Abstract
Objectives
Delafloxacin is an investigational anionic fluoroquinolone being developed to treat infections caused by Gram-positive and -negative organisms. This clinical trial evaluated the efficacy and safety of delafloxacin in the treatment of acute bacterial skin and skin structure infections (ABSSSIs).
Methods
In a double-blind, Phase 2 trial, 256 patients were randomized (1 : 1 : 1) to 300 mg of delafloxacin, 600 mg of linezolid or 15 mg/kg vancomycin (actual body weight), each administered intravenously twice daily for 5–14 days. Randomization was stratified by infection category. The primary endpoint was the investigator's assessment of cure, defined as complete resolution of baseline signs and symptoms at follow-up. Secondary endpoints included reductions in the total areas of erythema and induration and assessments of bacterial eradication. This trial has been registered at ClinicalTrials.gov under registration number NCT01283581.
Results
Cure rates were significantly greater with delafloxacin versus vancomycin (mean difference: −16.3%; 95% CI, −30.3% to −2.3%; P = 0.031); differences were significant for obese patients (BMI ≥30 kg/m2; mean difference: −30.0%; 95% CI, −50.7% to −9.3%; P = 0.009), but not for non-obese patients. Cure rates with delafloxacin and linezolid were similar. Using digital measurement, the percentage decrease in total erythema area was significantly greater with delafloxacin versus vancomycin at follow-up (−96.4% versus −84.5%; P = 0.028). There were no differences in bacterial eradication among the treatment groups. The most frequently reported treatment-emergent adverse events were nausea, diarrhoea and vomiting.
Conclusions
These data show that delafloxacin is effective in the treatment of ABSSSIs and is well tolerated.
Introduction
Delafloxacin is an investigational anionic fluoroquinolone being developed for oral or intravenous administration as an antibacterial agent against infections caused by Gram-positive organisms, including MRSA, Gram-negative organisms and anaerobes. The agent's broad-spectrum activity1–3 suggests a potential for investigation in a variety of infections, including acute bacterial skin and skin structure infections (ABSSSIs), complicated intra-abdominal infections, complicated urinary tract infections and bacterial pneumonia. In previous Phase 14 and Phase 25 studies, delafloxacin was well tolerated and exhibited clinical and microbiological efficacy at dosages of 300 and 450 mg intravenously every 12 h.6
Investigator's assessment of global clinical outcome has been a common efficacy measure in clinical studies of investigational antibiotics. Regulatory authorities have since expressed an interest in more objective assessment methods,7–9 leading to the issuance of a guidance by the FDA10 designed to assist sponsors on the conduct of clinical trials to assess the effects of treatments for ABSSSIs.
Here, we report the results of a Phase 2 clinical trial evaluating the efficacy, safety and tolerability of intravenous delafloxacin, linezolid and vancomycin in the treatment of ABSSSIs. The primary efficacy measure was cure rate based on investigators' assessments of clinical response with complete resolution of signs and symptoms. The study design was based on the 2010 draft FDA guidance for antibiotic development in ABSSSI.10 As such, this was an exploratory study to evaluate the utility, variability and measurement of several objective outcomes of clinical efficacy for use in future clinical trials in patients with ABSSSIs, including changes in erythema and induration surface areas, body temperature and serum levels of C-reactive protein (CRP) and IL-6. Other secondary endpoints were microbiological and clinical response rates in patients with MRSA infections.
Methods
Study design
This was a Phase 2, multicentre, stratified, randomized, double-blind trial designed per FDA10 and EMA guidelines.11,12 Patients were enrolled at 23 US centres beginning in February 2011 and the last contact with the final patient was in November 2011. Patients with ABSSSIs were randomized (1 : 1 : 1 ratio) to twice-daily intravenous treatment with 300 mg of delafloxacin, 600 mg of linezolid or 15 mg/kg vancomycin (actual body weight) for 5–14 days (per investigator judgement). Linezolid and vancomycin were chosen as comparators in accordance with guidelines established by the FDA10 and IDSA;13 both are indicated for use to treat infections caused by MRSA.14 The study drug was delivered in 5% dextrose (D5W) and was administered every 12 ± 1 h. Vancomycin levels were monitored in blood samples drawn from all patients on day 2 or 3 and on day 6 ± 1; dosing was adjusted based on achieving a target trough of 15–20 μg/mL.15 Patients with proven or presumptive Gram-negative infections could have aztreonam added to their treatment regimen in a blinded fashion at the investigator's discretion post-baseline. If an investigator ordered aztreonam for a patient in the delafloxacin group, the unblinded pharmacist was to supply a placebo infusion in place of aztreonam for that patient.
All study drug infusions were administered by study site personnel or as a home infusion service that had been trained on specifics of the study protocol. Scheduled study visits took place at screening, days 1 through to 14 of treatment (or until the last day of study drug administration), follow-up (day 14 ± 1 and ≥12 h after the final study drug dose) and late follow-up (days 21–28). Treatment was required to begin within 24 h after screening. Throughout the study, wound care (including any surgical procedures) was performed according to the study site's standards of practice. However, no antibacterial topical solutions or dressings were allowed, except for silver dressings for the treatment of burns. Hyperbaric oxygen therapy was not allowed. Unplanned debridement procedures completed >48 h after study entry dictated that the subject be considered a treatment failure and that study drug be discontinued.
