Abstract
The HIV epidemic in Malaysia is concentrated among people who inject drugs (PWID). Accurate estimates of HIV prevalence are critical for developing appropriate treatment and prevention interventions for PWID in Malaysia. In 2010, 461 PWID were recruited using respondent-driven sampling in Greater Kuala Lumpur, Malaysia. Participants completed rapid HIV testing and behavioral assessments. Estimates of HIV prevalence were computed for each of the three recruitment sites and the overall sample. HIV prevalence was 15.8% (95% CI: 12.5-19.2%) overall but varied widely by location: 37.0% (28.6-45.4%) in Kampung Baru, 10.3% (5.0-15.6%) in Kajang, and 6.3% (3.0-9.5%) in Shah Alam. Recruitment extended to locations far from initial interview sites but was concentrated around discrete geographic regions. We document the high prevalence of HIV among PWID in Greater Kuala Lumpur. Sustained support for community surveillance and HIV prevention interventions is needed to stem the HIV epidemic among PWID in Malaysia.
Keywords: HIV prevalence, people who inject drugs, Malaysia, surveillance, respondent-driven sampling
1. Introduction
The HIV epidemic in Malaysia is concentrated among marginalized populations, including people who inject drugs (PWID), sex workers, and men who have sex with men. HIV transmission initially occurred primarily among PWID: by 2000, 75% of cases were attributed to drug injection, mostly of heroin (1). After initially pursuing a strictly punitive approach toward PWID, Malaysia introduced evidence-based harm reduction programs in 2005, including needle exchange and opioid maintenance therapy (2). Supported primarily by the Ministry of Health and the Global Fund, needle exchange programs have been opened in all states in peninsular Malaysia (3). Methadone maintenance therapy is primarily provided by the Ministry of Health through specialist and primary care clinics supported by the National Anti-Drug Agency, which has scaled back its system of compulsory drug detention centers and increased access to subsidized methadone maintenance therapy in the community (4-6). The Ministry of Health fully subsidizes first-line antiretroviral therapy for HIV. New HIV infections among PWID now comprise 22% of reported incident cases; nevertheless, PWID comprise two thirds (66%) of all people living with HIV and continue to represent a key target for public health surveillance and intervention (1).
Disease surveillance among PWID in Malaysia is complicated by the hidden nature of the population. Drug injection is often hidden from public view because of stigma and criminalization, particularly in Malaysia, where even traces of illegal drugs in the urine can result in arrest and detention (7-9). Despite the burden of HIV among PWID, community surveillance is limited and only partial surveillance data are made public. Ministry of Health surveillance data suggest that HIV prevalence could be as high as 18.9% among PWID, but data disaggregated by state and methodological details are not publicly available (1). There are no published studies designed to estimate HIV prevalence among non-institutionalized PWID in Malaysia. Accurate estimates of HIV prevalence are critical for monitoring Malaysia’s dynamic HIV epidemic and deploying appropriate treatment and prevention interventions for PWID.
Respondent-driven sampling (RDS) was developed to recruit members of hidden populations such as PWID for which traditional probability sampling is not possible (10-12). Participants in RDS studies are given a small number of coupons that they use to recruit other subjects who meet study inclusion criteria. Subjects are remunerated for participation and for successful recruitment. RDS studies of PWID have often reported rapid accrual of large sample sizes (13). Because of its effectiveness in reaching hidden populations, RDS has been adopted for HIV surveillance among PWID by researchers and public health organizations worldwide (14,15). Traits, such as HIV status, of subjects recruited by RDS may not be statistically independent, so statistical approaches have been developed to estimate population prevalence from RDS data (10,11,16-18). Estimation of population prevalence from subjects recruited by RDS remains controversial, and researchers continue to disagree about whether and how estimates should be adjusted for possible differences in sampling probability (12,19-24). Statistical diagnostics have been proposed to help researchers determine whether data obtained by RDS conform to the assumptions made by statistical techniques for prevalence estimation (25).
To assess HIV prevalence among PWID, we used RDS to recruit PWID from Greater Kuala Lumpur. We present for the first time in the peer-reviewed literature estimates of HIV prevalence among non-institutionalized PWID in Malaysia, use diagnostic tools to evaluate whether some key sampling assumptions for RDS are likely to hold, and document heterogeneity in HIV prevalence across geographic regions. Our results represent the best available estimates of HIV prevalence and are critical for informing the public health response to the HIV epidemic among PWID in Greater Kuala Lumpur.
2. Methods
2.1. Eligibility and Recruitment
In 2010, 461 PWID were recruited to complete an in-depth survey and rapid HIV testing. Eligibility criteria were (i) injecting drugs in the previous 30 days (verified by examination of injection sites and knowledge of drug preparation methods), (ii) age 18 years or older, (iii) possessing a valid RDS coupon, and (iv) spending time in Greater Kuala Lumpur. Interviews were conducted in three private clinics frequented by PWID in Greater Kuala Lumpur, the area encompassing Kuala Lumpur proper and surrounding suburbs in Klang Valley. One interview site was located in a central urban area (Kampung Baru) and the other two were located in peri-urban areas approximately 30km west (Shah Alam) and south (Kajang) of the city center. After meeting with local harm reduction and addiction treatment experts, these particular sites were selected to maximize the chance of reaching PWID from all regions of Greater Kuala Lumpur. These experts confirmed a sizeable presence of PWID in these regions.
