Abstract
Carbamylated erythropoietin (cEpo), which is neuroprotective but lacks hematopoietic activity, has been attracting rising concerns. However, the cellular and molecular mechanisms involved in the process of neuroprotection of cEpo are not well known. Based on several recent reports, the neuroprotective effects of cEpo are illustrated, and signaling pathways involved in the different effects of erythropoietin and cEpo are discussed. These newly reported researches may shed new light on the development and application of cEpo, a prospective drug candidate for neuroprotection.
Keywords: carbamylated erythropoietin, neuroprotection, signaling pathway
Introduction
Erythropoietin (EPO) is a pleiotropic cytokine originally identified for its role in erythropoiesis.1 Although EPO was first recognized for its hematopoietic properties, much attention was attracted by its tissue protection function. The neuroprotective effect of EPO was shown in documented experimental reports.2–4 However, the fact that EPO increases hematocrit and hemoglobin levels precludes any possible clinical development of EPO as a drug candidate for treating diseases of the central nervous system (CNS).5 Since the stimulation of erythropoiesis is unwanted for neuroprotection, the development of EPO-like compounds with selective actions is attracting rising interest. One such compound, carbamylated EPO (cEpo), is derived from the carbamylation of EPO’s lysine residues. cEpo retains strong neuroprotective properties while lacking the hematopoietic complications of EPO.6 Thus, cEpo may possibly become a potential drug to treat diseases of the CNS, including neurodegenerative disorders (Parkinson’s and Alzheimer’s diseases), spinal cord injury, and stroke.7,8 In this review, the neuroprotective effects of cEpo and the underlying cellular and molecular mechanisms are discussed.
EPO and its Derivatives
EPO is a glycoprotein hormone mainly synthesized in fetal liver and adult kidneys and is also detected in multiple tissues including liver, lung, and brain.9,10 The molecular weight of EPO is ~34 kDa consisting of a single polypeptide chain of 165 amino acids with four oligosaccharide chains terminated by sialic acids.9 The four oligosaccharide chains are located at Asp-24, Asp-38, and Asp-8 through N-glycosylation linking and at Ser-126 through O-glycosylation linking. There are four α-helices in the secondary dimensional structure of EPO, which are helices A–D. Helices A, C, and D are hydrophobic and participate in the binding with the EPO receptor (EPOR), while the remaining helix B is hydrophilic and involved in tissue protection.11
In 2004, Leist et al reported that the carbamylation of EPO maintained the tissue-protective effects of EPO and removed its erythropoietic effects.6 The chemical modification resulted in a kind of non-hematopoietic EPO derivative, cEpo. It was the first report indicating that the erythropoietic effects and tissue-protective effects of EPO could be separated. Subsequent studies suggested that the erythropoietic effects and tissue-protective effects of EPO were mediated by different receptors.12 Based on these discoveries, many efforts have been made to design and validate EPO derivatives with tissue-protective effects but not with erythropoietic effects. These derivatives include modified EPO molecules and peptides that mimic the three-dimensional structure of EPO.13 Two major modified EPO molecules are now developed and verified to have tissue-protective effects, and they are cEpo and asialoerythropoietin (asialoEpo). Although the modification of EPO through carbamylation was first reported by Leist et al, the method of producing cEpo was described in a patent by Warren Pharmaceuticals.14 The eight lysine residues, together with the N-terminal alanine in the polypeptide chain of EPO, provide nine amino groups for carbamylation. The ideal carbamylated product was cEpo with ≤10% free primary amines on the lysines and the N-terminal amino acids. The invention documented in the patent by Warren Pharmaceuticals claims an efficient method to produce preferential carbamylation of the nine primary amino groups of lysine and N-terminal amino acids containing limited amounts of over- or under-cEpo isoforms. Accumulated research works indicated that cEpo was a promising neuroprotective agent to treat neural injury (discussed in detail in the following sections). asialoEPO was generated by enzymatic desialylation of EPO. The sialic acids at the end of the oligosaccharide chains of EPO help to maintain its stability in vivo. Therefore, the desialylation of EPO gives a short half-life of asialoEpo. The short half-life gives asialoEpo insufficient persistence time to significantly stimulate hematopoiesis, but a brief action time is needed for tissue-protective effects. asialoEpo was validated to have protective effects in several kinds of tissues including heart, kidney, and lungs.15–17 In this review, we focus on cEpo as a promising neural protective drug.