This trial has been registered at ClinicalTrials.gov under registration number NCT01283581.
Patients
Eligible patients were ≥18 years of age and had a diagnosis of ABSSSI (defined as cellulitis/erysipelas, wound infection, major cutaneous abscess or burn infection), characterized by ≥75 cm2 of erythema or induration, as determined by planimetry plus lymph node enlargement or at least one sign of systemic infection, specifically fever ≥38°C, lymphangitis, white blood cell count ≥15 000 cells/mm3 or serum CRP level >5.0 mg/L. Each patient was required to receive intravenous antibiotic therapy per the investigator's opinion. Patients were excluded for reasons including the following: hypersensitivities or allergies to quinolones, linezolid, vancomycin or vancomycin derivatives; concurrent skin conditions at the infection site; severely inadequate arterial blood supply to a limb containing the ABSSSI; severe immunocompromise; hypertension (≥180 mmHg systolic or ≥110 mmHg diastolic, confirmed within 20 min after the initial reading); body weight >140 kg; use of potentially effective systemic antibiotic therapy for >24 h within 14 days before enrolment unless objective evidence of documented clinical progression; and use of more than one dose of an antibiotic potentially effective against the ABSSSI under study within 24 h before study entry.
Randomization
Patients were randomized using the interactive web response system. Site-based randomization was stratified by infection category, with ≤30% of all patients having cutaneous abscess, and pre-treatment status, with ≤30% of all patients having received a single dose of an antibiotic potentially effective against the ABSSSI under study within 24 h before study entry.
Blinding
All study drug infusions were prepared by unblinded pharmacists, who obtained all treatment assignments from an interactive web response system and ensured blinding of study drug. To monitor pharmacy and drug administration records, certain clinical research organization personnel were also unblinded. Stratification/randomization was managed by an unblinded statistician who was not involved in the study conduct. Other parties involved in the study, including all patients, investigators, laboratories and sponsor personnel, remained unaware of the randomized treatment assignments.
Efficacy assessments
The primary efficacy measure was the investigator assessment of clinical response at the follow-up visit in the ITT population, defined as all randomized patients. Clinical response was categorized based on ABSSSI signs and symptoms: cure (complete resolution); improved (near resolution, some symptoms remain but no additional antibiotic treatment required); failure (additional non-study antibiotics required); or indeterminate (assessment was incomplete). Patients missing follow-up were considered indeterminate and mapped to failure in the ITT analysis. The cure rate was defined as the percentage of cures only in each treatment group; improved outcome was grouped with failure in the primary analysis. A post hoc analysis was conducted to assess the cure rate in obese (BMI ≥30 kg/m2) and non-obese (BMI <30 kg/m2) patients.
For exploratory objective assessments of clinical efficacy, the total areas of erythema and induration were obtained digitally (via planimetry readings of scanned acetate film tracings) and manually (with disposable rulers) at 12 h intervals through to day 5 and at follow-up. Clinical success was defined as cessation of expansion of erythema or induration (≤0% change from baseline in total area) accompanied by resolution or absence of fever, as assessed between 48 and 72 h after treatment inception and sustained through to 72 h. Resolution of fever was defined as three consecutive temperatures <37.7°C taken at 6 h intervals. CRP and IL-6 levels were obtained once daily through to day 5 and at day 10 and follow-up.
Microbiological assessments
At screening, specimens obtained from the ABSSSI site by biopsy, punch biopsy, aspiration or surgery were sent to a local laboratory for Gram staining, culture and in vitro susceptibility testing of microbial isolates.10 Each Gram-stained preparation was forwarded to a central microbiology laboratory, along with subcultures of identified bacterial isolates for confirmatory identification and testing. Blood cultures were also obtained at screening and daily thereafter, if positive, until the results were negative. In patients with materials available for culture, ABSSSI samples were collected at follow-up and late follow-up.
In microbiologically evaluable (ME) patients (see the Statistical analyses section), microbiological response was categorized as documented eradicated (baseline pathogen absent in follow-up cultures), presumed eradicated (no follow-up material available for culture, but the patient had a clinical response of cure), documented persisted (baseline pathogen present in follow-up cultures), presumed persisted (no follow-up material available for culture, but the patient had a clinical response of failure), superinfection (new pathogen cultured during therapy from the original site of infection, in the presence of infection signs or symptoms) or new infection (new pathogen cultured after therapy from the original site of infection, in the presence of infection signs or symptoms).
Safety/tolerability assessments
At each study visit, all patients were asked a non-directed question to elicit reports of adverse events (AEs). Each patient receiving >5 days of study drug treatment was telephoned 30 days after the final dose for follow-up of AEs. Safety assessments also included physical examinations, vital sign measurements, 12 lead electrocardiograms and clinical laboratory tests (including daily monitoring of blood glucose levels). Clinical laboratory tests were measured by a central laboratory (Eurofins, Global Central Laboratory, Chantilly, VA, USA). The CRP measurement method used Roche immunoturbidimetry and the IL-6 measurement method used R&D ELISA (ELISA processor).