Participants at each site were recruited using RDS (10). Outreach workers recruited two initial participants, or “seeds,” from each of the three sites who did not share the same peer group. Each participant was given three coupons that could be used to recruit eligible peers and 50RM (16USD) for participating and 25RM (8USD) for each eligible individual they recruited and who was successfully enrolled. Coupons were uniquely numbered to allow tracking of who recruited whom. Participants were interviewed at the interview site specific to their recruitment “chain,” the series of recruitments resulting from a given seed. To discourage repeat participation, we communicated to subjects that they could be uniquely identified from biometric measurements (ulnar length, wrist circumference), but these measurements were ultimately not used because the number of participants at each site was relatively small and research staff were confident they could identify those attempting to participate multiple times.
Sample size calculations indicated that we would need at least n=432 participants overall to estimate HIV prevalence with a 95% CI of +/− five percentage points, assuming an HIV prevalence of 17% (from earlier unpublished estimates) and a design effect of two (26).
2.2. Measures
2.2.1. Rapid HIV Testing
A rapid oral fluid antibody test (OraQuick ADVANCE® Rapid HIV-1/2, OraSure Technologies, Inc.) was administered to all participants, and those with a reactive oral fluid test were administered a confirmatory rapid whole-blood HIV antibody test (ACON HIV 1/2/0 Rapid Test Device, ACON Laboratories, Inc.). No discordance between tests was observed. All participants received pre-test and post-test counseling. Individuals with reactive test results were referred to local specialists at clinics where fully subsidized antiretroviral therapy was available.
2.2.2. Urine toxicology screening
To verify the validity of self-reported drug use, urine samples were collected from all participants and tested for opioids. Tests usually indicate use within the prior two to three days. To avoid encouraging drug use to gain access to the study, urine test results were not used to determine eligibility.
2.2.3. Questionnaire
Participants completed a 60-minute interviewer-administered questionnaire in their choice of Bahasa Malaysia or English that included a range of validated instruments and study-specific questions. Participants were asked a series of questions on personal network size, which is used in the RDS estimators (10). Network size was defined as the number of PWID from Greater Kuala Lumpur that participants knew by name, who also knew their name, and whom they had seen in the prior three months. A standard summation method using a series of questions about the number of contacts of different ethnicities, genders, and ages was used to improve participants’ recall of their aggregate network size (27). Opioid dependence was assessed using DSM-IV criteria with the Mini-International Neuropsychiatric Interview (28). Injection-related risk behaviors were assessed in multiple ways: participants were asked about receptive sharing episodes—using a needle or syringe that had been used previously by someone else—as well as whether they had any needle or syringe sharing partners.
2.2.4. Geographic information
To protect the privacy of participants, we collected data on what neighborhood participants lived in rather than exact addresses. Neighborhoods were coded into geographic coordinates of the neighborhood’s centroid. Since neighborhoods vary greatly in size, the spatial resolution of our location data also varies greatly. To achieve the same level of spatial resolution for all participants, we coarsened the residential data by classifying each individual into one of three geographic regions based on which interview site was closest to their place of residence. The ggmap package in R was used to query driving distances between locations from the Google Maps API (29,30).
2.3. Ethical Oversight
No identifying information was collected. All participants gave their informed consent to participate. This study was approved by institutional review boards at University of Malaya and Yale University.
2.4. Statistical Methods
Several estimators of population prevalence have been proposed for RDS data (16,17,31). Using the RDS package in R (32), we computed prevalence estimates using four commonly-used RDS estimators: Heckathorn's original estimator (RDS-I) (10,11), the Volz-Heckathorn estimator (RDS-II) (17), Gile's successive sampling estimator (RDS-SS) (31), and the sample mean. We estimate confidence intervals using Salganik's bootstrap for the RDS-I and RDS-II estimators, Gile's bootstrap for the RDS-SS estimator, and a conventional bootstrap for the sample mean, all implemented in the RDS package (32).
In the online appendix, we describe the population size estimate used as an input for the RDS-SS estimator and the insensitivity of HIV prevalence estimates to a range of population size estimates (Table A1). We use the full sample, including the six seeds, in all estimates. We stratify our estimates of HIV prevalence by recruitment site and present a pooled estimate for all three sites. We highlight the sample mean over RDS estimators, which differ little within each site in our study because HIV-infected and HIV-uninfected individuals have similar network sizes. Furthermore, assumptions about the sampling process necessary for the RDS estimators to be valid are controversial, and simulation and empirical studies have shown that even under ideal conditions these estimators can be biased with high sampling variance (20,21,23,33).
