200. [DOI] [PubMed] [Google Scholar]
- 12.Yideng J, Jianzhong Z, Ying H, et al. Homocysteine-mediated expression of SAHH, DNMTs, MBD2, and DNA hypomethylation potential pathogenic mechanism in VSMCs. DNA Cell Biol. 2007;26:603–11. doi: 10.1089/dna.2007.0584. [DOI] [PubMed] [Google Scholar]
- 13.Weisenberger DJ, Campan M, Long TI, et al. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–36. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang AS, Estecio MR, Doshi K, et al. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32:e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SA, Kang D, Nishio H, et al. Methylenetetrahydrofolate reductase polymorphism, diet, and breast cancer in Korean women. Exp Mol Med. 2004;36:116–21. doi: 10.1038/emm.2004.17. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Sun Y, Zhang L, et al. Detection of C677T mutation of MTHFR in subject with coronary heart disease by hairpin probe with enzymatic color on microarray. Biosens Bioelectron. 2011;28:84–90. doi: 10.1016/j.bios.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Li JJ, Li Q, Du HP, et al. Homocysteine Triggers Inflammatory Responses in Macrophages through Inhibiting CSE-H2S Signaling via DNA Hypermethylation of CSE Promoter. Int J Mol Sci. 2015;16:12560–77. doi: 10.3390/ijms160612560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YS, Zhi YF, Wang SR. Hypermethylation of estrogen receptor-alpha gene in atheromatosis patients and its correlation with homocysteine. Pathophysiology. 2009;16:259–65. doi: 10.1016/j.pathophys.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Yang X, Ma L, et al. Homocysteine-mediated cholesterol efflux via ABCA1 and ACAT1 DNA methylation in THP-1 monocyte-derived foam cells. Acta Biochim Biophys Sin (Shanghai) 2013;45:220–28. doi: 10.1093/abbs/gms119. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Jiao Y, Yang AN, et al. [The effect of miR-124 on homocysteine-induced atherosclerosis via promoter region DNA methylation in ApoE(−/−) mice]. Sheng Li Xue Bao. 2015;67:207–13. in Chinese. [PubMed] [Google Scholar]
- 21.Turunen MP, Aavik E, Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790:886–91. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Szabo GV. The role and importance of gene polymorphisms in the development of atherosclerosis. Interv Med Appl Sci. 2013;5:46–51. doi: 10.1556/IMAS.5.2013.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zawadzki C, Chatelain N, Delestre M, et al. Tissue factor pathway inhibitor-2 gene methylation is associated with low expression in carotid atherosclerotic plaques. Atherosclerosis. 2009;204:e4–14. doi: 10.1016/j.atherosclerosis.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Babic Bozovic I, Stankovic A, Zivkovic M, et al. Altered LINE-1 Methylation in Mothers of Children with Down Syndrome. PLoS One. 2015;10:e0127423. doi: 10.1371/journal.pone.0127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burghardt KJ, Pilsner JR, Bly MJ, Ellingrod VL. DNA methylation in schizophrenia subjects: gender and MTHFR 677C/T genotype differences. Epigenomics. 2012;4:261–68. doi: 10.2217/epi.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]