Abstract
Objectives
Candida species are major causes of invasive mycoses in immunocompetent and immunocompromised hosts. Treatment options are limited in the setting of antifungal resistance and increased rates of echinocandin-resistant Candida glabrata have been reported. The novel arylamidine T-2307 demonstrates potent in vitro antifungal activity against Candida species. Our objective was to evaluate the in vitro and in vivo activity of T-2307 against resistant C. glabrata.
Methods
In vitro activity was determined against 42 clinical C. glabrata isolates, including 17 echinocandin-resistant strains. Neutropenic ICR mice were inoculated intravenously with an echinocandin-resistant C. glabrata isolate (T-2307; caspofungin MICs ≤0.008 and 0.5 mg/L, respectively). Therapy with vehicle control, T-2307 (0.75, 1.5, 3 or 6 mg/kg subcutaneously once daily) or caspofungin (1 or 10 mg/kg intraperitoneally once daily) began 1 day post-challenge. Kidneys were collected on day 8 and fungal burden was assessed by counting cfu.
Results
T-2307 demonstrated potent in vitro activity against C. glabrata (geometric mean MIC 0.0135 mg/L), which was maintained against echinocandin-resistant isolates (geometric mean MIC 0.0083 mg/L). T-2307 also demonstrated in vivo efficacy in mice infected with echinocandin-resistant C. glabrata. Significant reductions in fungal burden were observed at each dosage level of T-2307 compared with control. Reductions in fungal burden were also observed with high-dose caspofungin.
Conclusions
T-2307 demonstrated potent in vitro activity against C. glabrata, including echinocandin-resistant isolates, which translated into in vivo efficacy against invasive candidiasis caused by an echinocandin-resistant C. glabrata strain. These results demonstrate the potential for T-2307 as therapy against echinocandin-resistant Candida.
Introduction
Candida glabrata is a common species isolated from patients with invasive candidiasis.1 The treatment of infections caused by this species may be limited, as many isolates may be resistant to fluconazole or require higher doses of this widely available azole.2 Treatment guidelines recommend the use of the echinocandins for invasive infections caused by C. glabrata due to their in vivo efficacy and good tolerability.2 However, echinocandin-resistant C. glabrata may be increasing at some institutions in the USA.3–5
T-2307 is a novel arylamidine structurally similar to pentamidine and causes the collapse of mitochondrial membrane potential.6–8 This agent has potent activity against Candida species, including azole-resistant isolates,6 and was recently shown to be efficacious against echinocandin-resistant Candida albicans.9 Our objective was to assess the in vitro activity of T-2307 against C. glabrata, including echinocandin-resistant isolates, and determine whether this translated into in vivo efficacy in a murine model of invasive candidiasis caused by resistant C. glabrata.
Materials and methods
Antifungals
T-2307 (Toyama Chemical Company, Ltd, Toyama, Japan) and caspofungin powder (Merck & Company, Inc., Whitehouse Station, NJ, USA) powders were dissolved in DMSO to prepare a stock solution for susceptibility testing with further dilutions in RPMI buffered with 0.165 M MOPS (pH 7.0). For in vivo studies, a solution of T-2307 was prepared in sterile physiological saline and the commercially available formulation of caspofungin was used.
Candida glabrata isolates and in vitro susceptibility testing
Forty-two C. glabrata clinical isolates from the University of Texas Health Science Center at San Antonio were used. This included 25 WT isolates and 17 that were echinocandin-resistant according to the CLSI M27-S4 clinical breakpoints.10 Antifungal susceptibility testing was performed according to the CLSI M27-A3 methods with MICs read after 24 h of incubation at both 50% and 100% inhibition of growth.11 In the in vivo model an echinocandin-resistant C. glabrata clinical isolate (05-62) with a defined Fks2p amino acid substitution (R1379S) was used. This resistant isolate was chosen as it results in consistent infection in the murine model described below.12,13 The isolate was sub-cultured three times on Sabouraud dextrose agar, each for 48 h at 37°C. Colonies were taken from the third subculture by scraping the surface of the agar plates and washing three times in sterile saline with 0.1% Tween 20. The cells were then collected by centrifugation and resuspended in sterile saline with 0.1% Tween to the target inoculum and the number of viable cells/mL was determined by counting cfu.