2.5. Data visualizations
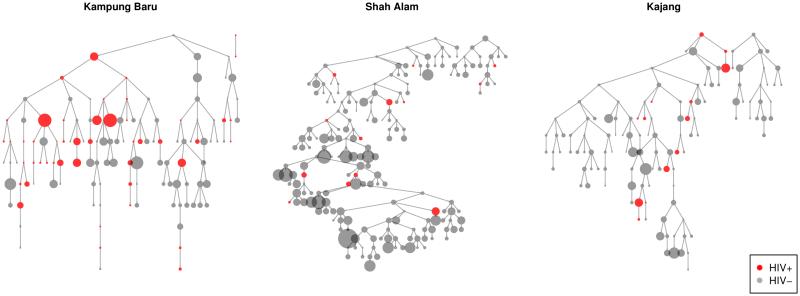
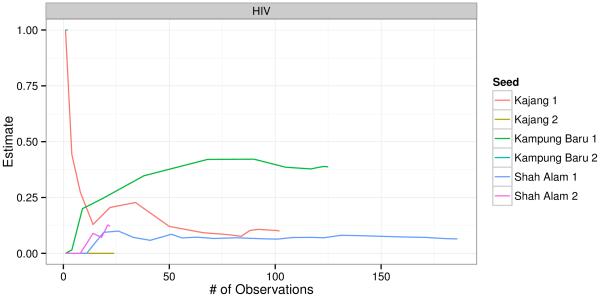
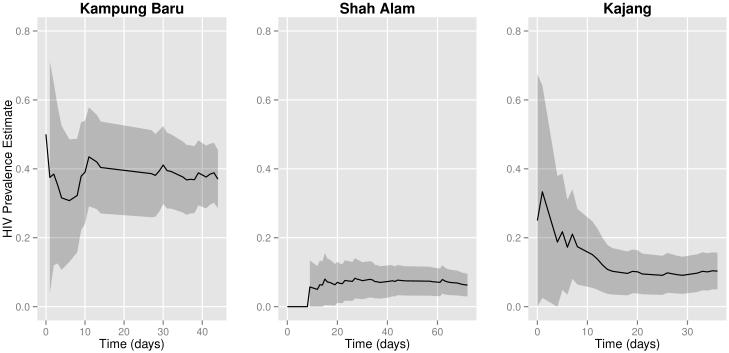
We present data visualizations to explore potential heterogeneity in HIV prevalence across recruitment sites and recruitment chains. The igraph package in R was used to plot recruitment graphs for each site (Figure 2) (34). Cumulative sample prevalence plots for each recruitment chain were created using the RDS package in R (Figure 3); the x-axis in these plots is the set of participants ordered by when they were recruited (25,32). We present our own cumulative prevalence plots for each site that show the cumulative HIV prevalence over time since beginning recruitment (Figure 1). Cumulative prevalence plots can provide visual evidence for whether the sample exhibits dependence from initial seed selection and whether recruitment reaches individuals with different characteristics over time (25).
Figure 2.
[Recruitment graphs by interview site. Vertex size is proportional to network size.]
Figure 3.
[Cumulative HIV prevalence by recruitment chain.]
Figure 1.
[Cumulative HIV prevalence estimates for each site from the sample mean. Shaded areas represent bootstrapped 95% confidence intervals.]
3. Results
3.1. Participant characteristics
We recruited a total of 461 participants, 127 (27.5%) from Kampung Baru, 208 (45.1%) from Shah Alam, and 126 (27.3%) from Kajang. All participants reported using opioids in the prior six months, and nearly all (99.1%) screened positive for opioid use on urine toxicology testing and met criteria for opioid dependence. The mean age was 39 years at all sites. The sample was predominantly male (12.6% women recruited at Kampung Baru and less than 1% at Shah Alam and Kajang) and Malay (87.4%, 94.7% and 86.4% at Kampung Baru, Shah Alam and Kajang, respectively). While 39.2% of participants from the Kajang site reported incomes below the poverty line, only 18.2% from Kampung Baru and 15.4% from Shah Alam did so. Drug use varied by site, with methamphetamine use in the past 6 months reported by 32.3% in Kampung Baru, 50.4% in Shah Alam and 40.1% in Kajang and with benzodiazepine use in the past 6 months reported by 33.9% at Kampung Baru, 34.6% in Shah Alam and 55.2% in Kajang. On average, participants at all sites reported injecting more than three times per day, and the vast majority were daily injectors (Table I). More detailed data on drug use and drug treatment are reported elsewhere (35,36).
Table I.
Participant characteristic by recruitment site
|
KAMPUNG
BARU |
SHAH
ALAM |
KAJANG | ALL SITES | |
|---|---|---|---|---|
| n = 127 (100%) |
n = 208 (100%) |
n = 126 (100%) |
n = 461 (100%) |
|
| DEMOGRAPHICS | ||||
| Age | ||||
| Mean (SD) | 39.4 (8.7) | 38.5 (9.3) | 38.7 (9.5) | 38.8 (9.2) |
| Gender | ||||
| Male | 111 (87.4%) | 207 (99.5%) | 125 (100%) | 443 (96.3%) |
| Female | 16 (12.6%) | 1 (0.5%) | 0 (0%) | 17 (3.7%) |
| Ethnicity | ||||
| Malay | 111 (87.4%) | 197 (94.7%) | 108 (86.4%) | 416 (90.4%) |
| Chinese | 5 (3.9%) | 3 (1.4%) | 4 (3.2%) | 12 (2.6%) |
| Indian | 11 (8.7%) | 7 (3.4%) | 13 (10.4%) | 31 (6.7%) |
| Other | 0 (0%) | 1 (0.5%) | 0 (0%) | 1 (0.2%) |
| Income + | ||||
| At or below poverty line | 23 (18.1%) | 32 (15.4%) | 49 (39.2%) | 104 (22.6%) |
| Education | ||||
| Completed secondary school | 56 (44.1%) | 96 (46.2%) | 65 (52.0%) | 217 (47.2%) |
| Personal network size | ||||
| Mean (SD) | 17.6 (24.3) | 26.1 (33.9) | 13.6 (17.2) | 20.3 (28.1) |
| DRUG USE | ||||
| Years of Drug Injection | ||||
| Mean (SD) | 15.8 (9.3) | 14.7 (9.0) | 14.9 (9.5) | 15.1 (9.2) |
| Injection frequency+ | ||||
| Injections per day, mean (SD) | 3.5 (1.6) | 3.1 (1.5) | 3.6 (1.8) | 3.4 (1.6) |
| Daily injector | 118 (92.9%) | 183 (88.0%) | 119 (95.2%) | 420 (91.3%) |
| HIV RISK BEHAVIORS+ | ||||
| Receptive syringe sharing | 10 (7.9%) | 61 (29.3%) | 19 (15.2%) | 90 (19.6%) |
| Receptive needle sharing | 81 (63.8%) | 49 (23.6%) | 72 (57.6%) | 202 (43.9%) |
| Cooker sharing | 48 (37.8%) | 105 (50.5%) | 48 (38.4%) | 201 (43.7%) |
| Any receptive sharing of needles or syringes |
81 (63.8%) | 64 (30.8%) | 76 (60.8%) | 221 (48.0%) |
| Any needle or syringe sharing partners | 11 (8.7%) | 79 (38.0%) | 19 (15.1%) | 109 (23.7%) |
| Other person administered injection | 47 (37.0%) | 109 (52.4%) | 52 (41.3%) | 208 (45.1%) |
| Injected with a stranger | 20 (15.7%) | 35 (16.8%) | 24 (19.0%) | 79 (17.1%) |
| No needle exchange use, prior 6 months |
20 (15.7%) | 189 (90.8%) | 68 (54.4%) | 277 (60.2%) |
| Unprotected sexual intercourse | 28 (22.1%) | 75 (36.1%) | 33 (26.4%) | 136 (29.6%) |
Assessed in prior 30 days unless otherwise noted.