Statistical analyses
The statistical analysis plan was prospectively defined prior to database lock and unblinding. Clinical efficacy outcomes were analysed using the ITT population, comprising all randomized patients. Microbiological outcomes were analysed in the ME population, comprising all patients with an identified baseline pathogen known to cause an ABSSSI who received at least eight study drug infusions or ≥80% of the total anticipated doses during the patient's treatment period (whichever was greater); did not receive any concomitant, systemic antibacterial therapy with activity against the identified pathogen; had no major protocol deviations; and had a follow-up visit at day 14 ± 1.
Continuous variables were compared among treatment groups using an analysis of covariance model, with study site, treatment, infection type and prior antimicrobial therapy as main effects and baseline value as the covariate. Categorical variables were compared separately between delafloxacin and each of the two comparator drugs by the Cochran–Mantel–Haenszel test stratified by infection type. Statistical significance was set at P < 0.05. Mean differences between treatments were expressed as vancomycin minus delafloxacin.
Sample size calculation
A sample size of 240 was based on clinical and practical considerations and not on a formal statistical power calculation. Simultaneous application of the study's stratifications, however, was found to cause an imbalance in the size of the treatment groups. This was because there was a large variation in the total number of patients enrolled by each site. Multiple stratifications performed at the site level in the randomization process combined with the variable enrolment by site produced an imbalance in the sizes of the treatment groups. The unblinded statistician therefore recommended increasing enrolment from 240 to 256 patients. All enrolment was completed in a blinded fashion.
Ethics
The study was conducted according to principles of the International Conference on Harmonisation Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. Before any study procedures, the study protocol was approved by the Institutional Review Boards and before enrolment, all prospective patients or their legally authorized representatives received a full explanation of the study and provided written informed consent.
Results
Patient flow
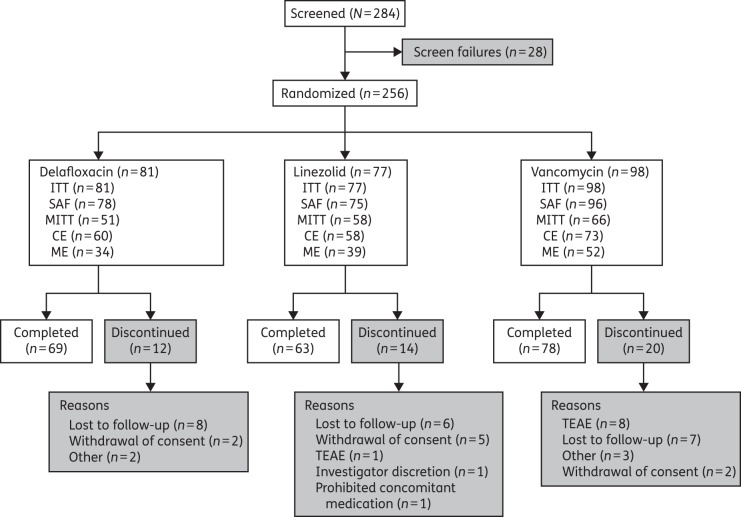
Of 284 patients screened, 256 were randomized (ITT population): 81, 77 and 98 to delafloxacin, linezolid and vancomycin, respectively (Figure 1). Because of the variable numbers enrolled at each site, there was an imbalance among treatment arms. Among the patients enrolled, 210 (82.0%) patients completed the study: 69 (85.2%), 63 (81.8%) and 78 (79.6%) in the delafloxacin, linezolid and vancomycin groups, respectively. The most frequent reasons for premature discontinuation were ‘lost to follow-up’ (21 patients), ‘patient withdrew consent’ (9 patients) and treatment-emergent AEs [TEAEs; 9 patients (1 linezolid and 8 vancomycin); Table S1, available as Supplementary data at JAC Online]. In all, 249 randomized patients received study drug (safety population), 175 had an identified baseline pathogen known to cause ABSSSIs (microbiological ITT population) and 125 were the ME population.
Figure 1.
CONSORT diagram of patient disposition. CE, clinically evaluable; MITT, microbiological ITT; SAF, safety.
Baseline characteristics
In general, patients' baseline characteristics were similar across treatment groups (Table 1). Overall, a majority of patients were men (59.4%) and Caucasian (76.2%). The mean (SD) age was 43.2 (15.1) years (median, 43.0; range, 18.0–91.0) and the mean (SD) BMI was 29.5 (6.6) kg/m2 (median, 28.3; range, 15.4–52.6). Obese patients (BMI ≥30 kg/m2) constituted 42.2% (108/256) of the ITT population. The most frequent ABSSSI category was cellulitis/erysipelas (44.9%), followed by major cutaneous abscess (28.5%), wound infection (25.0%) and burn infection (1.6%). Only 28.1% of patients had received antibacterial therapy prior to enrolment and randomization. The majority of patients enrolled with signs and symptoms consistent with systemic infection (fever, lymphangitis and/or elevated white blood cell count). Only three patients had only elevated CRP at baseline, without any other systemic sign of infection. The mean and median CRP levels at baseline were 50.8 and 24.0 mg/L, respectively.
Table 1.