Animal model and fungal burden
We utilized our established murine model of invasive candidiasis for this study.12,13 Outbred male ICR mice were used and had access to food and water ad libitum. Mice were rendered neutropenic by a single dose of 150 mg/kg 5-fluorouracil 1 day prior to inoculation. On day 0, mice were infected intravenously with C. glabrata at a target inoculum of 1 × 108 cfu/mouse. Twenty-four hours after inoculation, therapy was started with control (sterile physiological saline), T-2307 (0.75, 1.5, 3 or 6 mg/kg once daily by subcutaneous injection) or caspofungin (1 or 10 mg/kg once daily by intraperitoneal injection), and continued for 7 days. These doses were chosen based on our previous experience with both T-2307 and caspofungin against invasive candidiasis caused by echinocandin-susceptible and -resistant isolates of C. glabrata and C. albicans.9,13 On day 8, mice were humanely euthanized and the kidneys were removed, weighed and homogenized in sterile saline. Serial dilutions of the homogenates were prepared and plated on Sabouraud dextrose agar. Following incubation, colonies were counted and the number of cfu/g within the kidney tissue was calculated. This study was approved by the Institutional Animal Care and Use Committee at UTHSCSA (protocol number 10129x) and all animals were maintained in accordance with the Association for the Assessment and Accreditation of Laboratory Animal Care.
Data analysis
For the in vitro analysis, MIC ranges, MIC50 values, MIC90 values and geometric mean (GM) MIC values were determined. Differences in GM MICs were assessed for significance using a paired t-test. Differences in fungal burden were assessed for significance using ANOVA with Tukey's test for multiple comparisons. A P value of ≤0.05 was considered statistically significant.
Results
In vitro susceptibility
T-2307 demonstrated potent in vitro activity against C. glabrata, including echinocandin-resistant isolates. The MICs for all isolates ranged from ≤0.008 to >4 mg/L for a single isolate using the 50% inhibition endpoint, with the T-2307 MIC ≤0.008 mg/L for the majority of isolates (Table 1). Caspofungin MICs ranged from 0.06 to 4 mg/L and the GM MIC of caspofungin was significantly higher than that observed with T-2307 (0.3773 versus 0.0135 mg/L, respectively; P < 0.0001). The potency of T-2307 was also maintained against the echinocandin-resistant isolates (MIC range ≤0.008–0.015 mg/L; GM MIC 0.0083). In contrast, caspofungin MICs ranged from 0.5 to 4 mg/L for these 17 isolates (GM MIC 0.8849). The MICs of T-2307 and caspofungin for the strain used to infect mice (05-62) were ≤0.008 and 0.5 mg/L, respectively. As previously reported, T-2307 did not result in complete inhibition of growth, as reflected by the MICs of >4 mg/L when the 100% inhibition endpoint was used, which is consistent with the trailing effect observed with this agent.9,14 Thus, it is unknown whether the isolate with the elevated T-2307 MIC (>4 mg/L) was truly resistant to this agent or whether this was due to the trailing effect. However, no visual reductions in growth were observed against this isolate on two separate occasions and its growth rate was not reduced compared with the others.
Table 1.
MICs (mg/L) of T-2307 (0.008–4 mg/L) and caspofungin (0.015–8 mg/L) for 42 C. glabrata isolates, including caspofungin-susceptible (n = 25) and caspofungin-resistant (n = 17) strains
| Parameter | T-2307 | Caspofungin |
|---|---|---|
| All isolates (n = 42) | ||
| MIC range | ≤0.008 to >4 | 0.06–4 |
| MIC50 | ≤0.008 | 0.25 |
| MIC90 | ≤0.008 | 2 |
| GM MIC | 0.0135 | 0.3773 |
| Echinocandin-resistant isolates (n = 17) | ||
| MIC range | ≤0.008–0.015 | 0.5–4 |
| MIC50 | ≤0.008 | 0.5 |
| MIC90 | ≤0.008 | 2 |
| GM MIC | 0.0083 | 0.8849 |
MIC50 and MIC90, MICs at which 50% and 90% of the isolates were inhibited, respectively.