Engagement in HIV risk behaviors in the previous month was common. Unprotected sexual intercourse was reported by 22.1%, 36.1% and 26.4% of participants at Kampung Baru, Shah Alam and Kajang, respectively. Engaging in any receptive sharing of needle or syringes occurred among nearly two thirds of participants from the Kampung Baru (63.8%) and Kajang (60.8%) sites and slightly less than a third (30.8%) of those from the Shah Alam site. Yet more participants reported having any sharing partners in Shah Alam (38.0%) than in Kampung Baru (8.7%) or Kajang (15.1%). Needle exchange use varied widely by site, with 90.9% in Shah Alam, 54.4% in Kajang and 15.7% in Kampung Baru reporting not having received any injection equipment from a needle exchange in the prior six months.
3.2. HIV prevalence estimates
HIV prevalence varied considerably by site of recruitment: estimated prevalence was 37.0% (47/127, 95% CI: 28.6 - 45.4%) among participants interviewed in Kampung Baru, 6.3% (13/208, 95% CI: 3.0 - 9.5%) among those interviewed in Shah Alam, and 10.3% (13/126, 95% CI: 5.0 - 15.6%) among those interviewed in Kajang. Estimated confidence intervals from the sample mean and other RDS estimators were broadly overlapping, and point estimates from the different estimators did not differ appreciably within each site (Table II).
Table II.
HIV prevalence estimates, including seeds
| KAMPUNG BARU | SHAH ALAM | KAJANG | ALL SITES | |
|---|---|---|---|---|
| Reactive HIV Test | 47/127 | 13/208 | 13/126 | 73/461 |
| Sample mean (95% CI) | 37.0% (28.6 – 45.4) | 6.3% (3.0 – 9.5) | 10.3% (5.0 – 15.6) | 15.8% (12.5 – 19.2) |
| RDS-I estimator (95% CI) | 38.8% (25.0 – 52.6) | 7.2% (2.6 – 11.8) | 9.5% (0.0 – 22.1) | 20.0% (13.0 – 27.0) |
| RDS-II estimator (95% CI) | 40.8% (26.9 – 54.6) | 7.1% (2.6 – 11.7) | 8.0% (0.0 – 21.0) | 20.4% (13.6 – 27.3) |
| RDS-SS estimator (95% CI) | 40.8% (26.7 – 54.9) | 7.1% (2.5 – 11.7) | 8.1% (0.0 – 17.7) | 20.4% (13.1 – 27.7) |
Given considerable recruitment across neighborhoods (See Section 3.5 below), we also present a pooled estimate of HIV prevalence from all three sites. Using the sample mean, the estimated HIV prevalence overall was 15.8% (73/461, 95% CI: 12.5 - 19.2%).
3.3. Plotting changes in estimated HIV prevalence over time since initiating recruitment
Cumulative sample prevalence and bootstrapped confidence intervals at different times in the sampling process are shown in Figure 1. Each point represents the HIV prevalence estimated with the sample mean using all people recruited up to that time. Plots are stratified by interview site. Similar plots for all RDS estimators are presented in Figure A1 in the appendix. These plots show that the cumulative sample prevalence within each recruitment site remains relatively stable after the first two to three weeks of recruitment.
3.4. Recruitment networks
Figure 2 shows the recruitment chains with HIV status indicated by color and network size proportional to node size. HIV-infected individuals recruited 44% other HIV-infected individuals, while HIV-uninfected individuals recruited only 10% HIV-infected individuals. Applying a chi-squared test to the contingency table of recruiter and recruit HIV status shows that recruiter’s HIV status was not independent of recruit HIV status (p<0.001). Similar deviations from independence were observed between participants’ HIV status and the HIV status of recruiters one and two steps further up the recruitment chain (recruiter’s recruiters, p=0.004, recruiter’s recruiter’s recruiters, p=0.006).
One of the sampling assumptions for the RDS estimators is that recruitment ties between subjects represent reciprocal relationships (25). In our study, only 5 participants (1.1%) stated that they had received their coupon from a stranger. All other respondents reported receiving the coupon from a friend, acquaintance, or relative, which supports the assumption of reciprocation.
The longest recruitment chain extended 11 waves in Kampung Baru, 25 waves in Shah Alam, and 14 waves in Kajang.
3.5. Geographic distribution of recruitment
Supplementary Table A2 in the online appendix shows participants’ place of residence and the interview site closest to their place of residence. The vast majority of participants interviewed in Kampung Baru lived closest to Kampung Baru (124/127), as did most of those interviewed in Shah Alam (130/208) and some of those interviewed in Kajang (20/126). While only 28% (127/461) of all participants were interviewed in Kampung Baru, the majority of the sample (59%, 214/461) resided closest to the Kampung Baru site. While recruitment was concentrated around the Kampung Baru site, in some cases recruitment extended over a broad geographic area.