Patients' baseline characteristics (ITT population)
| Characteristic | Delafloxacin (n = 81) | Linezolid (n = 77) | Vancomycin (n = 98) |
|---|---|---|---|
| Male, n (%) | 49 (60.5) | 52 (67.5) | 51 (52.0) |
| Age (years) | |||
| mean (SD) | 39.7 (14.26) | 44.8 (14.91) | 44.8 (15.54) |
| median | 36.0 | 47.0 | 45.0 |
| Race, n (%) | |||
| Caucasian | 63 (77.8) | 58 (75.3) | 74 (75.5) |
| black or African American | 10 (12.3) | 15 (19.5) | 15 (15.3) |
| othera | 8 (9.9) | 4 (5.2) | 9 (9.2) |
| BMI (kg/m2) | |||
| mean (SD) | 29.4 (6.47) | 29.5 (6.37) | 29.6 (6.88) |
| median | 28.3 | 29.4 | 28.5 |
| Diabetic, n (%) | 5 (6.2) | 5 (6.7) | 16 (16.3) |
| ABSSSI category, n (%) | |||
| cellulitis/erysipelas | 39 (48.1) | 32 (41.6) | 44 (44.9) |
| major cutaneous abscess | 21 (25.9) | 24 (31.2) | 28 (28.6) |
| wound infection | 19 (23.5) | 19 (24.7) | 26 (26.5) |
| burn infection | 2 (2.5) | 2 (2.6) | 0 |
| Erythema total area (cm2; digital), median (range)b | 187.9 (7.5–1555.3) | 197.4 (6.1–4658.9) | 164.6 (0.0–1503.6) |
| Induration total area (cm2; digital), median (range)b | 67.3 (0.0–907.5) | 62.2 (0.0–4103.0) | 75.1 (0.0–1429.4) |
| Prior antibacterial therapy,c n (%) | 20 (24.7) | 24 (31.2) | 28 (28.6) |
| Identified pathogens, n (%) | |||
| none | 30 (37.0) | 20 (26.0) | 31 (31.6) |
| at least one | 51 (63.0) | 57 (74.0) | 67 (68.4) |
| S. aureus | 45 (55.6) | 53 (68.8) | 61 (62.2) |
| MRSA | 34 (42.0) | 37 (48.1) | 35 (35.7) |
| MSSA | 11 (13.6) | 16 (20.8) | 26 (26.5) |
| multiple pathogens | 6 (7.4) | 15 (19.5) | 8 (8.2) |
| Positive blood culture, n (%) | 0 | 6 (7.8) | 1 (1.0) |
| Fever, n (%) | 18 (22.2) | 18 (23.4) | 29 (29.6) |
| Serum CRP (mg/L),d mean (SD) | 46.6 (70.66) | 49.3 (56.14) | 55.2 (69.70) |
| Serum IL-6 (ng/L),e mean (SD) | 11.4 (15.35) | 14.5 (15.14) | 16.6 (17.93) |
| Blood glucose (mmol/L),f mean (SD) | 5.53 (2.365) | 6.27 (3.957) | 6.32 (3.138) |
aIncluding American Indian or Alaska Native, Asian and Native Hawaiian or other Pacific Islander.
bn = 75 delafloxacin, 73 linezolid and 93 vancomycin.
cA single dose of an antibiotic potentially effective against the ABSSSI under study, taken within the 24 h before study entry.
dn = 71 delafloxacin, 71 linezolid and 92 vancomycin.
en = 71 delafloxacin, 70 linezolid and 93 vancomycin.
fSafety population; n = 63 delafloxacin, 58 linezolid and 82 vancomycin.
Among the 175 patients (68.4% of 256) with identified baseline pathogens known to cause ABSSSI, 29 (11.3% of 256) had multiple pathogens. Overall, Staphylococcus aureus was the most frequently identified organism in 159 patients (90.9% of 175 patients with baseline pathogens). Among identified pathogens, 205 were Gram-positive, including 177 S. aureus strains, of which 119 (67.2%) were MRSA. Six identified pathogens were Gram-negative and three were anaerobes. Seven patients (six linezolid and one vancomycin) had positive baseline blood cultures.
Study drug exposure
The mean duration of treatment was 7.6, 7.4 and 7.8 days for delafloxacin, linezolid and vancomycin, respectively. Most patients were exposed to study drug for 6–14 days (66.7% delafloxacin, 72.0% linezolid and 74.0% vancomycin), whereas less than one-third received study drug for 1–5 days (30.8% delafloxacin, 28.0% linezolid and 24.0% vancomycin); only four patients (two each for delafloxacin and vancomycin) received study drug for >14 days.
Efficacy
Investigator's assessment of clinical response at follow-up in the ITT population is shown in Table 2. Delafloxacin had a cure rate of 70.4% compared with 64.9% in the linezolid group (mean difference: −5.4%; 95% CI, −20.0%–9.1%; P = 0.496) and 54.1% in the vancomycin group (mean difference vancomycin minus delafloxacin was −16.3%; 95% CI, −30.3% to −2.3%; P = 0.031). The cure rates were maintained at late follow-up: 76.5% (62/81), 68.8% (53/77) and 62.2% (61/98) for delafloxacin, linezolid and vancomycin, respectively. Results for cellulitis/erysipelas and major cutaneous abscess resembled the overall pattern (Table 3). Among patients with MRSA identified at baseline, the clinical cure rates were comparable among the treatment groups. Prior antibiotics did not appear to have a clinically relevant impact on outcomes (Table 3). In a post hoc analysis, the cure rate was statistically significantly higher in obese patients, but not in non-obese patients with delafloxacin versus vancomycin (78.8% versus 48.8%; mean difference: −30.0%; 95% CI, −50.7% to −9.3%; P = 0.009; Table 3).