The 50% inhibition endpoint is shown for both T-2307 and caspofungin.
Fungal burden
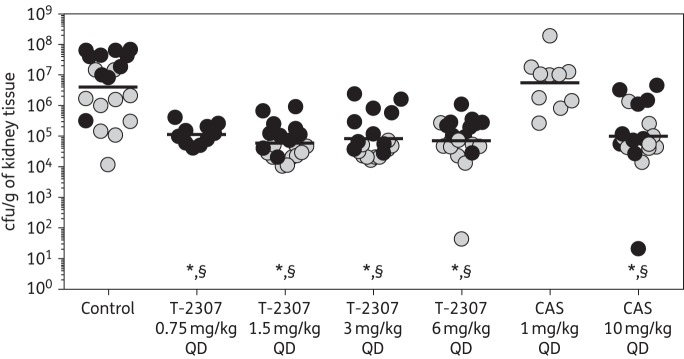
The in vitro potency of T-2307 translated into in vivo efficacy in our neutropenic murine model of invasive candidiasis. Significant reductions in fungal burden were observed with each T-2307 dose (range of mean log10 cfu/g 4.78–5.01) compared with control (6.60 log10 cfu/g; P ≤ 0.001 for all comparisons) (Figure 1). These results were reproducible for the T-2307 dose levels of 1.5, 3 and 6 mg/kg, which were evaluated in two independent experiments. Fungal burden with T-2307 was also significantly lower than that observed with 1 mg/kg caspofungin (6.75 log10 cfu/g; P ≤ 0.001 for all comparisons). In contrast, high-dose 10 mg/kg caspofungin also resulted in significant reductions in fungal burden compared with both control and the lower dose level of this echinocandin (5.00 log10 cfu/g; P < 0.001). When two low fungal burden outliers were removed from the 6 mg/kg T-2307 and 10 mg/kg caspofungin groups, the reductions in fungal burden compared with control and 1 mg/kg caspofungin were still significant (5.02 and 5.19 log10 cfu/g for 6 mg/kg T-2307 and 10 mg/kg caspofungin, respectively; P < 0.001 for both comparisons).
Figure 1.
Kidney fungal burden (cfu/g of kidney tissue) on day 8 in mice infected with C. glabrata 05-62 and treated with placebo by subcutaneous injection once daily as follows: control (physiological saline); T-2307 at doses of 0.75, 1.5, 3 or 6 mg/kg by subcutaneous injection once daily; or caspofungin at doses of 1 or 10 mg/kg by intraperitoneal injection once daily. Treatment began 1 day post-inoculation and continued for 7 days. Grey circles represent data from the first experiment and black circles represent data from the repeat experiment. Lines represent mean values. *P < 0.001 versus vehicle control; §P < 0.001 versus 1 mg/kg caspofungin. CAS, caspofungin; QD, once daily.