Supplementary Table A3 shows that while recruitment from each interview site extended to different neighborhoods across the city, recruitment was concentrated near recruitment sites, and recruitment in certain regions appears to have been structured geographically. More than half (57%, 41/72) of those residing closest to Shah Alam recruited individuals living closest to Kampung Baru. While participants living near Kajang primarily recruited others living closest to Kajang (80%, 92/115), 14% (16/115) also recruited individuals living closest to Kampung Baru. There were very few recruitment events between individuals living near Kajang and those living near Shah Alam. Only 3% (4/115) of those living near Kajang recruited people living near Shah Alam, and only 1% (1/72) of those living near Shah Alam recruited individuals from Kajang. Supplementary Figure A2 displaying the frequency of recruitments between disaggregated neighborhoods shows that recruitment occurred across many neighborhood pairs.
3.6. Cumulative sample prevalence plots for each recruitment chain
Figure 3 is a plot showing the cumulative sample prevalence of HIV separately for each recruitment chain. Three of the chains, one from each recruitment site, grew to include more than 25 respondents. By the time these chains accumulated 50 participants, the cumulative prevalence of HIV was similar in the longer chain from Kajang and the longer chain from Shah Alam. The longer chain from Kampung Baru has a noticeably higher prevalence by the time this chain includes 50 participants. This plot suggests that the prevalence in the final sample may be heavily influenced by the selection of the initial seeds and that the chain resulting from the first Kampung Baru seed may have sampled from a social network with high HIV prevalence, which could bias our estimates.
3.7. Network size distribution
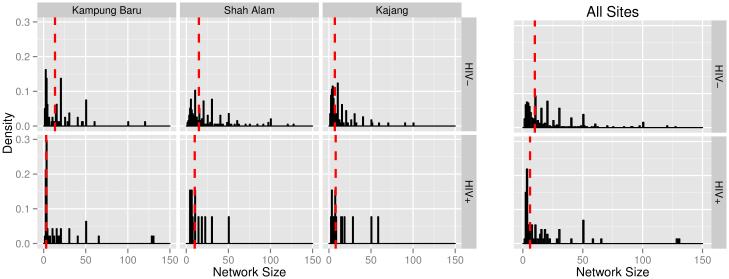
We present a summary of the network size distribution stratified by recruitment site and HIV status in Figure 4 and Table III. Network size reflects how many connections participants have to other PWID. Additionally, network size is incorporated in different ways by the RDS estimators, and differences in network size between HIV-infected and uninfected people are responsible for differences between adjusted and unadjusted estimates of HIV prevalence. With a median network size of 10 (S.D. 28), participants generally had numerous connections to other PWID. Self-reported network size is highly variable, and there is evidence of rounding to multiples of 10. Testing the difference in the distributions of network size between HIV-infected and HIV-uninfected individuals using the Wilcoxon rank-sum test, we find no difference within each site (Kampung Baru p=0.197, Shah Alam p=0.142, Kajang p=0.147; Table III). In the pooled sample, however, the distribution of network size differs between HIV-infected and HIV-uninfected individuals (HIV-uninfected median=10, HIV-infected median=6, p=0.005), which is why unadjusted and adjusted HIV prevalence estimates differ slightly.
Figure 4.
[Network size distribution by recruitment site and HIV status. Red lines indicate medians. Truncated at 150, excluding n=1 participant reporting a network size of 300.]
Table III.
Network size distribution by recruitment site and HIV status
| KAMPUNG BARU |
SHAH ALAM |
KAJANG | ALL SITES | |
|---|---|---|---|---|
| Median network size (SD), HIV-uninfected | 13.5 (21.6) | 15 (34.7) | 7 (17.1) | 10 (28.7) |
| Median network size (SD), HIV-infected | 3 (28.6) | 10 (13.3) | 8 (18.1) | 6 (24.6) |
| Median network size (SD), overall | 7 (24.3) | 15 (33.9) | 7 (17.2) | 10 (28.1) |
|
Test of difference between HIV+ and HIV−
(Wilcoxon rank-sum test statistic) |
p=0.197 (W=2137) |
p=0.142 (W=1576) |
p=0.147 (W=633) |
p=0.005
(W=17069) |
4. Discussion
The confidence interval for our pooled HIV prevalence estimate of 15.8% (95% CI: 12.5-19.2%) includes the Ministry of Health’s nationwide estimate of 18.9% and, more broadly, overlaps with estimates for other countries in East and Southeast Asia (1,37). Since nearly half of our participants were sampled from the Shah Alam site, the site with the lowest HIV prevalence, our estimate of 15.8% may be an underestimate of the HIV prevalence among PWID in Greater Kuala Lumpur. Previously published studies not employing methods for estimating population prevalence have reported high levels of HIV infection in their samples, including a 1993 study of 210 PWID undergoing detoxification at a hospital in northeast Malaysia where 30% of participants were HIV-infected (38), and a 2007 study that used snowball sampling to recruit 526 individuals from 5 different cities and reported a pooled sample prevalence of 43.9% without disaggregating by city (39). It is not possible to compare our estimates with these previous studies.
The high proportion of participants engaging in HIV risk behaviors in our sample has important implications for the future of the HIV epidemic in Kuala Lumpur. High levels of risk behaviors in Kampung Baru have the potential to facilitate continued transmission among people in this already high-prevalence region and also potentially among PWID from other regions who might inject drugs in Kampung Baru. The high proportion of participants from Shah Alam and Kajang engaging in HIV risk behaviors represents a potential threat for expansion of the epidemic among PWID in these areas. Furthermore, the frequency of recruitment events across neighborhood boundaries suggests that PWID are not geographically static. The potential for HIV transmission is augmented when PWID know other PWID in distant neighborhoods and are willing to travel significant distances to meet them, a behavior that is consistent with our data. Additionally, if participants underreported HIV risk behaviors due to social desirability bias, the prevalence of HIV risk behaviors could be even higher than our sample suggests.