Table 2.
Subjective clinical efficacy: investigator assessment of outcome at follow-up (ITT population)
| Response, n (%) |
|||
|---|---|---|---|
| delafloxacin (n = 81) | linezolid (n = 77) | vancomycin (n = 98) | |
| Curea | 57 (70.4) | 50 (64.9) | 53 (54.1)b |
| Improved | 11 (13.6) | 13 (16.9) | 26 (26.5) |
| Failure | 5 (6.2) | 3 (3.9) | 7 (7.1) |
| Indeterminate | 8 (9.9) | 11 (14.3) | 12 (12.2) |
aClinical success is defined as investigator assessment of cure only; improved, failure and indeterminate equate to failure.
bP < 0.05 versus delafloxacin, Cochran–Mantel–Haenszel test.
Table 3.
Subjective clinical efficacy: investigator assessment of outcome at follow-up in selected groups
| Cure rate, n/N (%) |
|||
|---|---|---|---|
| delafloxacin | linezolid | vancomycin | |
| Infection type (ITT) | |||
| cellulitis/erysipelas | 28/39 (71.8) | 24/32 (75.0) | 19/44 (43.2) |
| major cutaneous abscess | 15/21 (71.4) | 16/24 (66.7) | 15/28 (53.6) |
| wound infection | 12/19 (63.2) | 8/19 (42.1) | 19/26 (73.1) |
| burn infection | 2/2 (100) | 2/2 (100) | 0/0 |
| MRSA at baseline (MITT) | |||
| all | 19/29 (65.5) | 21/34 (61.8) | 21/32 (65.6) |
| cellulitis/erysipelas | 10/13 (76.9) | 9/12 (75.0) | 6/11 (54.5) |
| major cutaneous abscess | 6/11 (54.5) | 10/15 (66.7) | 8/13 (61.5) |
| wound infection | 3/5 (60.0) | 2/7 (28.6) | 7/8 (87.5) |
| burn infection | 0/0 | 0/0 | 0/0 |
| Prior antibiotic status (ITT) | |||
| prior use | 8/12 (66.7) | 8/16 (50.0) | 10/25 (40.0) |
| no prior use | 49/69 (71.0) | 42/61 (68.9) | 43/73 (58.9) |
| BMIa (ITT) | |||
| non-obese | 31/48 (64.6) | 30/43 (69.8) | 33/57 (57.9) |
| obese | 26/33 (78.8) | 20/34 (58.8) | 20/41 (48.8)b |
MITT, microbiological ITT.
aPost hoc analysis. Non-obesity was defined as BMI <30 kg/m2 and obesity as BMI ≥30 kg/m2 at baseline.
bP < 0.05 versus delafloxacin, Cochran–Mantel–Haenszel test.
Quantitative changes in erythema and induration
The proportion of patients with cessation of spread of erythema and induration at the 48–72 h visit ranged from 73% to 78% and 63% to 76%, respectively (Table 4), using digital measurement; differences among treatment groups were not statistically significant for either measure. The proportions of patients who had ≥20% reduction in erythema were not different among the treatment groups; however, the proportion of patients with ≥20% reduction in induration tended to be higher for those receiving vancomycin versus delafloxacin (69.5% versus 56.4%; P = 0.089) using digital measurement (Table 4). The decrease in erythema area expressed as the percentage decrease was statistically significantly greater with delafloxacin versus vancomycin at follow-up (−96.4% versus −84.5%; P = 0.028; Table 4). There were no differences among the treatment groups in any outcome using manual measurement (data not shown).
Table 4.
Objective clinical efficacy (ITT population) at 48–72 h after start of treatment
| Delafloxacin | Linezolid | Vancomycin | |
|---|---|---|---|
| Outcome/measurement technique | |||
| erythema/digital measurement | |||
| cessation of spread,a n/N (%) | 61/78 (78.2) | 56/75 (74.7) | 69/95 (72.6) |
| 20% reduction, n/N (%) | 58/78 (74.4) | 55/75 (73.3) | 65/95 (68.4) |
| percentage change in area at follow-up, mean (SD) | −96.4 (13.96) | −87.7 (39.22) | −84.5 (35.73)b |
| Induration/digital measurement | |||
| cessation of spread,a n/N (%) | 54/78 (69.2) | 47/75 (62.7) | 72/95 (75.8) |
| 20% reduction, n/N (%) | 44/78 (56.4) | 40/75 (53.3) | 66/95 (69.5) |
| percentage change in area at follow-up, mean (SD) | −73.5 (48.56) | −77.1 (47.02) | −84.8 (30.05) |
| Body temperature (°C)c | |||
| change from baseline to follow-up, mean (SD) | −0.2 (0.53) | −0.2 (0.59) | −0.2 (0.76) |
| Serum CRP (mg/L)d | |||
| change from baseline to follow-up, mean (SD) | −37.4 (64.90) | −38.1 (54.51) | −43.2 (64.90) |
| Serum IL-6 (ng/L)e | |||
| change from baseline to follow-up, mean (SD) | −7.9 (15.84) | −8.7 (19.11) | −9.7 (19.33)b |
aProportion of patients in whom erythema or induration had stopped expanding within 48–72 h, accompanied by resolution or continuing absence of fever.
bP < 0.05 versus delafloxacin, Cochran–Mantel–Haenszel test.
cn = 78 delafloxacin, 73 linezolid and 96 vancomycin.
dn = 71 delafloxacin, 69 linezolid and 92 vancomycin.
en = 71 delafloxacin, 68 linezolid and 93 vancomycin.