Discussion
T-2307 is an investigational antifungal that causes collapse of fungal mitochondrial membrane potential following uptake.7,8 Previous studies have demonstrated potent in vitro activity against Candida species, Cryptococcus neoformans and Aspergillus fumigatus, which has translated into in vivo efficacy in murine models of invasive disease caused by these species.6 We have recently demonstrated that T-2307 also maintains potent in vitro activity against echinocandin-resistant C. albicans, which translated into in vivo efficacy in a murine model of invasive candidiasis.9 This activity against resistant Candida is encouraging, because treatment options against such infections are limited. Recently, several institutions in the USA have reported increases in the incidence of echinocandin-resistant C. glabrata.3–5 Unfortunately, it has also been reported that many of these isolates may also be resistant to fluconazole.5,15
In the current study, T-2307 demonstrated potent in vitro and in vivo activity against echinocandin-resistant C. glabrata. The in vitro potency of this agent was similar between WT and echinocandin-resistant strains and each T-2307 dose resulted in significant reductions in tissue fungal burden compared with control and 1 mg/kg caspofungin. In fact, there did not appear to be a dose–response relationship, as the reductions in fungal burden observed with each dose of T-2307 were similar. The reductions in fungal burden that were observed in this study were not as great as those we recently reported when T-2307 was evaluated against invasive candidiasis caused by an echinocandin-resistant C. albicans isolate.9 This difference may be due to the use of a neutropenic model in the current study for mice infected with C. glabrata versus an immunocompetent model for the C. albicans study. We have previously demonstrated that the immune status (i.e. neutropenic versus non-neutropenic) may influence treatment outcomes in mice infected with C. albicans.13 Another explanation may be that that the doses used in the current study were not low enough to observe a dose–response relationship. Previous studies with T-2307 against invasive candidiasis caused by echinocandin-susceptible C. glabrata and C. albicans isolates did demonstrate a dose–response relationship, but with lower doses.6,14 In addition, the reduction in fungal burden observed against echinocandin-susceptible C. glabrata was similar to what we observed with higher doses of T-2307 against the resistant isolate.13
Overall, these results suggest that T-2307 may have potential for the treatment of invasive infections caused by echinocandin-resistant C. glabrata. Further in vivo studies with other isolates and pharmacokinetic/pharmacodynamics evaluations are warranted.
Funding
This project utilized preclinical services funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHS272201000018I and HHSN272201000038I—Task Orders A03 and A13, respectively.
Transparency declarations
N. P. W. has received research support from Astellas, Dow, F2G, Merck, Merz, Revolution Medicines and Viamet, and has served on advisory boards for Merck, Astellas, Toyama and Viamet. L. K. N. has received travel support from Viamet Pharmaceuticals, Inc. Y. F. and J. M. are employees of Toyama Chemical Co., Ltd. T. F. P. has received research grants to UT Health Science Center San Antonio from Astellas, Merck and Revolution Medicines, and has served as a consultant for Astellas, Merck, Revolution Medicines, Toyama, Viamet and Scynexis. All other authors: none to declare.
Acknowledgements
We would like to thank Arlene Farias for her assistance with the animal model.
References
- 1.Pfaller MA, Andes DR, Diekema DJ et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS One 2014; 9: e101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas PG, Kauffman CA, Andes D et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48: 503–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander BD, Johnson MD, Pfeiffer CD et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 2013; 56: 1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyda ND, John J, Kilic A et al. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis 2014; 59: 819–25. [DOI] [PubMed] [Google Scholar]
- 5.Pham CD, Iqbal N, Bolden CB et al. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother 2014; 58: 4690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsuyama J, Nomura N, Hashimoto K et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother 2008; 52: 1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishikawa H, Yamada E, Shibata T et al. Uptake of T-2307, a novel arylamidine, in Candida albicans. J Antimicrob Chemother 2010; 65: 1681–7. [DOI] [PubMed] [Google Scholar]
- 8.Shibata T, Takahashi T, Yamada E et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 2012; 56: 5892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiederhold NP, Najvar LK, Fothergill AW et al. The novel arylamidine T-2307 maintains in vitro and in vivo activity against echinocandin-resistant Candida albicans. Antimicrob Agents Chemother 2015; 59: 1341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Fourth Informational Supplement M27-S4. CLSI, Wayne, PA, USA, 2012. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts—Third Edition: Approved Standard M27-A3. CLSI, Wayne, PA, USA, 2008. [Google Scholar]
- 12.Brzankalski GE, Najvar LK, Wiederhold NP et al. Evaluation of aminocandin and caspofungin against Candida glabrata including isolates with reduced caspofungin susceptibility. J Antimicrob Chemother 2008; 62: 1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiederhold NP, Najvar LK, Bocanegra R et al. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob Agents Chemother 2007; 51: 1616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada E, Nishikawa H, Nomura N et al. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob Agents Chemother 2010; 54: 3630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Castanheira M, Lockhart SR et al. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 2012; 50: 1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]