We found that the distribution of network size differed significantly among HIV-infected and HIV-uninfected individuals, with a lower median network size among HIV-infected individuals. This runs counter to previous research showing that increased social ties to PWID is associated with HIV risk (40). One explanation is that since networks of PWID are not static (41,42), HIV infections could be long-standing and not related to current network characteristics. Alternatively, it is possible that HIV-infected individuals, such as those from the densely populated area around Kampung Baru, have more turnover in their networks and thus more casual or newly-formed ties, which have been associated with increased risk behaviors and also may be underreported (40-43).
Different levels of coverage of HIV prevention interventions may be required to impact transmission rates in regions with different levels of HIV prevalence (44). Regional differences in both HIV risk behaviors and prevalence in our sample may require different intervention strategies. In Kampung Baru, where HIV prevalence is already extremely high, high levels of coverage of needle exchange, opioid maintenance therapy, and antiretroviral therapy for HIV-infected PWID may be needed to achieve meaningful reductions in HIV prevalence. The Kampung Baru recruitment site was close to the oldest harm reduction organization in Malaysia, which may explain why a large proportion of participants sampled there reported accessing a needle exchange. Primary prevention interventions in settings such as Shah Alam and Kajang, with moderate HIV prevalence and high levels of HIV risk behaviors, are urgently needed to keep prevalence from increasing. Many participants from Kajang reported accessing a needle exchange while very few from Shah Alam did, likely due to recent establishment of a needle exchange program in Kajang and the absence of one in Shah Alam.
We observed frequent recruitment between coarsened geographic regions. Recruitment between individuals living near Shah Alam and those living near Kampung Baru was relatively frequent as was recruitment between individuals living near Kajang and those living near Kampung Baru Connections between PWID living in Shah Alam and Kajang and those living in Kampung Baru may be common because of Kampung Baru’s central location either as a transportation hub or because it may serve as a drug distribution center.
Recruitment occurred within and between diverse geographic regions, though many neighborhoods were not linked by recruitment, which could be due to barriers to recruitment, or bottlenecks, across geographic space (25), or to the limited sample size. Few recruitments occurred between people residing in Shah Alam and those residing in Kajang. This could have been due to our policy of allowing participants to be interviewed only at the site where the seed in their recruitment chain was interviewed. As recruitment chains extended to reach people who lived closer to other interview sites, greater geographic mixing might have occurred had we allowed interviews to be conducted at any research site. Limited recruitment between particular regions could be evidence of a violation of RDS sampling assumptions related to achieving a final sample that does not depend on the non-random initial selection of seeds (25). Previous studies have also documented the influence of geography on RDS recruitment, including how recruitment can be concentrated around study sites and may not cross certain geographic boundaries (45-48). Dependence within recruitment chains, of which we found evidence with respect to HIV status, also poses a threat to sampling assumptions (25). Differences in reported drug use between sites also suggests that these samples may have been drawn from heterogeneous sub-populations that could be separated by network or geographical bottlenecks. This heterogeneity across interview sites and recruitment chains highlights the potential role of the location of research sites and the selection of seeds in influencing estimates of HIV prevalence.
Our study has several additional limitations, some unique to our study and some shared by all RDS studies. In the absence of a sampling frame and with many aspects of the RDS recruitment process hidden to researchers, the source and magnitude of bias in estimates are difficult to characterize. In our study, it is possible that we undersampled women and non-Malay ethnic groups, but evaluating the representativeness of our sample is difficult without better data on the underlying population of PWID in Greater Kuala Lumpur. Few data are available on women who inject drugs in Malaysia or elsewhere (49). More than one third (36%) of participants reported knowing at least one woman who injected drugs and, on average, participants' reported networks included one woman who injected drugs for every eleven men, so it is likely that with no female participants in Kajang and only one in Shah Alam, women were undersampled. There is added stigma around drug use for women in Malaysia, which may have made women less likely to enter a setting frequented by PWID for fear of revealing their membership in the population (50,51). Additionally, underlying social networks of PWID may cluster by sex and participants may have been less able to recruit female members of their networks. Malays were overrepresented in our sample compared to their numbers in Greater Kuala Lumpur (52). Malays are also overrepresented among PWID in the criminal justice system and compulsory drug detention centers (4,53), but we may have undersampled ethnically Chinese and Indian Malaysians, possibly because of homophily in network formation or preferential recruitment by ethnicity. Additionally, although the vast majority of residents of Greater Kuala Lumpur can communicate in Bahasa Malaysia or English, not offering research interviews in any other language could have discouraged participation by non-Malay ethnic groups.
Participants in our sample had numerous connections to other PWID, making RDS an effective means for rapidly recruiting PWID in Greater Kuala Lumpur. In the absence of adequate HIV prevention efforts including sufficient access to safe injection equipment, however, social ties may also serve as injection equipment sharing partnerships, representing potential avenues of disease transmission.
5. Conclusion
Regular active surveillance of community-dwelling PWID is necessary to effectively monitor the HIV epidemic in Malaysia and elsewhere, and surveillance data and methodology should be publicly available. Sustained support for expanding evidence-based HIV prevention interventions, including needle exchange, methadone maintenance therapy, and antiretroviral therapy for PWID, is needed to stem the epidemic of HIV in Malaysia. Site-specific differences in HIV prevalence may necessitate the implementation or expansion of different types of prevention efforts.