Other objective outcomes
At baseline, fever was identified in 18 of 81 patients (22.2%) in the delafloxacin group, 18 of 77 (23.4%) in the linezolid group and 29 of 98 (29.6%) in the vancomycin group. Among them, fever resolved in 7 of 18 patients (38.9%) in the delafloxacin group, 5 of 18 (27.8%) in the linezolid group and 14 of 29 (48.3%) in the vancomycin group.
At follow-up, mean serum CRP and IL-6 levels decreased from baseline in all groups (Table 4). The decrease in serum IL-6 was significantly greater with vancomycin versus delafloxacin (−9.7 versus −7.9 g/L; P = 0.042); the decrease in serum CRP was comparable in all groups.
Microbiological efficacy
In the ME population, comprising 125 patients, microbiological responses were classified (Table 5) as presumed eradicated in 88.2%, 82.1% and 80.8% of delafloxacin, linezolid and vancomycin recipients, respectively; with the exception of 1 patient (linezolid, documented persisted), all others were classified as presumed persisted. Classifications of the 72 ME population patients with MRSA isolated at baseline mirrored those of the overall ME population. No new infections or superinfections were identified (Table 5).
Table 5.
Microbiological efficacy (ME population)
| Response rate, n/N (%) |
|||
|---|---|---|---|
| delafloxacin | linezolid | vancomycin | |
| All ME patients | |||
| documented eradicated | 0 | 0 | 0 |
| presumed eradicated | 30/34 (88.2) | 32/39 (82.1) | 42/52 (80.8) |
| documented persisted | 0 | 1/39 (2.6) | 0 |
| presumed persisted | 4/34 (11.8) | 6/39 (15.4) | 10/52 (19.2) |
| new infection/superinfection | 0 | 0 | 0 |
| ME patients with MRSA isolated at baseline | |||
| documented eradicated | 0 | 0 | 0 |
| presumed eradicated | 18/21 (85.7) | 20/25 (80.0) | 23/26 (88.5) |
| documented persisted | 0 | 1/25 (4.0) | 0 |
| presumed persisted | 3/21 (14.3) | 4/25 (16.0) | 3/26 (11.5) |
| new infection/superinfection | 0 | 0 | 0 |
Study-drug MIC required to inhibit growth of 50% and 90% of organism (MIC50 and MIC90) values were calculated for S. aureus, MRSA and MSSA, and are summarized in Table S2; those for other pathogens could not be obtained because too few isolates were collected. All Gram-positive bacteria tested were sensitive to delafloxacin, linezolid and vancomycin.
Safety
Among study drug recipients, 74.4% of the delafloxacin group, 72.0% of the linezolid group and 64.6% of the vancomycin group reported one or more TEAEs. Among the types most frequently reported (Table S3), nausea was more common in the delafloxacin and linezolid groups, diarrhoea and vomiting were more common in the delafloxacin group and pruritus was more common in the vancomycin group. There were no reports of Clostridium difficile diarrhoea.
Five patients (two delafloxacin and three vancomycin) experienced a total of six TEAEs related to liver toxicity. None of these events caused treatment discontinuation or serious AEs (SAEs). One subject in the vancomycin group had one TEAE each of elevated ALT and AST; both TEAEs were assessed as probably related to the study drug. Three subjects had TEAEs of ‘liver function test abnormal’; two were in the delafloxacin group (one assessed as possibly related to study drug and one as probably related) and one was in the vancomycin group (assessed as possibly related to study drug). One of these subjects (in the delafloxacin group) had ALT and AST values that were flagged high at screening. The subject had further increases at follow-up and late follow-up. The subject's bilirubin values remained within normal limits. One subject in the vancomycin group had a TEAE of ‘hepatic enzymes increased’, assessed as possibly related to study drug.
No patient had a TEAE related to hypoglycaemia. Conversely, a TEAE of hyperglycaemia occurred in two, one and two patients in the delafloxacin, linezolid and vancomycin groups, respectively; a TEAE of increased blood glucose occurred in one linezolid-treated patient (the only patient with diagnosis of diabetes) and two patients in the vancomycin group. All hyperglycaemia TEAEs were classified as mild. The study drug was delivered in D5W, which may have had an impact on hyperglycaemia reports; additionally, these were non-fasting measurements. No deaths occurred during the study.