Neuroprotective Effect of cEpo
cEpo has gained rising attentions in recent years. After proposing the carbamylation of EPO for the purpose of removing its erythropoietic effects, Leist et al conducted a preliminary experiment suggesting the neuroprotective effects of cEpo in a wide range of animal models of neurotoxicity including ischemic stroke, sciatic nerve compression, spinal cord depression, and peripheral diabetic neuropathy.6 They also underwent in vivo studies in mice and rats showing that no erythropoietic effects were observed after high doses of cEpo use for long periods. Since then, several reports showed that cEpo could improve functional outcomes in multiple infarct ischemic strokes, spinal cord hemisection, and focal cerebral ischemia.7,18,19 The neuroprotective effects of cEpo may related to its anti-inflammatory and antiapoptotic effects in the model of traumatic brain injury (TBI),20 spinal cord hemisection,18 and focal cerebral ischemia.19 In the last five years, an increasing number of studies proved that cEpo was neuroprotective in many CNS diseases including Alzheimer’s disease,21 Parkinson’s disease,22 periventricular leukomalacia,23 spinal cord ischemia/reperfusion injury,24 and TBI.25 Till now substantial experimental data have been gained to support cEpo as a promising agent to treat neural injury. (Articles on this issue were summarized in Table 1.) However, these results have only revealed the neuroprotective effects of cEpo in a wide range of CNS diseases, and systematic research work focusing on a specific disease needs to be performed to determine whether further clinical development is justified.
Table 1.
Articles on the neuroprotective effects of cEpo.
| ARTICLE | YEAR | AUTHOR |
|---|---|---|
| Carbamylated erythropoietin mediates retinal neuroprotectiona in streptozotocin-induced early-stage diabetic rats | 2015 | Liu X, et al |
| Memory Improvement in the AbetaPP/PS1 Mouse Model of Familial Alzheimer’s Disease Induced by Carbamylated-Erythropoietin is Accompanied by Modulation of Synaptic Genes | 2015 | Armand-Ugon M, et al |
| Carbamylated erythropoietin ameliorates hypoxia-induced cognitive and behavioral defects with the generation of choline acetyltransferase-positive neurons | 2013 | Ding J, et al |
| The neuroprotective and neurorescue effects of carbamylated erythropoietin Fc fusion protein (CEPO-Fc) in a rat model of Parkinson’s disease | 2013 | Thomas Tayra J, et al |
| Beneficial effects of carbamylated erythropoietin against oxygen-glucose deprivation/reperfusion-induced astrocyte swelling: proposed molecular mechanisms of action | 2012 | Tang Z, et al |
| Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury | 2012 | Xiong Y, et al |
| Neuroprotective potential of erythropoietin and its derivative carbamylated erythropoietin in periventricular leukomalacia | 2011 | Liu W, et al |
| Reduced brain edema and functional deficits after treatment of diffuse traumatic brain injury by carbamylated erythropoietin derivative | 2011 | Bouzat P, et al |
| Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury | 2011 | Simon F, et al |
| Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury | 2011 | Xiong Y, et al |
| Therapeutic window for nonerythropoietic carbamylated-erythropoietin to improve motor function following multiple infarct ischemic strokes in New Zealand white rabbits | 2008 | Lapchak PA, et al |
| Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury | 2008 | Adembri C, et al |
| Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat | 2007 | King VR, et al |
| Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia | 2007 | Wang Y, et al |
| Carbamylated erythropoietin reduces radiosurgically-induced brain injury | 2006 | Erbayraktar S, et al |
Mechanisms Involved in the Neural Protection of cEpo
The underlying mechanisms involved in the neural protection of cEpo have not been clarified well. Receptors and several signaling pathways were identified to play a role in the neural protection of cEpo.