Supplementary Material
Acknowledgements
This work was supported by NIH career development (FLA: NIDA K24 DA017072, AZ: NIDA K01 DA037826, FC: NCATS KL2 TR000140), research (NIDA FLA, AK: NIDA R01 DA032106, FC: NIMH/CIRA P30MH062294), and training (ARB: T32 GM07205, NIMH T32 MH020031, NIDA F30 DA039716) grants as well as University Malaya’s High Impact Research Grant (AK: E-000001-20001) and the Yale Downs Fellowship (ARB). OraSure Technologies, Inc. provided discounted rapid HIV tests. Funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- 1.Malaysia Country Responses to HIV/AIDS AIDS/STD Section, Diseases Control Division, Ministry of Health, Government of Malaysia. 2014 Reporting period: January 2013 - December 2013. [Google Scholar]
- 2.Kamarulzaman A. Impact of HIV prevention programs on drug users in Malaysia. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009;52:S17–S19. doi: 10.1097/QAI.0b013e3181bbc9af. [DOI] [PubMed] [Google Scholar]
- 3.Malaysian AIDS Council, Malaysian AIDS Foundation 2013 Annual Report. 2014 [Google Scholar]
- 4.Fu JJ, Bazazi AR, Altice FL, Mohamed MN, Kamarulzaman A. Absence of antiretroviral therapy and other risk factors for morbidity and mortality in Malaysian compulsory drug detention and rehabilitation centers. PloS one. 2012;7(9):e44249. doi: 10.1371/journal.pone.0044249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degenhardt L, Mathers BM, Wirtz AL, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010-2012? A review of the six highest burden countries. International Journal of Drug Policy. 2014;25(1):53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Ghani MA, Brown SE, Khan F, et al. An exploratory qualitative assessment of self-reported treatment outcomes and satisfaction among patients accessing an innovative voluntary drug treatment centre in Malaysia. Int J Drug Policy. 2015 Feb;26(2):175–182. doi: 10.1016/j.drugpo.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin H, Earnshaw VA, Wickersham JA, et al. An assessment of health-care students' attitudes toward patients with or at high risk for HIV: implications for education and cultural competency. AIDS Care. 2014;26(10):1223–1228. doi: 10.1080/09540121.2014.894616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Act 234: Dangerous Drugs Act 1952. 2006. Revised.
- 9.Act 283: Drug Dependents (Treatment and Rehabilitations) Act 1983. 2006. Revised.
- 10.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Social Problems. 1997:174–199. [Google Scholar]
- 11.Salganik MJ, Heckathorn DD. Sampling and estimation in hidden populations using respondent-driven sampling. Sociological methodology. 2004;34(1):193–240. [Google Scholar]
- 12.Gile KJ, Handcock MS. Respondent-Driven Sampling: An Assessment of Current Methodology. Sociological methodology. 2010 Aug;40(1):285–327. doi: 10.1111/j.1467-9531.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malekinejad M, Johnston LG, Kendall C, Kerr LRFS, Rifkin MR, Rutherford GW. Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS and Behavior. 2008;12(1):105–130. doi: 10.1007/s10461-008-9421-1. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention HIV infection and HIV-associated behaviors among injecting drug users-20 cities, United States. MMWR. 2009;2012;61(8):133. [PubMed] [Google Scholar]
- 15.Introduction to HIV, AIDS and sexually transmitted infection surveillance >World Health Organization, UNAIDS. 2010 [Google Scholar]
- 16.Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Social Problems. 2002;49(1):11–34. [Google Scholar]
- 17.Volz E, Heckathorn DD. Probability based estimation theory for respondent driven sampling. Journal of Official Statistics. 2008;24(1):79–97. [Google Scholar]
- 18.Gile KJ, Handcock MS. Network model-assisted inference from respondent-driven sampling data. arXiv preprint arXiv:1108.0298. 2011 doi: 10.1111/rssa.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heimer R, et al. Critical issues and further questions about respondent-driven sampling: comment on Ramirez-Valles. AIDS and Behavior. 2005;2005;9(4):403–408. doi: 10.1007/s10461-005-9030-1. [DOI] [PubMed] [Google Scholar]
- 20.White RG, Lansky A, Goel S, et al. Respondent driven sampling--where we are and where should we be going? Sexually Transmitted Infections. 2012;88(6):397–399. doi: 10.1136/sextrans-2012-050703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel S, Salganik MJ. Assessing respondent-driven sampling. Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6743–6747. doi: 10.1073/pnas.1000261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salganik MJ. Commentary: respondent-driven sampling in the real world. Epidemiology. 2012;23(1):148–150. doi: 10.1097/EDE.0b013e31823b6979. [DOI] [PubMed] [Google Scholar]
- 23.McCreesh N, Frost S, Seeley J, et al. Evaluation of respondent-driven sampling. Epidemiology. 2012;23(1):138–147. doi: 10.1097/EDE.0b013e31823ac17c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford FW. The graphical structure of respondent-driven sampling. arXiv:1406.0721. 2014 doi: 10.1177/0081175016641713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gile KJ, Johnston LG, Salganik MJ. Diagnostics for respondent-driven sampling. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2015 Jan;178(1):241–269. doi: 10.1111/rssa.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salganik MJ. Variance estimation, design effects, and sample size calculations for respondent-driven sampling. Journal of Urban Health. 2006;83(1):98–112. doi: 10.1007/s11524-006-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarty C, Killworth PD, Bernard RH, Johnsen EC, Shelley GA. Comparing two methods for estimating network size. Human Organization. 2001;60(1) [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. 34–57. quiz. [PubMed] [Google Scholar]
- 29.Kahle D, Wickam H. ggmap: Spatial visualization with ggplot2. R package Version 2.3. The R Journal. 2013;5(1):144–161. http://journal.r-project.org/archive/2013-1/kahle-wickham.pdf [Google Scholar]
- 30.R Core Team R: A Language and Environment for Statistical Computing. 2015 http://www.R-project.org/
- 31.Gile KJ. Improved Inference for Respondent-Driven Sampling Data With Application to HIV Prevalence Estimation. Journal of the American Statistical Association. 2011;106(493):135–146. [Google Scholar]
- 32.Handcock MS, Fellows IE, Gile KJ. RDS: Respondent-Driven Sampling. R package version 0.7-2. 2015 http://CRAN.R-project.orgpackage=RDS.