The three treatment groups were generally similar with regard to changes from baseline in haematology, serum chemistry, urinalysis and glucose measurements. In all, 13 patients (5.2% of 249) had SAEs: 5 patients (6.4% of 78), 2 patients (2.7% of 75) and 6 patients (6.3% of 96) in the delafloxacin, linezolid and vancomycin groups, respectively (Table S4); none was considered to be related to study drug. Reported SAEs of convulsions occurred in patients with a history of seizure disorder. There were no clinically relevant changes in the QT interval or clinically significant electrocardiogram abnormalities.
Discussion
The investigational fluoroquinolone delafloxacin was evaluated against two antibiotics approved in the USA and Europe for ABSSSIs (linezolid and vancomycin). Delafloxacin was significantly more effective than vancomycin for treating ABSSSIs by the primary endpoint, the investigator assessment of cure at the follow-up visit. This outcome appears to have been driven in part by statistically better outcomes in obese patients with delafloxacin. The improved outcome was maintained over the study period. Outcome with delafloxacin was not significantly different from either linezolid or vancomycin in the treatment of patients with MRSA.
The reason for the statistically higher cure rate for delafloxacin over vancomycin is unclear. Vancomycin was dosed according to recommendations by the IDSA and the American Society of Health-System Pharmacists;15 however, dosing issues do not appear to have contributed to the lower cure rate. Vancomycin exposure as monitored by trough levels or pharmacokinetic/pharmacodynamic assessment (ratio of free-drug AUC : MIC) did not correlate with clinical outcome. There was a high percentage of patients classified as cured or improved among those with an AUC : MIC ratio of ≥200, which suggests that the majority of patients had free-drug AUC : MIC ratios that were on the plateau of a pharmacokinetic/pharmacodynamic relationship for clinical response and thus had sufficient vancomycin exposure.
Assessment of objective measures by digital and manual determinations 48–72 h after the start of therapy revealed that all three agents were comparable in either cessation of spread or 20% reduction of total areas of erythema and induration, although digital measurements appeared to be associated with less variability. The present study was conducted in accordance with the FDA- and EMA-issued guidances for assessment of antibiotics in the treatment of ABSSSIs in terms of efficacy criteria and endpoints.10–12 Historically, antibiotic trials have defined clinical success using investigator assessments that typically encompassed both cured and improved patients, in whom either all or most signs and symptoms were resolved, respectively. In this study, the protocol specified cure, the most stringent definition of clinical success, and excluded improved patients as the primary endpoint; improved outcome was mapped to failure in the primary analysis.
As previously stated, the statistically superior outcome in obese patients treated with delafloxacin appears to have contributed to the overall outcome compared with vancomycin. Obesity is a risk factor for ABSSSI and is also a predictor of poor outcomes and increased treatment cost.16–19 Pharmacotherapy of obese patients presents additional challenges to physicians and pharmacists.20 Based on these considerations, the potential for delafloxacin to improve cure rates in obese patients may provide a therapeutic advantage and thus may be clinically meaningful.
In the present study, delafloxacin had an acceptable safety profile and was well tolerated; the most common TEAEs were similar to those observed in a prior Phase 2 study.5 Hypoglycaemia has been associated with some fluoroquinolone antibiotics and, in a previous Phase 2 study,5 single low serum glucose values were observed in 4.1% and 17.6% of patients given 300 and 450 mg of delafloxacin intravenously, respectively; all but one patient in the 450 mg group was asymptomatic. In the present study, there were no AEs of hypoglycaemia in patients receiving delafloxacin and the decrease in blood glucose from baseline to the last recorded value was not statistically significant. Together, these data suggest that delafloxacin poses a low risk of hypoglycaemia.
This study is limited by the size of the database and larger Phase 3 studies would more definitively characterize the safety and efficacy of delafloxacin. The finding of an increased cure rate in obese patients with delafloxacin treatment, while notable, was not a prospectively defined outcome and merits further study in a prospective fashion.
In summary, the present trial provides objective and subjective evidence to expand the findings from a previous Phase 2 study and thus supports continued study of delafloxacin as a treatment for ABSSSIs and infections due to MRSA. Delafloxacin met the primary endpoint of the trial and appears to be well tolerated. The statistically significant clinical benefit seen with delafloxacin compared with vancomycin, particularly in obese patients, warrants further study. With the challenges for physicians and pharmacists in treating obese patients, any therapeutic advantage in these patients could be particularly clinically meaningful.
Funding
This work was supported by Melinta Therapeutics (formerly Rib-X Pharmaceuticals, Inc.). Editorial assistance for this paper was funded by Melinta Therapeutics.
Transparency declarations
J. K. reports grants from Rib-X Pharmaceuticals, Inc., during the conduct of the study, and grants from Durata Therapeutics, Inc., Affinium Pharmaceuticals, Ltd, Trius Therapeutics, Inc., Tetraphase Pharmaceuticals, Achaogen, Inc. and Cempra, Inc., outside the submitted work. L. E. L., E. D. and S. K. C. are employees of Melinta Therapeutics. J. P. reports grants from Rib-X Pharmaceuticals, Inc., during the conduct of the study, and grants from The Medicine Shoppe® pharmacy, outside the submitted work. E. H. is a statistical consultant for Melinta Therapeutics. P. M.: none to declare.
Editorial assistance was provided by Cory Hussar, PhD, of The Curry Rockefeller Group, LLC.