The classical EPOR is a homodimer of two identical EPOR subunits, which was initially identified to mediate erythropoiesis. The extrahematopoietic activity of EPO is supposed to involve a different type of receptor, named the β common receptor (βcR). βcR is a heterodimer consisting of one EPOR monomer and CD131, the cytokine β common subunit, shared by receptors for granulocyte macrophage colony-stimulating factor, interleukin (IL)-3, and IL-5.26 However, Um et al.27 reported that EPO exerted its antiapoptotic effects on differentiated SH-SY5Y and PC-12 cells through its binding to the classical homodimeric EPOR complex, and βcR expression was not detected in these cell lines. The direct evidence on the receptors involved in the action of EPO neuroprotection was obtained by assays of receptor inhibition and competition. The assays showed that the antiapoptotic effect of EPO was completely blocked by anti-EPOR addition and only blunted in the presence of anti-βcR incubation.28 The study suggests that both the homodimeric EPOR and the heterodimetic βcR may participate in the neuroprotective activity of EPO in some types of neural cells. In that study, the inhibition and competition assays were also used to detect the receptors involved in the neuroprotective activity of cEpo. Consequently, the experiment confirmed that cEpo did not bind to the classical EPOR and may only bind to the βcR,28 that is, cEpo exerts its functions through binding to the βcR but not to the classical EPOR. In an in vitro test, Sturm et al found that cEpo increased the expression of frataxin, a mitochondrial protein related to Friedreich’s ataxia, both in human erythroleukemic K562 cells (expressing EPOR) and human monocytic leukemia THP-1 cells (not expressing EPOR).29 The result indicates that cEpo upregulates frataxin expression without the involvement of binding to the classical EPOR. Recently, Ding et al proposed that cEpo increased cell proliferation in primary neurons from embryonic mice after oxygen–glucose deprivation but lacked proliferative activity in Neuro-2a cells both in vitro and in vivo.30 The nonsupport of cEpo for the proliferation of Neuro-2a cells could be attributed to the lack of CD131 in the cell lines, suggesting the involvement of CD131 in the function of cEpo in non-erythroid cells. This lack of agreement on the receptors involved in cEpo and EPO actions suggests that their different effects may not only exist in the binding action to different receptors.
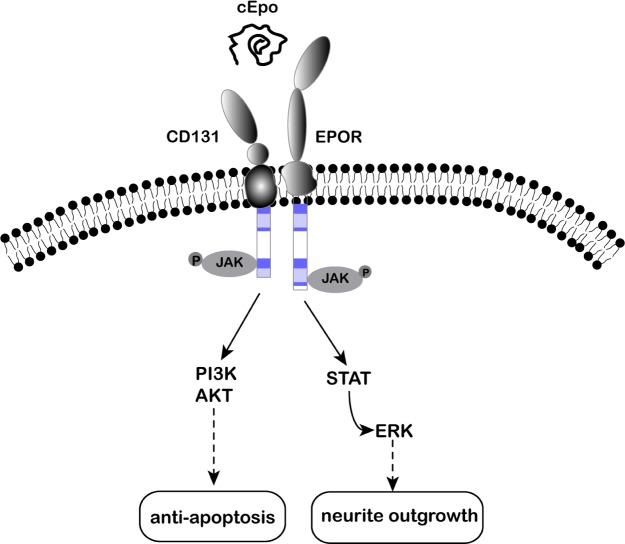
With respect to neuroprotection, cEpo appears to induce antiapoptotic signaling pathways similar to those stimulated by EPO. In SH-SY5Y neuroblastoma cells, similar Jak2- and PI3K-mediated mechanisms for the antiapoptotic actions of EPO and cEpo were observed.28 In the activity of promoting neurite outgrowth and neuronal spine formation, cEpo increases the expression of two well-characterized postsynaptic molecules, Shank2 and Shank3, by upregulating CREB-binding protein (CBP)/E1A-associated protein (p300), a histone acetyltransferase that acetylates histones and in turn modulates neuronal functions. The signaling pathways from cEpo to CBP/p300 involve the phosphorylation of the signal transducer and activator of transcription (STAT)-3, the extracellular signal-regulated kinase (Erk), and Akt,31 whereas in UT-7 and TF-1 cells, cEpo induced Jak2 phosphorylation but did not produce a significant activation of cell-proliferating signals, such as Erk1/2, nuclear factor-κB, and STAT-5. Reasonably, the different effects of EPO and cEpo may involve some critical molecules in the signaling pathways. Recently, Chamorro et al demonstrated that cEpo blocked phosphorylation of the forkhead transcription factor FOXO3a and failed to induce subsequent p27kip1 downregulation in UT-7 and TF-1 cells. In contrast, the activation of cell proliferation by EPO was consistent with the increase in FOXO3a phosphorylation and the downregulation of its downstream target p27kip1. The level and time course of phosphorylation of some signal factors, including Jak2, Akt, Erk1/2, and FOXO3a, was another possible reason accounting for the different effects of cEpo and EPO.32 The result from Chamorro et al showed that Akt and Erk1/2 phosphorylation was strongly induced after 15 minutes of EPO treatment and remained high after 60 minutes in UT-7 cells. On the contrary, after cEpo treatment, Akt and Erk phosphorylations were significantly lower at 15 minutes and barely detectable at 60 minutes. In general, these reports identified certain signal pathways involved in the neuroprotection of cEpo but still cannot illustrate the underlying mechanisms (Fig. 1).