- 33.Wejnert C. An empirical test of respondent-driven sampling: point estimates, variance, degree measures, and out-of-equilibrium data. Sociological methodology. 2009;39(1):73–116. doi: 10.1111/j.1467-9531.2009.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csardi Gabor, Nepusz T. The igraph software package for complex network research. InterJournal. 2006 Complex Systems. [Google Scholar]
- 35.Bazazi AR, Zelenev A, Fu JJ, Yee I, Kamarulzaman A, Altice FL. High prevalence of non-fatal overdose among people who inject drugs in Malaysia: Correlates of overdose and implications for overdose prevention from a cross-sectional study. Int J Drug Policy. 2014 Dec 2; doi: 10.1016/j.drugpo.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijay A, Bazazi AR, Yee I, Kamarulzaman A, Altice FL. Treatment readiness, attitudes toward, and experiences with methadone and buprenorphine maintenance therapy among people who inject drugs in Malaysia. Journal of Substance Abuse Treatment. 2015 doi: 10.1016/j.jsat.2015.01.014. e-Pub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008 Nov 15;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Crofts N. HIV infection among injecting drug users in north-east Malaysia, 1992. AIDS Care. 1993;5(3):273–281. doi: 10.1080/09540129308258610. [DOI] [PubMed] [Google Scholar]
- 39.Vicknasingam B, Narayanan S, Navaratnam V. The relative risk of HIV among IDUs not in treatment in Malaysia. AIDS Care. 2009;21(8):984–991. doi: 10.1080/09540120802657530. [DOI] [PubMed] [Google Scholar]
- 40.De P, Cox J, Boivin JF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007 Nov;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 41.Costenbader EC, Astone NM, Latkin CA. The dynamics of injection drug users' personal networks and HIV risk behaviors. Addiction. 2006 Jul;101(7):1003–1013. doi: 10.1111/j.1360-0443.2006.01431.x. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann JP, Su SS, Pach A. Changes in network characteristics and HIV risk behavior among injection drug users. Drug Alcohol Depend. 1997 Jun 6;46(1-2):41–51. doi: 10.1016/s0376-8716(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 43.Rudolph AE, Fuller CM, Latkin C. The importance of measuring and accounting for potential biases in respondent-driven samples. AIDS and behavior. 2013;17(6):2244–2252. doi: 10.1007/s10461-013-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vickerman P, Platt L, Jolley E, Rhodes T, Kazatchkine MD, Latypov A. Controlling HIV among people who inject drugs in Eastern Europe and Central Asia: Insights from modelling. International Journal of Drug Policy. 2014 Nov;25(6):1163–1173. doi: 10.1016/j.drugpo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Toledo L, Codeco CT, Bertoni N, et al. Putting respondent-driven sampling on the map: insights from Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr. 2011 Aug;57(Suppl 3):S136–143. doi: 10.1097/QAI.0b013e31821e9981. [DOI] [PubMed] [Google Scholar]
- 46.McCreesh N, Johnston LG, Copas A, et al. Evaluation of the role of location and distance in recruitment in respondent-driven sampling. International journal of health geographics. 2011;10(1):56. doi: 10.1186/1476-072X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young AM, Rudolph AE, Quillen D, Havens JR. Spatial, temporal and relational patterns in respondent-driven sampling: evidence from a social network study of rural drug users. Journal of epidemiology and community health. 2014;68(8):792–798. doi: 10.1136/jech-2014-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu P, Yang Y, Ma X, et al. Respondent-driven sampling to recruit in-country migrant workers in China: A methodological assessment. Scandinavian journal of public health. 2012;40(1):92–101. doi: 10.1177/1403494811418276. [DOI] [PubMed] [Google Scholar]
- 49.Springer SA, Larney S, Mehrjerdi ZA, Metzger D, Altice FL, Shoptaw S. Drug treatment as HIV prevention among women and girls who use drugs from a global perspective: Progress, gaps and future directions. J.Acquir.Immune Defic.Syndr. 2015 doi: 10.1097/QAI.0000000000000637. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MInistry of Health Malaysia, UNICEF Women and girls: confronting HIV and AIDS in Malaysia. 2008 http://www.unicef.org/malaysia/Women_and_AIDSReport2008%281%29.pdf
- 51.Wickersham JA, Loeliger K, Marcus R, Pillai V, Kamarulzaman A, Altice FL. Correlates of Lifetime and Active Injection Drug Use Among Women in Malaysia. American Journal of Drug and Alcohol Abuse. 2015 doi: 10.3109/00952990.2015.1101467. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Department of Statistics Malaysia 2010 Population and housing census of Malaysia: population distribution and basic demographic characteristics. 2011 [Google Scholar]
- 53.Bachireddy C, Bazazi AR, Kavasery R, Govindasamy S, Kamarulzaman A, Altice FL. Attitudes toward opioid substitution therapy and pre-incarceration HIV transmission behaviors among HIV-infected prisoners in Malaysia: implications for secondary prevention. Drug Alcohol Depend. 2011 Jul 1;116(1-3):151–157. doi: 10.1016/j.drugalcdep.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.