Author contributions
Conception and design: J. K., P. M., L. E. L., E. H., E. D., S. K. C. and J. P.
Acquisition, analysis or interpretation of data: J. K., P. M., L. E. L., E. H., E. D., S. K. C. and J. P.
Drafting the work and revising it critically for important intellectual content and giving final approval of the version to be published: J. K., P. M., L. E. L., E. H., E. D., S. K. C. and J. P.
Supplementary data
Tables S1 to S4 are available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
This study was presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, USA, 2012 (Abstract L1-1663).21
We gratefully acknowledge the contributions of Scott Hopkins and Jarrod Longcor to the design and oversight of this study.
References
- 1.Nilius AM, Shen LL, Hensey-Rudloff D et al. . In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob Agents Chemother 2003; 47: 3260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almer LS, Hoffrage JB, Keller EL et al. . In vitro and bactericidal activities of ABT-492, a novel fluoroquinolone, against Gram-positive and Gram-negative organisms. Antimicrob Agents Chemother 2004; 48: 2771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harnett SJ, Fraise AP, Andrews JM et al. . Comparative study of the in vitro activity of a new fluoroquinolone, ABT-492. J Antimicrob Chemother 2004; 53: 783–92. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence L, Benedict M, Hart J et al. . Pharmacokinetics (PK) and safety of single doses of delafloxacin administered intravenously in healthy human subjects. In: Abstracts of the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2011. Abstract A2-045A American Society for Microbiology, Washington, DC, USA. [Google Scholar]
- 5.O'Riordan W, Mehra P, Manos P et al. . A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis 2015; 30: 67–73. [DOI] [PubMed] [Google Scholar]
- 6.Rubino CM, Bhavnani SM, Burak E et al. . Pharmacokinetic-pharmacodynamic target attainment analyses supporting delafloxacin phase 3 dose regimen decisions. In: Abstracts of the Fiftieth Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2010. Abstract A1-681 American Society for Microbiology, Washington, DC, USA. [Google Scholar]
- 7.Spellberg B, Fleming TR, Gilbert DN. Executive summary: workshop on issues in the design and conduct of clinical trials of antibacterial drugs in the treatment of community-acquired pneumonia. Clin Infect Dis 2008; 47 Suppl 3: S105–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spellberg B, Talbot GH, Brass EP et al. . Position paper: recommended design features of future clinical trials of antibacterial agents for community-acquired pneumonia. Clin Infect Dis 2008; 47: S249–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Center for Drug Evaluation and Research (CDER). Guidance for Industry. Community-Acquired Bacterial Pneumonia: Developing Drugs for Treatment. 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm123686.pdf. [Google Scholar]
- 10.Center for Drug Evaluation and Research (CDER). Guidance for Industry. Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment. 2013. http://www.fda.gov/downloads/Drugs/…/Guidances/ucm071185.pdf. [Google Scholar]
- 11.Committee for Medicinal Products for Human Use, European Medicines Agency. Guideline on the Evaluation of Medicinal Products Indicated for Treatment of Bacterial Infections. 2011. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003417.pdf. [Google Scholar]
- 12.Committee for Human Medicinal Products, European Medicines Agency. Addendum to the Guideline on the Evaluation of Medicinal Products Indicated for Treatment of Bacterial Infections. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/11/WC500153953.pdf. [Google Scholar]
- 13.Liu C, Bayer A, Cosgrove SE et al. . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52: e18–55. [DOI] [PubMed] [Google Scholar]
- 14.Gorwitz RJ, Jernigan DB, Powers JH et al. . Strategies for Clinical Management of MRSA in the Community: Summary of an Experts' Meeting Convened by the Centers for Disease Control and Prevention. 2006. http://www.cdc.gov/mrsa/pdf/MRSA-Strategies-ExpMtgSummary-2006.pdf. [Google Scholar]
- 15.Rybak M, Lomaestro B, Rotschafer JC et al. . Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66: 82–98. [DOI] [PubMed] [Google Scholar]
- 16.Carratalà J, Rosón B, Fernández-Sabé N et al. . Factors associated with complications and mortality in adult patients hospitalized for infectious cellulitis. Eur J Clin Microbiol Infect Dis 2003; 22: 151–7. [DOI] [PubMed] [Google Scholar]
- 17.Halilovic J, Heintz BH, Brown J. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect 2012; 65: 128–34. [DOI] [PubMed] [Google Scholar]
- 18.Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes 2013; 37: 333–40. [DOI] [PubMed] [Google Scholar]
- 19.Sreeramoju P, Porbandarwalla NS, Arango J et al. . Recurrent skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus requiring operative debridement. Am J Surg 2011; 201: 216–20. [DOI] [PubMed] [Google Scholar]
- 20.Pai MP, Bearden DT. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 2007; 27: 1081–91. [DOI] [PubMed] [Google Scholar]
- 21.Longcor J, Hopkins S, Lawrence L et al. . Results of a Phase 2 study of delafloxacin (DLX) compared to vancomycin (VAN) and linezolid (LNZ) in acute bacterial skin and skin structure infections (ABSSSI). In: Abstracts of the Fifty-second Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2012. Abstract L1-1663. American Society for Microbiology, Washington, DC, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.