Figure 1.
Signaling pathways involved in the biological activity of cEpo.
cEpo retains the ability of EPO to protect neuronal cells, while it is unable to support erythroid cell survival and proliferation. The neuroprotective effects of cEpo have been confirmed in accumulated research work. To date, no toxicity has been reported regarding cEpo. These advantages make cEpo a promising drug candidate for many CNS diseases. However, the underlying mechanisms of the neuroprotection of cEpo have not been clarified well. The identification of key factors involved in the neuroprotection of cEpo may shed new light on the elucidation of the signaling pathways. Reports on the preclinical trials and clinical trials of cEpo are rare due to the lack of systematic experimental evidences for a certain disease. Further studies on these areas should be carried out to provide substantial research data for the clinical application of cEpo, such as therapeutic windows, dose, and toxicity of long-term usage. The future of cEpo for neuronal diseases is promising, and its safety and efficacy, as well as the molecular mechanism of neuroprotection, are significant issues to be clarified.
Footnotes
ACADEMIC EDITOR: Gabor Mocz, Editor in Chief
PEER REVIEW: Seven peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,277 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: JMC. Wrote the first draft of the manuscript: JMC. Contributed to the writing of the manuscript: JMC, ZY, XZ. Developed the structure and arguments for the paper: JMC. Made critical revisions: JMC, XZ. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Lombardero M, Kovacs K, Scheithauer BW. Erythropoietin: a hormone with multiple functions. Pathobiology. 2011;78(1):41–53. doi: 10.1159/000322975. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi R, Brines M, Lauria G, et al. Protective effect of erythropoietin and its carbamylated derivative in experimental cisplatin peripheral neurotoxicity. Clin Cancer Res. 2006;12(8):2607–2612. doi: 10.1158/1078-0432.CCR-05-2177. [DOI] [PubMed] [Google Scholar]
- 3.Erbaş O, Çınar BP, Solmaz V, et al. The neuroprotective effect of erythropoietin on experimental Parkinson model in rats. Neuropeptides. 2015;49:1–5. doi: 10.1016/j.npep.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Gu L, Xu H, Wang F, et al. Erythropoietin exerts a neuroprotective function against glutamate neurotoxicity in experimental diabetic retina. Invest Ophthalmol Vis Sci. 2014;55(12):8208–8222. doi: 10.1167/iovs.14-14435. [DOI] [PubMed] [Google Scholar]
- 5.Torup L. Neuroprotection with or without erythropoiesis; sometimes less is more. Br J Pharmacol. 2007;151(8):1141–1142. doi: 10.1038/sj.bjp.0707287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305(5681):239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 7.Lapchak PA, Kirkeby A, Zivin JA, et al. Therapeutic window for nonerythropoietic carbamylated-erythropoietin to improve motor function following multiple infarct ischemic strokes in New Zealand white rabbits. Brain Res. 2008;1238:208–214. doi: 10.1016/j.brainres.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Mofidi A, Bader A, Pavlica S. The use of erythropoietin and its derivatives to treat spinal cord injury. Mini Rev Med Chem. 2011;11(9):763–770. doi: 10.2174/138955711796355267. [DOI] [PubMed] [Google Scholar]
- 9.Lapchak PA. Carbamylated erythropoietin to treat neuronal injury: new development strategies. Expert Opin Investig Drugs. 2008;17(8):1175–1186. doi: 10.1517/13543784.17.8.1175. [DOI] [PubMed] [Google Scholar]
- 10.Collino M, Thiemermann C, Cerami A, et al. Flipping the molecular switch for innate protection and repair of tissues: long-lasting effects of a non-erythropoietic small peptide engineered from erythropoietin. Pharmacol Ther. 2015;151:32–40. doi: 10.1016/j.pharmthera.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Brines M. The therapeutic potential of erythropoiesis-stimulating agents for tissue protection: a tale of two receptors. Blood Purif. 2010;29(2):86–92. doi: 10.1159/000245630. [DOI] [PubMed] [Google Scholar]
- 12.Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101(41):14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brines M, Patel NS, Villa P, et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci U S A. 2008;105(31):10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren Pharmaceuticals WO2006/014466. Novel carbamylated epo and method for its production. 2006
- 15.Sonoda A, Nitta N, Tsuchiya K, et al. Asialoerythropoietin ameliorates bleomycin-induced acute lung injury in rabbits by reducing inflammation. Exp Ther Med. 2014;8(5):1443–1446. doi: 10.3892/etm.2014.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeyama T, Takemura G, Kanamori H, et al. Asialoerythropoietin, a nonerythropoietic derivative of erythropoietin, displays broad anti-heart failure activity. Circ Heart Fail. 2012;5(2):274–285. doi: 10.1161/CIRCHEARTFAILURE.111.965061. [DOI] [PubMed] [Google Scholar]
- 17.Nakazawa J, Isshiki K, Sugimoto T, et al. Renoprotective effects of asialoerythropoietin in diabetic mice against ischaemia-reperfusion-induced acute kidney injury. Nephrology (Carlton) 2010;15(1):93–101. doi: 10.1111/j.1440-1797.2009.01170.x. [DOI] [PubMed] [Google Scholar]
- 18.King VR, Averill SA, Hewazy D, et al. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci. 2007;26(1):90–100. doi: 10.1111/j.1460-9568.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Zhang ZG, Rhodes K, et al. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151(8):1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adembri C, Massagrande A, Tani A, et al. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med. 2008;36(3):975–978. doi: 10.1097/CCM.0B013E3181644343. [DOI] [PubMed] [Google Scholar]
- 21.Armand-Ugon M, Aso E, Moreno J, et al. Memory improvement in the AbetaPP/PS1 mouse model of familial Alzheimer’s disease induced by carbamylated-erythropoietin is accompanied by modulation of synaptic genes. J Alzheimers Dis. 2015;45(2):407–421. doi: 10.3233/JAD-150002. [DOI] [PubMed] [Google Scholar]
- 22.Thomas Tayra J, Kameda M, Yasuhara T, et al. The neuroprotective and neurorescue effects of carbamylated erythropoietin Fc fusion protein (CEPO-Fc) in a rat model of Parkinson’s disease. Brain Res. 2013;1502:55–70. doi: 10.1016/j.brainres.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Liu W, Shen Y, Plane JM, et al. Neuroprotective potential of erythropoietin and its derivative carbamylated erythropoietin in periventricular leukomalacia. Exp Neurol. 2011;230(2):227–239. doi: 10.1016/j.expneurol.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon F, Scheuerle A, Gröger M, et al. Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury. Intensive Care Med. 2011;37(9):1525–1533. doi: 10.1007/s00134-011-2303-4. [DOI] [PubMed] [Google Scholar]
- 25.Xiong Y, Mahmood A, Zhang Y, et al. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J Neurosurg. 2011;114(2):549–559. doi: 10.3171/2010.10.JNS10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy JM, Young IG. IL-3, IL-5, and GM-CSF signaling: crystal structure of the human beta-common receptor. Vitam Horm. 2006;74:1–30. doi: 10.1016/S0083-6729(06)74001-8. [DOI] [PubMed] [Google Scholar]
- 27.Um M, Gross AW, Lodish HF. A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19(3):634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Chamorro ME, Wenker SD, Vota DM, et al. Signaling pathways of cell proliferation are involved in the differential effect of erythropoietin and its carbamylated derivative. Biochim Biophys Acta. 2013;1833(8):1960–1968. doi: 10.1016/j.bbamcr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Sturm B, Helminger M, Steinkellner H, et al. Carbamylated erythropoietin increases frataxin independent from the erythropoietin receptor. Eur J Clin Invest. 2010;40(6):561–565. doi: 10.1111/j.1365-2362.2010.02292.x. [DOI] [PubMed] [Google Scholar]
- 30.Ding J, Li QY, Yu JZ, et al. The lack of CD131 and the inhibition of Neuro-2a growth by carbamylated erythropoietin. Cell Biol Toxicol. 2015;31(1):29–38. doi: 10.1007/s10565-015-9292-y. [DOI] [PubMed] [Google Scholar]
- 31.Choi M, Ko SY, Lee IY, et al. Carbamylated erythropoietin promotes neurite outgrowth and neuronal spine formation in association with CBP/p300. Biochem Biophys Res Commun. 2014;446(1):79–84. doi: 10.1016/j.bbrc.2014.02.066. [DOI] [PubMed] [Google Scholar]
- 32.Chamorro ME, Maltaneri RE, Vittori DC, et al. Protein tyrosine phosphatase 1B (PTP1B) is involved in the defective erythropoietic function of carbamylated erythropoietin. Int J Biochem Cell Biol. 2015;61:63–71. doi: 10.1016/j.biocel.2015.01.019. [DOI] [PubMed] [Google Scholar]